Abstract
Based on garden cress significantly used for phytoremediation, the antioxidant system included antioxidant-phenolic compounds and antioxidant-enzymes of 6-day-garden cress sprouts (GCS) were assessed as potential bio-indicators for cadmium (Cd) and lead (Pb) contamination. Total phenolic and flavonoid contents of GCS germinated under Cd and Pb treatments (25–150 mg kg−1) gradually increased with increasing concentration of metals and peaked by 2.0, 2.6, and 2.5, 2.3 folds at 150 mg kg−1, respectively. By using DPPH, ABTS, and PMC antioxidant assays, the total antioxidant activity of phenolic compounds of GCS increased 6.1, 13.0, and 5.8-fold for Cd and 5.9, 14.6, and 8.2-fold for Pb at 150 mg kg−1, respectively. The antioxidant enzymes of GCS (POD, CAT, GR, and GST) were significantly activated in response to Cd and Pb stress, and two new electrophoretic POD bands were detected. GCS was absorbed 19.0% and 21.3% of Cd and Pb at 150 mg metal kg−1, respectively. In conclusion, the approaches of the antioxidant defense system of GSC could potentially be used as bio-indicator for monitoring Cd and Pb contamination in a short time of germination process.
Similar content being viewed by others
Introduction
Heavy metals are some of the most pervasive and severe environmental pollutants in the world. Among them, cadmium (Cd) and lead (Pb) are the most common and hazardous; both can have harmful effects on human health. Cd is a potent nephron toxin, a class one carcinogen, and is associated with many severe diseases including teratogenic consequences, hypertension, liver damage, lung damage, renal malfunction, urine protein spills, and disruption of protein metabolism. Acute and/or persistent exposure to Pb is also related to substantial harm to the kidney, nervous system, reproductive system, liver, and brain in addition to metabolic toxicity and enzyme inhibitors1,2,3. Cd and Pb, among other heavy metals, flow and leak into the soil–plant system from many sources such as sewage sludge, ash, inorganic fertilizers, atmospheric deposition, and base and precious mining activity, as well as waste byproducts from petrochemical industries3. Wastes like ash and sewage sludge contain large quantities of rapidly biodegradable organic materials, subsequently causing the release of hazardous heavy metals into the environment4.
Various concentrations of Pb and Cd were measured in different Egyptian soils collected from some regions, ranging between 32.22–88.67 mg Pb kg−1 and 11.35–28.88 mg Cd kg−15. The average concentration of Pb and Cd in these studied agricultural soils was greater than their maximum allowable concentrations reported by WHO6 as 84 and 4 mg Kg−1, respectively. These indicate a serious Pb and Cd pollution danger affecting soil quality and requiring more attention. However, El-Hassanin et al.7 reported concentrations of Pb and Cd in samples collected from other Egyptian soils ranging from 1.77 to 7.33 mg Pb kg−1 and 0.40 to 1.66 mg Cd kg−1. Moreover, the Cd pollution in some soils via phosphate fertilizers, atmospheric deposition, and solid waste leaching reach 77, 74, and 344 mg kg1 in the soil, respectively8.
To address the heavy metal soil contamination problem, several physical and chemical remediation technologies have been developed9. However, these remediation technologies are typically expensive, cause damage to the soil, and produce conditions that are unsuitable for plant growth. Phytoremediation is a promising, clean, and cheap technology, receiving attention from soil scientists and the general public. Several phytoremediation approaches have been used for the remediation of heavy metals from polluted soils. Some plant species were used in phyto-stabilization to reduce heavy metal migration in belowground soil, phyto-extraction to absorb heavy metals from containment soil, phyto-volatilization to release heavy metals into the atmosphere, and phyto-filtration to adsorb heavy metals from groundwater9,10. Phytoextraction is the most significant phytoremediation method used nowadays, as a permanent solution, for removing heavy metals from contaminated soil. In phytoextraction, the heavy metals were taken up by plant roots, translocated to their aerial parts, and then sequestrated in the different plant tissues11. Hyperaccumulator plants can collect great amounts of heavy metals in their aboveground tissues without any signs of phytotoxicity. The heavy metal hyperaccumulator plants can collect 100 times more than ordinary plants under the same conditions. Currently, metal hyperaccumulators include more than 450 plants from at least 45 angiosperm families12.
