Abstract
The addition of protonating acids to e-cigarette liquid formulations (e-liquids) enhances nicotine bioavailability in e-cigarette use. However, little is known about the impact of different combinations of protonating acid on nicotine pharmacokinetics. The objectives of this study were to compare pharmacokinetics of nicotine absorption following use of a closed-system e-cigarette, containing e-liquids with two different nicotine levels and with different ratios of three common protonating acids—lactic, benzoic and levulinic. In a randomised, controlled, crossover study, nicotine pharmacokinetics and product liking were assessed for prototype e-liquids used in a Vuse e-cigarette containing either 3.5% or 5% nicotine and varying ratios of lactic, benzoic and/or levulinic acid. During an 8-day confinement period, 32 healthy adult current cigarette smokers/e-cigarette dual users used a single study e-liquid each day during 10-min fixed and ad libitum use periods after overnight nicotine abstinence. For most comparisons, Cmax and AUC0–60 following both fixed and ad libitum puffing were significantly higher for e-liquids containing 5% nicotine compared with 3.5% nicotine. However, Cmax and AUC0–60 were not statistically different for 5% nicotine e-liquids containing varying ratios of lactic, levulinic and benzoic acid when compared to an e-liquid containing lactic acid only. Mean scores for product liking were similar for all e-liquid formulations assessed, regardless of nicotine concentration, acid content, and whether the product was used in a fixed or ad libitum puffing regimen. While e-liquid nicotine concentration significantly affected users’ nicotine uptake, the different combinations of benzoic, levulinic and lactic acid in the e-liquids assessed had limited impact on nicotine pharmacokinetics and product liking scores.
Similar content being viewed by others
Introduction
The fundamental principle of tobacco harm reduction is that the health burden of combustible cigarette smoking at both the individual and population levels can be reduced by encouraging smokers to switch to nicotine and tobacco products that support combustible cigarette displacement1. While not risk free, smokers who switch to alternative tobacco and nicotine products reduce exposure to the smoke toxicants responsible for the morbidity and mortality associated with cigarette smoking, as compared to continued cigarette smoking1,2. One such alternative product is the electronic cigarette (e-cigarette), which electrically heats a liquid formulation (e-liquid) to produce an inhalable vapour3. Studies have shown e-cigarette vapour contains far fewer and substantially lower levels of harmful toxicants compared with cigarette smoke2,4,5,6, and research has indicated significant reductions in biomarkers of toxicant exposure when smokers switch completely to e-cigarettes7,8,9,10,11,12. Although not marketed for this purpose, a growing body of evidence stemming from interventional and observational studies suggest that e-cigarettes are effective in supporting smoking cessation13,14,15,16,17,18,19,20,21,22,23. Furthermore, population modeling has shown that reductions in smoking prevalence supported by exclusive e-cigarette use offer large improvements in population health by reducing smoking-related mortality24,25.
It has been proposed that alongside offering sensorial performance and some subjective effects similar to those experienced during combustible cigarette smoking, e-cigarettes that more closely match the nicotine delivery from combustible cigarettes are likely to have greater acceptance and are also likely to be more effective for adult smokers in providing a complete substitute for cigarette smoking26,27,28,29. In a recent study of adult smokers with no plans to quit smoking, participants randomised to using an e-cigarette containing 36 mg/ml nicotine were significantly more likely than those randomised to 0 mg/ml nicotine to report at least 28 days cigarette smoking abstinence after 24 weeks of use, and were also significantly more likely than those using an e-cigarette containing 8 mg/ml nicotine to report at least one or more days of cigarette smoking abstinence and more total days of cigarette smoking abstinence throughout the study30. Similar effects have been reported for other nicotine products, with improved nicotine delivery profiles associated with better cigarette smoking cessation support and relapse prevention31,32.
There are several means by which nicotine delivery to e-cigarette users can be increased, such as increasing the e-liquid nicotine concentration28,33, increasing the aerosol mass by applying greater power to the heating coil28, and/or adding protonating acids to the e-liquid28,33. Due to the lower volatility of protonated nicotine compared with unprotonated nicotine, e-liquids with protonated nicotine result in greater amounts of nicotine remaining in the aerosol particles until they reach the highly vascularised lung alveoli for absorption. Conversely, e-liquids with the more volatile unprotonated nicotine results in nicotine evaporation from the aerosol particles earlier and absorption therefore occurs mainly in the oral cavity and upper respiratory tract33. It was recently demonstrated that e-cigarette liquid protonation increases nicotine absorption, such that increasing either the nicotine content or the level of benzoic acid28 or lactic acid33 in the e-liquid increased nicotine bioavailability. Therefore, including protonating acids in e-cigarettes has the potential to more closely match nicotine delivery from combustible cigarettes, which may encourage more current smokers to use e-cigarettes as a complete substitute for combustible cigarettes.