The common signs of heavy metal toxicity are plant growth inhibition, photosynthesis reduction, and disturbances of respiration and nitrogen metabolism13,14. In addition, heavy metals cause oxidative stress/damage through the overproduction of reactive oxygen species (ROS), which attack plant cells to cause lipid peroxidation, protein oxidation, nucleic acid damage, and ultimately cell death15. To combat such oxidative damage, plants can alter the activities of various antioxidant enzymes, including ascorbate peroxidase (APX), catalase (CAT), glutathione reductase (GR), guaiacol peroxidase (POD), glutathione S-transferase (GST), and overproduce antioxidant phenolic compounds16. These changes in antioxidant enzymes and nonenzymatic antioxidant phenolic compounds are considered biomarkers for monitoring soil heavy metal contamination13,17.
Garden cress (Lepidium sativum L.) is a fast-growing edible plant that belongs to the Brassicaceae family. It grows in many countries, is native to Egypt, and is used for several culinary and medicinal purposes. It can be grown in any type of climate under various soil conditions and can be sown and harvested several times during the year18. In addition, its short vegetation period and high sensitivity to slight changes in environmental conditions make it suitable for environmental stress investigations19. Several studies reported that the full garden cress plant can be used for phytoextraction of residual soil ecotoxicity and the edible parts of the plant accumulated relatively high concentrations of heavy metals19,20,21,22. Some of the above studies used the enhancement of antioxidant-phenolic compounds and antioxidant enzymes as bio-indicators for plants cultivated in metal-polluted soil, but the plants take a long time to cultivate. However, our recent study showed that 6-day- garden cress sprouts possessed the highest content of antioxidant-phenolic compounds along with the highest activities of antioxidant enzymes23. Therefore, this study was conducted to assess the garden cress sprouts (GCS) antioxidant defense system, including the total phenolic content, total flavonoid content, and total antioxidant activity of their phenolic compounds, and variation in their antioxidant enzyme activities (POD, CAT, GR, and GST) as potential bio-indicators for monitoring the cadmium (Cd) and lead (Pb) contamination at various concentrations after 6 days of germination (a short period of germination). This study will provide important information on the potential of garden cress six-day sprouts as Cd/Pb indicators within a very short time.
Materials and methods
Chemical reagents
Folin Ciocalteu reagent, ammonium molybdate, 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2-azinobis (3-ethylbenzo-thiazoline-6-sulfonic acid) (ABTS), Guaiacol, H2O2, 1-Chloro-2,4-dinitrobenzene (CDNB), nicotinamide adenine dinucleotide phosphate (NADPH), oxidized glutathione (GSSG) were purchased from Sigma-Aldrich Co.
Seed source
The seeds of L. sativum garden cress plants were obtained from the Agriculture Research Center, Cairo, Egypt. The formal identification of the plant is confirmed in a voucher sample (Ser. No. 5220) deposited at Herbarium, Agriculture Research Center. Experimental research and field studies on the L. sativum-garden cress plant (either cultivated or wild), including the collection of plant material, complied with relevant institutional, national, and international guidelines and legislation.
Germination of garden cress seeds in contaminated media
Germination of garden cress seeds was carried out according to Abdel-Aty et al.23. Garden cress seeds were surface-sterilized with 0.1% (v/v) sodium hypochlorite solution after which they were extensively washed with distilled water. CdCl2 and PbCl2 were individually dissolved in distilled water and mixed with cotton as a growth medium at different concentrations ranging from 25 to 150 mg kg−1 dry mass. The washed seeds were then spread in Petri dishes (5 cm in diameter) covered with moistened and contaminated cotton and allowed to germinate in the dark at 25–30°C. The garden cress seeds were watered daily with deionized water. Sprouted seeds were collected on day 6 and oven-dried overnight at 45°C before being ground.
Germination index determination
The germination index (GI) of the garden cress seeds has been examined over 6 days of germination under different Cd and Pb concentrations (25–150 mg/kg), GI was measured according to the Salehzade et al. equation24: GI = Σ (ni / Di). Where ni is the number of germinated garden cress seeds at day ‘i’ and Di is the day ‘i’
Methanol extracts of 6-day-garden cress sprouts (GCS)
Two grams of each treatment of dried GCS were added to a flask containing 80% methanol (20 mL) and continuously shaken (120 rpm) overnight at 25–30°C. Each methanol extract was filtered three times using Whatman-one filter paper23.
Determination of total phenolic content
The Folin–Ciocalteu method of Velioglu et al.25 was used to determine total phenolic content. A mixture containing each methanol extract (50 µL), distilled water (850 µL), and Folin–Ciocalteu reagent (100 µL) was incubated at 25–30°C for 5 min. A sodium carbonate solution (20%) was then added to the mixture, which was maintained for 30 min at 25–30°C. The absorbance of each mixture was recorded at 750 nm. A gallic acid equivalent (GAE) standard curve was used to calculate total phenolic content, which was expressed as mg GAE 100 g DW−1.
Determination of total flavonoid content
Methanol extract (250 µL), 5% NaNO2 solution (75 µL), and distilled water (1.25 mL) were incubated at 25–30°C for 5 min. Aluminum chloride solution (10%) and NaOH solution (1.0 M) were then added to the mixture, which was maintained for 10 min at 25–30 °C. The absorbance of each mixture was recorded at 510 nm26. A catechin equivalent (CE) standard curve was used to calculate total flavonoid content, which was expressed as mg CE 100 g DW−1.
Assays of antioxidant activity
DPPH assay
Methanol extract (50 µL) was added to 950 µL of 0.1 mM of DPPH (1, 1-diphenyl-2-picrylhydrazyl) solution and incubated for 30 min at 25–30°C in the dark27. The absorbance of the mixture was recorded at 517 nm. The DPPH radical-scavenging activity percentage = (Control absorbance− sample absorbance/control absorbance) ×100.
ABTS assay
Methanol extract (10 µL) was added to 990 µL of ABTS (2,2-azino-bis (3-ethylbenzo-thiazoline-6-sulfonic acid) reagent and incubated for 1 min at 25–30°C28. The absorbance of the mixture was recorded at 734 nm. The ABTS radical-scavenging activity percentage was calculated using the formula given for the DPPH assay (above).
The IC50 value is the phenolic concentration required to scavenge 50% of either DPPH or ABTS free radicals.
PMC assay
A phosphor-molybdenum complex (PMC) antioxidant assay was conducted according to Prieto et al.29 to determine antioxidant activity. The methanol extract (50 µL) was incubated with 950 µL of 28 mM sodium phosphate, 4 mM ammonium molybdate, and 600 mM sulfuric acid for 45 min at 90°C. The mixture was then cooled before absorbance was read at 695 nm. The EC50 was considered the phenolic concentration equivalent to 0.5 OD at 695 nm.
Total antioxidant activity
Following the work of Abdel-Aty et al.23, total antioxidant activity was calculated as follows: total antioxidant activity = total phenolic content (mg GAE 100 g DW−1)/mg IC50 or mg EC50.
Preparation of crude enzyme extract
According to the method of Abdel-Aty et al.23, one gram of GCS was homogenized with 10 mL of extraction buffer (50 mM Tris–HCl, pH 7.0 containing 1 mM EDTA) using a glass mortar under cooled conditions. After centrifugation (12,000 g for 12 min at −4ºC), each supernatant (crude enzyme extract) was stored at −20ºC until further use.
Assays of antioxidant enzymes
Peroxidase (POD; EC 1.11.1.7) activity was estimated following the method described by Miranda et al.30. The assay was performed in 50 mM sodium acetate buffer (pH 5.5) in the presence of two substrates, 8 mM H2O2 and 40-mM guaiacol, as well as crude enzyme extract. The increase in the absorbance at 1.0 per min at 470 nm was considered as one unit. Catalase (CAT; EC 1.11.1.6) activity was estimated using the method described by Bergmeyer31. The assay was performed in 75 mM phosphate buffer (pH 7.0) in the presence of 25 mM H2O2 as a substrate as well as crude enzyme extract. The decrease in absorbance at 0.1 per min at 240 nm was considered as one unit. Glutathione-S-transferase (GST; EC 2.5.1.18) activity was estimated according to the method described by Habig et al.32 The assay was conducted in 0.1 M potassium phosphate buffer (pH 6.5) and 1.6 mM GSH, with 1 mM CDNB added to the crude enzyme extract. The increase in absorbance was read at 340 nm. The enzyme concentration that converted 1 µM CDNB per min was considered as one unit. Glutathione reductase (GR; EC 1.6.4.2) activity was estimated using the method described by Zanetti33. The reaction was based on the oxidation of NADPH in the presence of GSSG as a substrate. The reaction mixture included 50 mM potassium phosphate buffer (pH 7.0) containing 1 mM EDTA, GSSG, NADPH, and crude enzyme extract. The enzyme concentration that oxidized 1 µmol of NADPH per min was considered as one unit.