The addition of various different protonating acids to e-liquid formulations is becoming more prevalent, with lactic, benzoic and levulinic acids recently identified as the three most commonly used among “top sellers”34. It is also increasingly acknowledged that this impacts nicotine delivery and pharmacokinetics from both e-cigarettes28,33 and other inhaled nicotine devices27, although little is known about the relative impact of combining different protonating acids on nicotine pharmacokinetics. The objectives therefore of the present study were to determine and compare the pharmacokinetics of nicotine absorption into the blood of cigarette smokers/e-cigarette dual users when using a closed-system e-cigarette filled with e-liquids containing various nicotine levels and with various ratios of lactic, levulinic and/or benzoic acids.
Methods
Study design
This was a randomised, controlled, crossover clinical study carried out at a single site operated by Simbec-Orion in Merthyr Tydfil, Wales, U.K. The study was registered on the ISRCTN registry (ISRCTN80837297). The experimental protocol was approved by the NHS Health Research Authority, Wales Research Ethics Committee 1 (reference number 20/WA/0264) prior to study commencement. The study was conducted in accordance with the protocol and under the principles of both the International Council for Harmonisation (ICH) of Technical Requirements for Pharmaceuticals for Human Use Guideline for Good Clinical Practice (GCP) E6(R2) and European Union General Data Protection Regulation (GDPR). Written informed consent was obtained from all participants prior to participation in the study and before undergoing any study procedures, including screening assessments. All participants were informed that they were free to quit smoking/using e-cigarettes and withdraw from the study, or to withdraw their consent to participate, at any time.
Participants
Potential participants attended a screening visit, and 32 healthy male or female participants who met the inclusion criteria were enrolled in the study to ensure that a minimum of 24 participants completed the study. Participants were 21–65 years of age, inclusive, and were generally healthy as determined by clinical laboratory and physical evaluations including haematology, clinical chemistry, urinalysis, serology, urine drug and alcohol screen, medical history, physical examination, lung function tests, vital signs assessment and 12-lead ECG. All participants had at least a one year history of cigarette smoking and e-cigarette use and were current dual users of combustible cigarettes (smoking ≥ 6 mg ISO tar cigarettes and a maximum of 21 per week) and e-cigarettes (regular use of e-cigarettes with an e-liquid nicotine strength of at least 18 mg/ml). A urine cotinine threshold of ≥ 200 ng/ml was used to confirm nicotine product use status. For female participants, a negative serum pregnancy test at screening and a urine pregnancy test at check-in to the clinical site was required. Women of childbearing potential were required to use an accepted form of contraception during and for 30 days after the study.
The main exclusion criteria were pregnancy or breastfeeding (women only); self-reported non-inhalation of cigarette smoke/e-cigarette vapour; participants who did not use a flavoured e-liquid (since a flavoured e-liquid was used in the study); significant history of urticaria or asthma; blood donation of ≥ 450 ml within the three months before the participant’s first study visit, or plasma donation in the seven days prior to screening, or platelets donation in the six weeks prior to screening; participants who are planning to quit smoking or e-cigarette use; or use of any medication or substance that aids smoking cessation in the 30 days prior to the study.
Investigational products
The products examined in this study were Vype ePod 2 (now Vuse ePod 2, referred to throughout as Vuse) closed-system e-cigarettes (Nicoventures Trading Ltd, London, UK) with prototype e-liquid formulations containing either 3.5% or 5% nicotine and various proportions of lactic, levulinic and/or benzoic acid (Table 1). Participant’s usual brand of combustible cigarettes was also assessed in this study.
Study procedures
At screening, participants underwent testing to ensure that they met all inclusion and no exclusion criteria and completed tobacco and nicotine product use history questionnaires and the Fagerstrom Test for Cigarette Dependence35. Participants who passed screening were admitted to the clinic site the day before the first study product use session (Day-1) and were confined to the site until the last assessments at the end of the study (i.e., for a period of approximately eight days). On admission to the clinic site, participants underwent eligibility criteria review including vital signs assessments, physical examination if indicated, a test for active COVID-19 infection, and a urine pregnancy test (women only). Subsequently, participants underwent a product use session to familiarise themselves with the study e-cigarette device, where they used the product ad libitum for a minimum three-hour duration. In this session, participants would ask clinic staff for the study product, and they were provided with one of two different e-liquid formulations in an alternating manner. The 5% nicotine, EPOD2.0_VFB50_1VA e-liquid formulation was used first, followed by the 5% nicotine, EPOD2.0_VFB50_2VI e-liquid formulation. For the remainder of the familiarisation session the alternating sequence was repeated until the initiation of the first 12-h abstinence period prior to product use the following day.
Upon completion of the product familiarisation, participants began an eight-day study period in which they used one of eight assigned study products on each day. The order in which participants used the study products was set by a predetermined randomisation sequence produced using a computer generated pseudo-random permutation procedure (SAS version 9.4). The randomisation code was produced based on a Williams Latin square design for an 8 × 8 crossover study with eight sequences and four participants per sequence.