Determination of peroxidase by gel electrophoresis
To identify peroxidase isozyme variations between the control and metal treatments of GCS, native polyacrylamide gel electrophoresis (native-PAGE) was performed according to the method of Stegemann et al.34 with a slight modification. The substrate solution contained benzidine HCl (0.25 g), 4 mL of glacial acetic acid, and distilled water (added up to a total volume of 50 mL). After electrophoresis, the gel was placed into the substrate solution along with 10 drops of hydrogen peroxide. The gel was incubated at room temperature until bands appeared.
Determination of total heavy metals
Before metal determination, all samples were digested using nitric acid, an acceptable matrix for consistent recovery of metals that are compatible with the analytical method35. All heavy metal analyses were performed on an Agilent 5100 Inductively Coupled Plasma–Optical Emission Spectrometer with Synchronous Vertical Dual View. For each series of measurements, an intensity calibration curve was constructed that was composed of a blank and three or more standards from Merck (Germany). The accuracy and precision of the metal’s measurements were confirmed using external reference standards from Merck; standard reference materials for trace elements in water and quality control samples from the National Institute of Standards and Technology were used to confirm the instrument reading.
All experimental procedures were carried out in compliance with relevant guidelines.
Statistical analysis
Data were analyzed using one-way ANOVA followed by Tukey’s post hoc test; these tests were conducted in GraphPad Prism version 5. Data are presented as means ± SD (n = 4) and differences were considered significant at p < 0.01.
Results and discussion
Total phenolic and flavonoid contents
Table 1 screens the total phenolic and flavonoid contents of GCS grown in media contaminated with Cd and Pb at various concentrations (25–150 mg kg−1). The total phenolic and total flavonoid contents of untreated GCS as a control (1600 ± 51 mg GAE 100 g−1 DW and 231±6.6 mg CE 100 g−1 DW, respectively) significantly increased (p < 0.01) and reached their highest levels at the highest doses of Cd (3196±54 mg GAE 100 g−1 and 590±10.2 mg CE 100 g−1 DW, respectively) and Pb (3960 ± 76 mg GAE 100 g−1 and 522 ± 10.5 mg CE 100 g−1 DW, respectively) by 2.0, 2.6 and 2.5, 2.3-fold, respectively. Similarly, over-expression of phenolics and flavonoids was detected at 2.48–2.50-fold and 1.5–2.0-fold, respectively, above the levels in controls in C4 weed subjected to aluminum stress36. Accumulation of antioxidant-phenolic compounds in tissues facilitates the ability of plants to tolerate and detoxify Cd and Pb stress via their metal chelating activity and/or their antioxidant activity37. The hydroxyl and carboxylic groups of the phenolic compounds assist with the binding of metals. In addition, flavonoids, as an important class of antioxidant-phenolic compounds, aid the detoxification of ROS free radicals that are induced by heavy metal stress. Rice, as a cereal plant, responds to cadmium stress by accumulating antioxidant-phenolic compounds38. An increase in phenolic compounds is correlated with increases in Cd and Pb concentrations, which suggests that de novo synthesis of soluble phenolic compounds and/or hydrolysis of conjugated phenolic compounds occurs under heavy metal stress39. In addition, the increase in the soluble phenolic compounds that are used in the lignin biosynthesis of cell walls to create physical barriers against the harmful action of heavy metals has also been reported40. From such observations, we can conclude that the total phenolic and flavonoid contents of GCS are highly sensitive markers for indicating Pb and Cd contamination in soils, even when concentrations are low.
Antioxidant activities
Most of the recently identified phenolic compounds of GCS have potent antioxidant activity23. Therefore, the antioxidant activity of GCS germinated in media contaminated with Cd and Pb was evaluated by various antioxidant assays. In Table 2, IC50 values using DPPH and ABTS methods and EC50 values using the PMC method of untreated GCS (0.0098, 0.0065, and 0.007 mg GAE mL−1, respectively) gradually and significantly decreased (p < 0.01) and reached to their lowest values (0.0041, 0.0011 and 0.0021 mg GAE mL−1, respectively) at highest concentrations of Cd and Pb, respectively. Low IC50 and EC50 values reflect a high antioxidant activity. Additionally, the total antioxidant activity of GCS gradually and significantly increased relative to the control levels (p < 0.01) to reach maximum levels for Cd by 6.1-, 13.0-, and 5.8-fold and for Pb by 5.9-, 14.6-, and 8.2-fold at the highest concentrations of Cd and Pb (Table 3). This increase in antioxidant activity may be attributed to increases in the concentration of antioxidant-phenolic compounds that were associated with increasing Cd and Pb concentrations in growth media. ABTS and DPPH free radicals were tested in all antioxidation reactions of organic residues that were combined with ROS radicals41,42. Therefore, the antioxidant activities of GCS could be used as potential bioindicators for Cd and Pb toxicity. In previous studies, the antioxidant activity of rice was found to increase during Cd stress, which facilitated metal chelating and enhanced Cd tolerance38. Total antioxidant activity increased from 1.2- to 1.7-fold in Malva parviflora roots and leaves under different Cd concentration treatments43. Additionally, ABTS and DPPH free radical scavenging activity as well as PMC reduction activity gradually increased in C4 weed grown under aluminum treatments36. In contrast, chickpeas grown under different heavy metals treatments had an antioxidant activity that remained lower than that of the control, according to DPPH or ABTS assays, but that slightly increased as the concentration of accumulated metal increased44.
Antioxidant enzymes
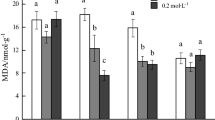
Cd and Pb can cause the overproduction of ROS free radicals and induce oxidative damage to plant tissues. Maintaining the balance between ROS free radicals and activation of the antioxidative system under heavy metal stress is a critical protective mechanism in plants that diminishes oxidative damage in polluted tissues15. Therefore, in the present work, the potential role of the antioxidant enzymes (POD, CAT, GR, and GST) in response to oxidative stress in GCS under Cd and Pb treatments was investigated; the results are presented in Fig. 1. POD activity gradually increased (p < 0.01) with increasing of Cd and Pb concentrations till reached highest activity (355 and 451 U g−1, respectively) compared to the control (153 U g1) (Fig. 1A). The results observed a strong correlation between the phenolic content and POD activity of GCS germinated under Cd and Pb treatments. POD is involved in removing H2O2 as a harmful ROS-free radical by oxidation of phenolic compounds45,46,47,48. Similarly, increasing Hg accumulation in garden cress shoots was correlated with an increase in POD activity and changes in total carotenoid content19. Furthermore, POD participates in the polymerization of phenolic compounds for lignin synthesis to build a barrier that protects against toxic metal ions49.
In the antioxidant defense system, CAT also stopped the hyper-accumulation of H2O2 by converting it to water and oxygen. Here, compared with control CAT activity (100 U g−1), an increase in Cd and Pb treatment concentrations caused a significant increase (p < 0.01) in CAT activity, which peaked (250 and 190 U g−1, respectively) at 75 mg metal kg−1 (Fig. 1B). Similarly, CAT and POD activities were significantly enhanced in vetiver grass, Populus nigra, Cajanus cajan50,51,52, and Malva parviflora43 grown in Cd- and/or Pb-contaminated soils. This considerable increase in CAT activity could be induced by excessive production of H2O2 under 75 mg Cd and Pb kg−1. However, the CAT activity was reduced under higher concentrations of Cd and Pb (100 and 150 mg kg−1), which may be due to severe oxidative damage occurring under much higher Cd and Pb levels. The severity of the Cd/Pb stress may suppress enzyme synthesis or alter the enzyme's assembly53. Interestingly, this decline in CAT activity was compensated by the increase in POD activity to remove H2O2. Many previous investigations on some plant species have revealed that variations in antioxidant enzyme activities were related to the severity of Cd/Pb stress53,54.
The important GSH-utilizing enzymes, GST and GR, were also evaluated in the current study. These enzymes largely participate in the efficient metabolism of ROS free radicals and their products55. Hence, they tightly control heavy metal-induced oxidative stress in plants. The activities of GST and GR enzymes significantly increased relative to control levels (239 and 900 U g−1, respectively) in all Cd and Pb treatments, and the activities reached their peak at 75 metal mg kg−1 treatments (Cd: 700 and 566 U g−1, respectively; Pb: 2,330 and 2,318 U g−1, respectively) (Fig. 1C and D). An increase in GST activity was previously reported in pumpkin, rice, and Cicer arietinum in response to either Cd or Pb stress17,56,57. Our results revealed a strong correlation between the phenolic compound levels and GST activity of GCS under Cd and Pb treatments, suggesting that GST not only removes toxic ROS radicals but also transports the phenolic compound chelating-metal complexes to the vacuoles58. In a previous study, GR activity increased in Brassica napus leaves under Cd stress59. In contrast, GR activity decreased in Ceratophyllum demersum and mung bean seedlings in response to Cd stress60,61. Such variations in GR activity and responses may have been due to differences in plant genotypes58. In the results described above, there was a direct relationship between Cd and Pb concentrations and POD, CAT, GST, and GR activities, which indicates that these four enzymes of the antioxidant defense system could be used as bio-indicators for Cd and Pb stress. In addition, the GCS can cope with the oxidative damage induced by Pb and Cd.