Before the product use sessions on each study day, participants were required to abstain from using any nicotine or tobacco products for a period of at least 12 h. On the day participants were randomised to smoke their usual brand cigarettes, participants smoked a single cigarette by taking ad libitum puffs over a period of 10 min. During this session, the number of puffs taken was counted. If the cigarette was finished before the end of the 10-min session no further cigarette smoking was allowed and the time the product was finished was recorded. Participants did not smoke combustible cigarettes using the fixed-puff regime, as the purpose of this regime was to isolate the impact of different protonating acid combinations contained in the prototype e-liquids on resulting PK profiles. On the days participants were randomised to use the study e-cigarettes, participants underwent two product use sessions. The first session used a “fixed-puff” regimen (one puff every 30 s for 10 min, 21 total puffs), which was followed by a six-hour period of nicotine abstinence. After this period, participants underwent a further product use session in which they took ad libitum puffs over a 10-min period, during which puffs were counted. The purpose of the ad libitum regime was to evaluate the resulting PK profiles for the prototype e-liquids and combustible cigarette products under participants’ typical usage patterns.
For all product use sessions (excluding the familiarisation session), blood samples for nicotine pharmacokinetic analysis were taken before, during and after product use. After completion of blood sampling on each study day, participants were allowed to use their own nicotine products (combustible cigarettes and/or e-cigarettes) ad libitum until 12 h prior to the next product use session. After the last study day, participants were discharged from the clinic site and a follow-up phone call was conducted with all participants 5–7 days after discharge.
Blood sampling for nicotine pharmacokinetics
Venous blood samples (2.7 ml) were collected into lithium heparin monovette tubes at − 5 (baseline), 5, 8, 10, 15, 30, and 60 min relative to the first puff of each product use session (either fixed or ad libitum). The collected samples were processed by centrifugation at 1600g/3500 rpm at 4 °C for 10 min no later than 60 min after collection. Two equal aliquots (each containing approximately 1 ml) of plasma were transferred to storage tubes and stored at approximately -20 °C within 120 min after collection.
Plasma nicotine analysis was performed by liquid chromatography with tandem mass spectrometry detection (LC–MS–MS) using the instrument in turbo ionspray, positive ion Multiple Reaction Monitoring (MRM) mode. Two LC–MS–MS systems were used, AB Sciex API4000 and API5000 triple quadrupole atmospheric pressure ionisation mass spectrometers. An automated injection of samples took place using a Shimadzu SIL-20AC autosampler for each system. The API 5000 utilised a Shimadzu LC-20AD pump and the API4000 a Shimadzu LC-20AB pump. LC–MS–MS separation was performed using a Betasil-Silica-100 column using an isocratic method. The following MRM transitions were monitored: nicotine: m/z 163.0 → m/z 130.0, typical retention time 2.1 min; and nicotine − D4 (internal standard): m/z 167.0 → m/z 134.0, typical retention time 2.1 min. The measured method calibration range was 0.498–49.832 ng/ml. Samples above the upper limit of quantification (ULOQ) were diluted 1 in 10 and reanalysed. Each batch contained test samples, a reagent blank, a standard blank (i.e., a matrix blank with the internal standard omitted), and a standard zero (i.e., a matrix blank with the internal standard).
Subjective effects assessment
At the end of each e-cigarette product use session (60 min relative to first puff), participants completed a single product liking questionnaire to evaluate the subjective effect of study product use. Answers were given by participants responding to the question “Can you tell me how much you like this nicotine product?” by making a vertical mark on a 100 mm horizontal line, with the boundaries set as “Very much” and “Not at all”. A product liking questionnaire was not completed for the usual brand cigarette product use session.
Safety assessments
Adverse events (AEs) were recorded and monitored throughout the study from the signing of informed consent form through to the post-study follow-up visit. AEs were defined as any untoward medical occurrence in a participant to whom a product has been administered, including occurrences which are not necessarily caused by or related to that product. An AE could be any unfavorable and unintended sign, including abnormal laboratory findings considered to be clinically significant, symptom, or disease temporarily associated with the use of a study product, whether or not considered related to the product. An unexpected adverse reaction was defined as an adverse reaction where the nature or severity was not consistent with the applicable product information (e.g., documented in the Investigator’s brochure). A product-emergent adverse event (PEAE) was defined as an AE not present prior to the use of a study product or an AE already present that worsened in intensity or frequency following the use of a study product.
Serious adverse events (SAEs) were defined as any untoward medical occurrence that resulted in death, was life-threatening, required hospitalisation or prolongation of existing hospitalisation, resulted in persistent or significant disability or incapacity, was a congenital anomaly or birth defect, or was a medically significant event that required treatment/intervention to prevent one of the listed outcomes. All serious adverse events (SAEs) and AEs that had not resolved by the end of the study would be followed up by the Investigator until resolution or until the Investigator believed there would be no further change, whichever was earlier.