Electrophoretic pattern of peroxidase activity
Fig. 2 shows the electrophoretic patterns of peroxidase in GCS germinated under Cd and Pb treatments (25–150 mg kg−1) compared with untreated controls. Two peroxidase isozymes were detected in untreated control and the intensity of these bands increased gradually with increasing Cd and Pb concentrations; moreover, two new peroxidase isozymes appeared. This observation explains why POD activity gradually increased in all Cd and Pb treatments to cope with ROS that caused stress-related damage to plant tissues.
Bioaccumulation of Cd and Pb in GCS
The contents of Cd and Pb absorbed by GCS showed a significant increase (p < 0.01) with increasing Cd and Pb at 25, 50, 75, 100, and 150 mg kg−1 in the order of 6.0, 8.0, 11.0, 14.0, 19.0 and 7.0, 10.0, 13.5, 16.0, 21.3%, respectively (Fig. 3). Thus, GCS seem to have the ability to take up Cd and Pb from contaminated media as phytoremediator in a short germination period (6 days). A previous study reported that garden cress plants could absorb (through their shoots and roots) 48% of Pb from soil contaminated with a 300-mg kg−1 concentration after a 30-day culture20. Another study found that garden cress reduced 100-mg kg−1 Hg by 33% in contaminated growth media after six repeated phytoextraction processes19. Additionally, chickpea seedlings produced maximum uptake of Pb (~3.8%) from contaminated soil at a Pb concentration of 250 mg kg−144.
Visual changes of GCS and germination index
During six days of germination, the GCS did not exhibit any noticeable visual changes when subjected to different concentrations of Cd and Pb, even under substantially higher concentrations of both. In addition, the germination index (GI) of the GCS was conducted throughout 6 days of germination under different Cd and Pb concentrations (25–150 mg/kg), as seen in Fig. 4, and the results showed that the GI was unaffected by these heavy metals even at their higher doses. One could conclude that the potent antioxidant defense system of GCS could easily counter the damaging effects of Cd and Pb toxicity. Some plants exposed to toxic levels of Cd and Pb decrease germination and interfere with seedling physiological processes62 such as cowpea, soybean, lettuce, and sugar beet8. While parsley seedlings that grew under considerably higher Cd concentrations didn't show any signs of visual changes63.
Bio-indicators collective data
All of the tested parameters that were measured in GCS germinated under the lowest Cd and Pb concentrations (25 mg kg−1) observed fold increase values of 1.5–2.2-fold (Table 4). At the highest Cd and Pb concentrations (150 mg kg−1), the fold-increase values of these parameters were slightly higher (1.7–2.9-fold), but the expected substantial increase did not occur, except for total antioxidant activity (5.8–14.6-fold) (Table 4). These observations suggest that all the tested parameters of GCS responded strongly to lower concentrations of Cd and Pb and the antioxidant activity of phenolic compounds of GCS can be considered a potent bioindicator for monitoring Cd and Pb at both low and high concentrations. It is important to conduct these experiments outdoors in varied soils under different environmental conditions in order to study more realistic changes in the GCS-antioxidant system and the accumulation and distribution of these heavy metals.
Conclusion
In the present work, GCS was germinated with different concentrations of Cd and Pb (25–150 mg kg−1) under in vitro conditions. The phenolic and flavonoid levels and antioxidant capacity were enhanced several folds and correlated with the Cd and Pb concentrations. The activities of antioxidant enzymes (POD, CAT, GR, and GST) were strongly increased under Cd and Pb stress. In addition, new peroxidase isozymes appeared in the electrophoretic profile. Further, considerable amounts of Cd and Pb were absorbed by the tissues of GCS. Moreover, all the investigated parameters of GCS responded considerably to lower levels of Cd and Pb, and the GCS-antioxidant activity was a potent bioindicator to monitor Cd and Pb at low and high levels. Based on these findings, we conclude that metal stress alters the biochemical parameters of GCS, which could be used as bio-monitors for Cd and Pb soil contaminants within short germination periods.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Sari, A. & Tuzen, M. Biosorption of Pb (II) and Cd (II) from aqueous solution using green alga (Ulva lactuca) biomass. J. Hazard. Mater. 152, 302–308 (2008).
Anayurt, R. A., Sari, A. & Tuzen, M. Equilibrium, thermodynamic and kinetic studies on biosorption of Pb (II) and Cd (II) from aqueous solution by macrofungus (Lactarius scrobiculatus) biomass. Chem. Eng. J. 151, 255–261 (2009).
Sari, A. & Tuzen, M. Kinetic and equilibrium studies of Pb (II) and Cd (II) removal from aqueous solution onto colemanite ore waste. Desalination 249, 260–266 (2009).
Antonkiewicz, J. et al. Phytoextraction of heavy metals after application of bottom ash and municipal sewage sludge considering the risk of environmental pollution. J. Environ. Manag. 306, 114517 (2022).
Al-Naggar, Y., Naiem, E., Mona, M., Giesy, J. P. & Seif, A. Metals in agricultural soils and plants in Egypt. Toxicol. Environ. Chem. 96, 730–742 (2014).
WHO guidelines for the safe use of wastewater, excreta, and greywater/World Health Organization. Volume 2 wastewater use in agriculture (2006).
El-Hassanin, A. S., Samak, M. R., Abdel-Rahman, G. N., Abu-Sree, Y. H. & Saleh, E. M. Risk assessment of human exposure to lead and cadmium in maize grains cultivated in soils irrigated either with low-quality water or freshwater. Toxicol. Rep. 7, 10–15 (2020).
Haider, F. U. et al. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Safety. 211, 111887 (2021).
Marques, A. P., Rangel, A. O. & Castro, P. M. Remediation of heavy metal contaminated soils: phytoremediation as a potentially promising clean-up technology. Crit. Rev. Envon. Sci. Technol. 39, 622–654 (2009).
Ernst, W. H. Phytoextraction of mine wastes–options and impossibilities. Chem. Erde. Geochem. 65, 29–42 (2005).
Ali, H., Khan, E. & Sajad, M. A. Phytoremediation of heavy metals-concepts and applications. Chemosphere 91, 869–881 (2013).
Yan, A. et al. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 11, 359 (2020).
Benavides, M. P., Gallego, S. M. & Tomaro, M. L. Cadmium toxicity in plants. Braz. J. Plant Physiol. 17, 21–34 (2005).
Wang, Z., Zhang, Y., Huang, Z. & Huang, L. Antioxidative response of metal accumulator and non-accumulator plants under cadmium stress. Plant Soil 310, 137–149 (2008).
Sharma, P., Jha, A. B., Dubey, R. S. & Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Botany 217037, 26 (2012).
Hossain, M. A., Piyatida, P., da Silva, J. A. T. & Fujita, M. Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Botany 872875, 37. https://doi.org/10.1155/2012/872875 (2012).
Hossain, M.A., Hossain, M.D., Rohman, M.M., da Silva, J.A.T., & Fujita, M. (2012b) Onion major compounds (flavonoids, organosulfurs) and highly expressed glutathione-related enzymes: possible physiological interaction, gene cloning and abiotic stress response in Onion Consumption and Health, Aguirre, C.B. and Jaramillo, L.M., Eds., Nova Science Publishers, New York, NY, USA
Nehdi, I. A., Sbihi, H., Tan, C. P. & Al-Resayes, S. I. Garden cress (Lepidium sativum Linn.) seed oil as a potential feedstock for biodieasel production. Bioresour. Technol. 126, 193–197 (2012).
Smolinska, B. & Szczodrowska, A. Antioxidative response of Lepidium sativum L. during assisted phytoextraction of Hg contaminated soil. New Biotechnol. 34, 74–83 (2017).
Cathrine, L. F. & Navab, M. Soil decontamination with using garden cress. J. Sci. Res. Develop. 1, 1–6 (2014).
Marchand, C. et al. Pilot scale aided-phytoremediation of a co-contaminated soil. Sci. Total Environ. 618, 753–764 (2018).
Souri, M. K., Alipanahi, N., Hatamian, M., Ahmadi, M. & Tesfamariam, T. Elemental profile of heavy metals in garden cress, coriander, lettuce and spinach, commonly cultivated in Kahrizak, South of Tehran- Iran. Open Agric. 3, 32–37 (2018).
Abdel-Aty, A. M., Salama, W. H., Fahmy, A. S. & Mohamed, S. A. Impact of germination on antioxidant capacity of garden cress: New calculation for determination of total antioxidant activity. Sci. Hortic. 246, 155–160 (2019).