Sample size and statistical methods
From previous studies, it had been observed that Cmax least squares means (LSM) of smoking participants can reach values around 13–14 ng/ml in plasma with coefficients of variation (CVs) between 40 and 60% among the different arms. Based on these data, a sample size calculation was performed using the PROC POWER function in SAS (version 9.4; Cary, NC, USA) to assess superiority between the 5% nicotine EPOD2.0_VFB50_2VH and 5% nicotine EPOD2.0_VFB50_1VA e-liquids. This comparison assumed superiority to have a ratio between the Cmax (maximum plasma nicotine concentration) > 1 with a ratio between means of 1.43, β = 0.2 and α = 0.05. Based on these assumptions, 24 participants completing the study was determined to be the minimum number required to successfully demonstrate superiority, providing an actual power of 0.811.
Nicotine concentration data were analysed using SAS. Prior to the calculation of derived pharmacokinetic (PK) parameters, concentration value(s) below the lower limit of quantification (LLOQ) were assigned a value of zero if the timepoint was prior to product use and LLOQ/2 otherwise.
The nicotine PK parameters Cmax and AUC0–60 (area under the nicotine concentration-versus-time curve from time zero to 60 min after the start of study product use during each session) and Tmax (time to the maximum plasma nicotine concentration during each product use session) were determined using WinNonlin Phoenix v8.2 from the individual concentration versus time data using non-compartmental methods. Demographic and PK parameters presented here are for the PK populations, which included 29–31 participants (depending on the parameter/arm) who used the randomised product, had sufficient plasma nicotine concentration–time profiles, did not use a concomitant medication which rendered the concentration profile unreliable, and did not violate the protocol in a way that may invalidate or bias the PK results. If three or more nicotine concentration values were missing or below the limit of quantification in a single use session, the participant was excluded from the PK population for that study product. Participants would also have been excluded from the PK population if the baseline (− 5 min) nicotine concentration value was higher than the post-first puff Cmax value for that study product.
Derived PK parameters were listed and summarised for each product by gender and overall. Descriptive statistics presented were N (total study population completing each arm), n (PK set for each parameter/arm), arithmetic mean, geometric mean (with the exception of Tmax), standard deviation (SD), coefficient of variation (CV%), minimum, median and maximum. Area under the curve (AUC0–60) was calculated using the Linear Up-Log Down method. Following logarithmic transformation, Cmax and AUC0–60 values were subjected to an analysis of covariance (ANCOVA) including fixed effects for sequence, period and product and a random effect of participant nested within sequence, with baseline %Cmax as a covariate. Point estimates and 90%/95% confidence intervals (CI) were constructed for the comparisons of interest between each of the products using the residual mean square error obtained from the ANOVA. The point and interval estimates were back-transformed to give estimates of the ratios of the geometric LSM and corresponding 1-sided (superiority) or 2-sided 90%/95% CIs, as appropriate. In addition, estimated geometric LSM and 95% CI were presented for each product.
Statistical comparisons were performed to test the following hypotheses: the addition of different acid ratios to the e-liquid results in different plasma nicotine compared to lactic acid only; higher concentrations of nicotine in the e-liquid increases the level of plasma nicotine compared to lower concentrations; a fixed puffing protocol yields a different plasma nicotine pharmacokinetic profile compared with that of an ad libitum puffing protocol; and the plasma nicotine concentrations for each e-cigarette are not higher than those obtained from a cigarette.
For the product liking subjective effects measure, data were listed by participant and summarised by product using descriptive statistics (N, n, mean, SD, minimum, median and maximum).
Results
Participant demographics
32 participants met eligibility requirements and were enrolled into the study. The majority (30) of randomised participants (93.8%) completed the study according to the protocol. Two participants were withdrawn; one used study products on Days 1–6 before requesting to be withdrawn and the other participant used study products on Day 1 before requesting to be withdrawn due to an adverse event (presyncope attributed to blood withdrawal).
Basic participant demographic and tobacco/nicotine product history details are presented in Table 2; participants were all white and predominantly male (approximately 81%). All participants currently smoked less than 10 cigarettes per day, with a mean (SD) number smoked per day of 2.14 (0.588). All participants smoked factory-manufactured cigarettes with some also smoking roll-your-own cigarettes and cigarillos. All participants had been smoking for at least two years and had been using e-cigarettes for at least one year, were daily e-cigarette users, and the predominant form of e-cigarette used was a refillable tank.
Nicotine pharmacokinetics
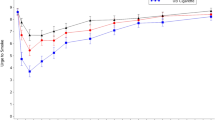
Mean plasma nicotine concentration–time curves during fixed and ad libitum puffing of the Vuse e-cigarettes with different prototype e-liquid formulations and participants usual brand combustible cigarette (ad libitum puffing only) are shown in Fig. 1A,B, respectively. Descriptive statistics for the PK parameters Cmax, AUC0–60 and Tmax are presented in Table 3. For each prototype e-liquid formulation, mean plasma nicotine concentration rose during e-cigarette use and reached a peak at the end of the 10-min fixed and ad libitum puffing sessions. For the usual brand cigarettes, mean plasma nicotine concentration rose during product use and reached a peak before the end of the 10-min ad libitum puffing session. Median Tmax was 10 min for all e-cigarette products assessed in both puffing regimens, except for EPOD2.0_VFB35_2VB fixed puffing which had a median Tmax of 15 min. The median Tmax for participants usual brand combustible cigarette was 8 min (Table 3).
Plasma nicotine concentrations over time during fixed (a) and ad libitum (b) Puffing. Each point shows the geometric mean plasma nicotine concentration at each nominal timepoint. Error bars are not shown for clarity; for variability estimates refer to Table 3. Figures with error bars are provided in Supplementary Fig. S1. N = 29–31 (arm dependent) A: Fixed-puffing regimen; B: Ad Libitum puffing regimen.
The PK parameters Cmax and AUC0–60 during ad libitum puffing on the study e-cigarettes were all higher than those for usual brand cigarettes, regardless of the e-liquid nicotine concentration or acid composition (Table 3). For the different e-liquid formulations, Cmax and AUC0–60 values were lower for the 3.5% nicotine concentration compared with the 5% nicotine concentration, regardless of acid composition or puffing regimen.
Statistical comparisons assessing the impact of the different e-liquid formulations on Cmax and AUC0–60 during fixed puffing are presented in Table 4. For the e-liquids containing 5% nicotine, Cmax and AUC0–60 following use of e-liquids containing lactic, levulinic and/or benzoic acid at varying levels, compared with those following use of the e-liquid containing lactic acid alone, were not significantly different (p > 0.05) across all e-liquid formulations assessed. Expectedly, for all but one comparison (Cmax for 3.5% nicotine EPOD2.0_VFB35_3VE) Cmax and AUC0–60 were significantly lower (p < 0.05) following use of the e-liquid formulations containing 3.5% nicotine compared with the e-liquid containing 5% nicotine and lactic acid only (Table 4). This impact of e-liquid nicotine concentration is demonstrated further in the statistical comparisons presented in Table 5; for the majority of the comparisons for Cmax and for all comparisons for AUC0–60, increasing the e-liquid nicotine concentration from 3.5 to 5% evoked significant increases (p < 0.05) in these PK parameters.
During the ad libitum puffing sessions, participants took a similar number of puffs on each of the study e-cigarettes (Supplementary Table S1), with mean (SD) puffs ranging from 25.7 (9.98) to 28.1 (11.93). For usual brand cigarettes, participants took approximately half the number of puffs than with e-cigarettes, with a mean (SD) of 12.9 (3.52) (Supplementary Table S1). Statistical analysis of the nicotine pharmacokinetic data (Supplementary Table S2) showed that similar to fixed puffing, the effect on Cmax and AUC0–60 of the addition of levulinic and/or benzoic acid at varying levels, compared with the eliquid containing lactic acid alone, was not significant (p > 0.05) across all 5% nicotine eliquid formulations assessed. Cmax and AUC0–60 were significantly lower for both e-liquid formulations containing 3.5% nicotine compared with the formulation containing 5% nicotine and lactic acid only (p < 0.05; Supplementary Table S2). Further statistical comparisons of ad libitum puffing data (Supplementary Table S3) showed that, similar to fixed puffing, for the majority of the comparisons for Cmax and for all comparisons of AUC0–60, increasing the e-liquid nicotine concentration from 3.5 to 5% evoked significant (p < 0.05) increases in these pharmacokinetic parameters. Additionally, Cmax and AUC0–60 were significantly higher (p < 0.05) for all e-cigarette products compared with participants usual brand combustible cigarettes (Supplementary Table S4). When comparing the effect of puffing regimens, Cmax and AUC0–60 were significantly higher (p < 0.05) in ad libitum compared to fixed puff use across all e-cigarette products evaluated (Supplementary Table S5).
Subjective effects
Data for the product liking subjective effect assessment are presented in Table 6. Generally, mean scores for product liking were similar for all e-liquid formulations assessed, regardless of nicotine concentration, of acid content, and of whether the product was used in a fixed or ad libitum puffing regimen.
Adverse events
There were a total of 11 PEAEs reported by 10 participants (31.3%) during the study, none of which were serious or severe and were all classed as mild or moderate events. The majority of PEAEs (six participants, 18.8%) had no reasonable possible relationship to product use. Four participants (12.5%) reported PEAEs considered to have a reasonable possible relationship to product use (one occurrence of mild dizziness and three occurrences of mild presyncope), all of which were completely resolved. It was notable that the presyncope and dizziness related events tended to occur following the fixed puffing regimen e-cigarette use. One participant was withdrawn from the study because of moderate presyncope related to blood sampling, which was completely resolved, and which was not considered to be of reasonable possible relationship to product use.
Discussion
Within both cigarette smoke and e-cigarette vapour, the alkaloid nicotine is initially distributed in both the gas and particulate phases and deposition of nicotine mostly occurs either directly from the gas phase or following the evaporation of nicotine from the particulate phase36,37. The nicotine molecule itself contains basic nitrogen atoms that can be protonated, and as such three forms of nicotine (unprotonated, monoprotonated and diprotonated) are found in e-cigarette vapour37. Only the unprotonated form of nicotine is able to evaporate from the particulate phase and deposit in the respiratory tract, from where it enters the bloodstream. Furthermore, only the unprotonated nicotine can pass through the cellular lipid bilayers as it is lipophilic, whereas hydrophilic protonated nicotine cannot. The addition of organic acids to e-cigarette liquids alters the gas/particulate equilibrium of nicotine in the vapour resulting in a longer retention period of nicotine in the particulate phase without changing the aerosol mass of nicotine38. Thus, most of the inhaled nicotine is more likely to be deposited deeper in the respiratory tract39 where there is both a greater surface area for absorption and a greater blood flow. Through this physicochemical mechanism, acid inclusion in e-liquids increases nicotine blood absorption while reducing sensorial impact40, since less nicotine evaporates in the oral cavity/upper respiratory tract28,37,41. This may provide nicotine delivery and some sensorial experiences that more closely matches cigarette smoking and therefore improve the acceptability of e-cigarettes as a complete substitute for combustible cigarettes in current smokers.
A number of different acids have been used to protonate nicotine in e-liquids, though the most commonly used organic acids are lactic, levulinic and benzoic acid34. While the degree of protonation may impact nicotine uptake28, previous data have also suggested that the inclusion of various single organic acids in e-liquids may differentially impact nicotine absorption and heart rate (a physiological impact of nicotine)42. Our data suggest that nicotine uptake is not affected when using varying combinations of lactic, benzoic and levulinic acids. In accordance with this, we also found similarity in product liking when using the different prototype e-liquids which suggests that both the product liking derived from nicotine delivery as well as the sensorial impact of the aerosol were similar for the acid combinations used in the assessed e-liquids. While the e-liquid acid constituent formulation did not affect nicotine delivery, the level of nicotine inclusion appeared to be the primary driver of differences in nicotine pharmacokinetic parameters. Furthermore, greater nicotine delivery was seen during the ad libitum puffing session, which along with puff count data showing that participants took greater numbers of puffs during ad libitum use than they were allowed to during fixed use supports that user behaviour is also a driver of nicotine uptake.
It was found that nicotine absorption was higher in all e-cigarette products evaluated than the usual brand cigarettes during ad libitum use. It was also observed that participants when smoking their usual brand cigarettes took approximately half the number of puffs in the session compared to e-cigarette products. Furthermore, it was found that the Tmax associated with cigarette use was eight minutes, before the end of the 10-min use session and earlier than the Tmax for e-cigarette products. This indicated that the single cigarette allowed during the use session was fully completed prior to the end of the use session. This was further verified by reviewing the times the usual brand cigarette session was started and when the product was finished, which showed all participants finished the cigarette before the end of the 10-min session (data not shown). This shorter use time cannot be excluded as a contributing factor to the lower Cmax and AUC0–60 parameters.
Interpretation of data from this study is subject to some limitations. Firstly, it was carried out among combustible cigarette and e-cigarette dual users in the UK and as such our findings may not be generalisable to other groups such as other geographic populations, smokers using e-cigarettes for the first time, a group whose nicotine delivery is different compared with accustomed e-cigarette users43,44, or smokers/e-cigarette users acclimated to different levels of nicotine. Secondly, e-cigarette use was limited to either a fixed or ad libitum use period of a brief and defined length and as such, while acute nicotine absorption was not impacted by e-liquid formulation acid content, nicotine uptake over longer periods of time cannot be inferred. Thirdly, this study only assessed the impact on nicotine pharmacokinetics of varying e-liquid levels of three organic acids; commercially-available e-liquids may contain other acids such as salicylic, malic and tartaric acid34 and findings from this study cannot be extrapolated to other acids or acid combinations. Lastly, since limiting participants to a single cigarette resulted in substantially less puffs and earlier Tmax compared to the e-cigarette products in the ad libitum use session, the pharmacokinetic parameters assessed are not directly comparable in this study and duration of product use must be considered.
Conclusion
While it has been previously shown that using single protonating acids increases nicotine pharmacokinetics, data from this study demonstrate that while the nicotine concentration and the pattern of use (fixed vs ad libitum) affected nicotine delivery, varying the ratios that lactic, benzoic and levulinic protonating organic acids were combined in e-cigarette liquids was without significant effect on nicotine pharmacokinetics and did not notably impact product liking scores. This suggests that e-cigarette products designed within the range of protonating acid combinations assessed in this study would produce a similar nicotine delivery without negatively impacting product liking as a product designed with a single protonating acid. This can be used in designing e-cigarette products that more closely match nicotine delivery and sensorial performance of combustible cigarettes, and therefore more likely to have greater acceptance and are also likely to be more effective for adult smokers in providing a complete substitute for cigarette smoking.
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
References
Royal College of Physicians. Nicotine Without Smoke. Tobacco Harm Reduction. A Report by the Tobacco Advisory Group of the Royal College of Physicians. (Royal College of Physicians, 2016).
Public Health England. Evidence Review of e-Cigarettes and Heated Tobacco Products 2018. A Report Commissioned By Public Health England (Phe Publications, 2018).
Breland, A. et al. Electronic cigarettes: What are they and what do they do?. Ann. N. Y. Acad. Sci. 1394(1), 5–30 (2017).
Margham, J. et al. Chemical composition of aerosol from an e-cigarette: a quantitative comparison with cigarette smoke. Chem. Res. Toxicol. 29(10), 1662–1678. https://doi.org/10.1021/Acs.Chemrestox.1666b00188 (2016) (Epub 02016 Sep 00118).
National Academies of Sciences Engineering and Medicine. Public Health Consequences of E-cigarettes. (National Academies Press, 2018).
Theron, A. et al. Electronic cigarettes: Where to from here?. J. Thorac. Dis. 11(12), 5572–5585. https://doi.org/10.21037/Jtd.22019.21011.21082 (2019).
Shahab, L. et al. Nicotine, carcinogen, and toxin exposure in long-term e-cigarette and nicotine replacement therapy users: A cross-sectional study. Ann. Intern. Med. 166(6), 390–400 (2017).
Goniewicz, M. et al. Comparison of nicotine and toxicant exposure in users of electronic cigarettes and combustible cigarettes. JAMA Netw. Open 1(8), E185937 (2018).
Smith, D. et al. Differences in exposure to nicotine, tobacco-specific nitrosamines, and volatile organic compounds among electronic cigarette users, tobacco smokers, and dual users from three countries. Toxics. 8(4), 88. https://doi.org/10.3390/Toxics8040088 (2020).
Cohen, G. et al. Changes in biomarkers of cigarette smoke exposure after 6 days of switching exclusively or partially to use of the Juul system with two nicotine concentrations: A randomized controlled confinement study in adult smokers. Nicotine Tob. Res. 23(10), 2153–2161 (2021).
Mcewan, M. et al. A randomized controlled study in healthy participants to explore the exposure continuum when smokers switch to a tobacco heating product or an e-cigarette relative to cessation. Toxicol. Rep. 8, 994–1001. https://doi.org/10.1016/J.Toxrep.2021.1005.1003 (2021).
Morris, P. et al. Reductions in biomarkers of exposure to selected harmful and potentially harmful constituents following exclusive and partial switching from combustible cigarettes To Myblu(™) electronic nicotine delivery systems (Ends). Intern. Emerg. Med. 26(10), 021–02813 (2021).
Mcrobbie, H. et al. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst. Rev. 12, Cd010216 (2014).
Beard, E. et al. Association between electronic cigarette use and changes in quit attempts, success of quit attempts, use of smoking cessation pharmacotherapy, and use of stop smoking services in England: Time series analysis of population trends. Br. Med. J. 354, I4645 (2016).
Zhu, S. et al. E-cigarette use and associated changes in population smoking cessation: evidence from us current population surveys. BMJ 358, J3262 (2017).
Giovenco, D. & Delnevo, C. Prevalence of population smoking cessation by electronic cigarette use status in a national sample of recent smokers. Addict. Behav. 76, 129–134 (2018).
Levy, D. et al. The relationship of e-cigarette use to cigarette quit attempts and cessation: insights from a large, nationally representative US survey. Nicotine Tob. Res. 20(8), 931–939 (2018).
Km, B. et al. E-Cigarette initiation and associated changes in smoking cessation and reduction: The population assessment of tobacco and health study, 2013–2015. Tob. Control 28(1), 42–49 (2019).
Hajek, P. et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N. Engl. J. Med. 380(7), 629–637 (2019).
Hajek, P. et al. E-cigarettes compared with nicotine replacement therapy within the Uk stop smoking services: The Tec Rct. Health Technol. Assess. 23(43), 1–82 (2019).
Beard, E. et al. Association of prevalence of electronic cigarette use with smoking cessation and cigarette consumption in England: A time-series analysis between 2006 and 2017. Addiction 115(5), 961–974 (2020).
Grabovac, I., Oberndorfer, M., Fischer, J. et al. Effectiveness of electronic cigarettes in smoking cessation: A systematic review and meta-analysis. Nicotine Tob. Res. (2020).
Hartmann-Boyce, J. et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst. Rev. 9(9), Cd010216 (2021).
Levy, D. et al. Potential deaths averted in USA by replacing cigarettes with e-cigarettes. Tob. Control 27(1), 18–25 (2018).
Mendez, D. & Warner, K. A magic bullet? The potential impact of e-cigarettes on the toll of cigarette smoking. Nicotine Tob. Res. 23, 654–661 (2020).
Rose, J. et al. Pulmonary delivery of nicotine pyruvate: Sensory and pharmacokinetic characteristics. Exp. Clin. Psychopharmacol. 18(5), 385–394 (2010).
Teichert, A. et al. Evaluation of nicotine pharmacokinetics and subjective effects following use of a novel nicotine delivery system. Nicotine Tob. Res. 20(4), 458–465 (2018).
Ebajemito, J. et al. A randomised controlled single-centre open-label pharmacokinetic study to examine various approaches of nicotine delivery using electronic cigarettes. Sci. Rep. 10(1), 19980 (2020).
Hajek, P. et al. Nicotine delivery and users’ reactions to Juul compared with cigarettes and other e-cigarette products. Addiction 115(6), 1141–1148 (2020).
Foulds, J. et al. Effect of electronic nicotine delivery systems on cigarette abstinence in smokers with no plans to quit: Exploratory analysis of a randomized placebo-controlled trial. Nicotine Tob. Res. 376, 342 (2021).
Stead, L. et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst. Rev. 11, Cd000146 (2012).
Food And Drug Administration. "Pmta Coversheet: Technical Project Lead Review (Tpl) For The Iqos Heated Tobacco Product." Retrieved 23rd September 2020. (2019). https://www.Fda.Gov/Media/124247/Download.
O’connell, G. et al. A randomised, open-label, cross-over clinical study to evaluate the pharmacokinetic profiles of cigarettes and e-cigarettes with nicotine salt formulations in US adult smokers. Intern. Emerg. Med. 14(6), 853–861 (2019).
Harvanko, A. et al. Characterization of nicotine salts in 23 electronic cigarette refill liquids. Nicotine Tob. Res. 22(7), 1239–1243 (2020).
Fagerström, K. Determinants of tobacco use and renaming the Ftnd to the Fagerstrom test for cigarette dependence. Nicotine Tob. Res. 14(1), 75–78 (2012).
Jf, P. A consideration of the role of gas/particle partitioning in the deposition of nicotine and other tobacco smoke compounds in the respiratory tract. Chem. Res. Toxicol. 14(11), 1465–1481 (2001).
Duell, A., Pankow, J. & Peyton, D. Nicotine in tobacco product aerosols: “It’s Déjà Vu all over again”. Tob. Control 29(6), 656–662 (2020).
Talih, S. et al. Effect of free-base and protonated nicotine on nicotine yield from electronic cigarettes with varying power and liquid vehicle. Sci. Rep. 10(1), 16263 (2020).
Pichelstorfer, L., Hofmann, W., Winkler-Heil, R., Yurteri, C. & Mcaughey, J. Simulation of aerosol dynamics and deposition of combustible and electronic cigarette aerosols in the human respiratory tract. J. Aerosol. Sci. 99, 125–132 (2016).
Gholap, V. et al. Nicotine forms: Why and how do they matter in nicotine delivery from electronic cigarettes?. Expert. Opin. Drug Deliv. 17(12), 1727–1736 (2020).
Leventhal, A. et al. Effect of exposure to e-cigarettes with salt vs free-base nicotine on the appeal and sensory experience of vaping: A randomized clinical trial. JAMA Netw. Open 4(1), E2032757 (2021).
Bowen, A., Chenyue, X. Nicotine salt formulation for aerosol devices and methods thereof. United States Patent 9215895b2 (2015).
Farsalinos, K. et al. Nicotine absorption from electronic cigarette use: Comparison between experienced consumers (vapers) and naïve users (smokers). Sci. Rep. 5, 11269 (2015).
Hajek, P., Goniewicz, M., Phillips, A., et al. Nicotine intake from electronic cigarettes on initial use and after 4 weeks of regular use. Nicotine Tob. Res. 17(2), 175–179 (2015).
Acknowledgements
The authors wish to thank Simbec-Orion (Merthyr Tydfil, Wales, UK) for their management and conduct of the clinical phase of the study and for providing bioanalytical services. We also gratefully acknowledge Ian M. Fearon, PhD of whatIF? Consulting Ltd (Harwell, UK) for his support with the preparation of this manuscript.
Funding
The study was supported by British American Tobacco (Investments) Limited, the manufacturer of Vuse e-cigarettes.
Author information
Authors and Affiliations
Contributions
J.F. was the Project Lead on this study and was principally responsible for all aspects of the study design, protocol development, study conduct and manuscript writing. M.McE., J.E., K.T., S.B.W., and G.H. all contributed to the technical development of the study design and the protocol, as well as reviewing and editing the manuscript. J.T. provided study design, power calculations and statistical analysis expertise.
Corresponding author
Ethics declarations
Competing interests
All authors are current or former employees of British American Tobacco (Investments) Limited, RAI Services Company or Reynolds American Inc.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Frosina, J., McEwan, M., Ebajemito, J. et al. Assessing the impact of protonating acid combinations in e-cigarette liquids: a randomised, crossover study on nicotine pharmacokinetics. Sci Rep 13, 10563 (2023). https://doi.org/10.1038/s41598-023-37539-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-37539-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.