Salehzade, H., Shishvan, M. I., Ghiyasi, M., Forouzin, F. & Siyahjani, A. A. Effect of seed priming on germination and seedling growth of wheat (Triticum aestivum L). Res. J. Biol. Sci. 4(5), 629–631 (2009).
Velioglu, Y. S., Mazza, G., Gao, L. & Oomah, B. D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 46, 4113–4117 (1998).
Zhishen, J., Mengcheng, T. & Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 64, 555–559 (1999).
Ao, C., Li, A., Elzaawely, A. A., Xuan, T. D. & Tawata, S. Evaluation of antioxidant and antibacterial activities of Ficus microcarpa L. fil. extract. Food Control 19, 940–948 (2008).
Re, R. et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med. 26, 1231–1237 (1999).
Prieto, P., Pineda, M. & Aguilar, M. Spectrophotometric quantitation of antiox-idant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem. 269, 337–341 (1999).
Miranda, M. V., Fernandez Lahor, H. M. & Cascone, O. Horseradish peroxidase extraction and purification by aqueous two-phase partition. Appl. Biochem. Biotechnol. 53, 147–154 (1995).
Bergmeyer, H. U. Methods of enzymatic analysis Vol. 1, 438 (Academic press, 1974).
Habig, W. H., Pabst, M. J. & Jakoby, & W.B.,. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 249, 7130–7139 (1974).
Zanetti, G. Rabbit liver glutathione reductase. Purification and properties. Arch. Biochem. Biophy. 198, 241–246 (1979).
Stegemann, H., Afify, A.M.R., & Hussein, K.R.F. Cultivar identification of dates (Phoenix dactylifera) by protein patterns. In Second International Symposium of Biochemical Approaches to Identification of Cultivars. Braunschweing, West Germany; pp 44 (1985).
APHA (American Public Health Association), AWWA (American Water Works Association), and WEF (Water Environment Federation). Standard methods for the examination of water and wastewater, 23rd ed. (Rice, E. W., Baird, R. B., Eaton, A. D., Clesceri, L. S. eds.) Washington DC (2017).
Sarkar, B., Saha, I., De, A. & Ghosh, A. Aluminium accumulation in excess and related antioxidation responses in C4 weed (Amaranthus viridis L.). Physiol. Mol. Biol. Plants 26, 1583–1598 (2020).
Bhattacharya, A., Sood, P. & Citovsky, V. The roles of plant phenolics in defense and communication during agrobacterium and rhizobium infection. Mol. Plant Pathol. 11, 705–719 (2010).
Sebastian, A. & Prasad, M. N. V. Cadmium minimization in rice. Rev. Agron. Sustain. Dev. 34, 155–173 (2014).
Parry, A. D., Tiller, S. A. & Edwards, R. The effects of heavy metals and root immersion on isoflavonoid metabolism in Alfalfa (Medicago sativa L.). Plant Physiol. 106, 195–202 (1994).
Diaz, J., Bernal, A., Pomar, F. & Merino, F. Induction of shikimate dehydrogenase and peroxidase in pepper (Capsicum annum L.) seedlings in response to copper stress and its relation to lignification. Plant Sci. 161, 179–188 (2001).
Barakat, A. Z., Bassuiny, R. I., Abdel-Aty, A. M. & Mohamed, S. A. Diabetic complications and oxidative stress: The role of phenolic-rich extracts of saw palmetto and date palm seeds. J. Food Biochem. 44, e13416 (2020).
Salama, W. H., Abdel-Aty, A. M. & Fahmy, A. S. Rosemary leaves extract: Anti-snake action against Egyptian Cerastes cerastes venom. J. Tradit. Complemen. Med. 8, 465–475 (2018).
Zoufan, P., Jalali, R., Hassibi, P., Neisi, E. & Rastegarzadeh, S. Evaluation of antioxidant bio-indicators and growth responses in Malva parviflora L. exposed to cadmium. Physiol. Mol. Biol. Plants 24, 1005–1016 (2018).
Bhagyawant, S. S. et al. Variations in the antioxidant and free radical scavenging under induced heavy metal stress expressed as proline content in chickpea. Physiol. Mol. Biol. Plants 25, 683–696 (2019).
Passardi, F., Penel, C. & Dunand, C. Performing the paradoxical: How plant peroxidases modify the cell wall. Trends Plant Sci. 9, 534–540 (2004).
Mohamed, S. A., Abdel-Aty, A. M., Mohamed, B. H., Mohamed, O. E. & Fahmy, A. S. Ficus sycomorus latex: A thermostable peroxidase. Afr. J. Biotechnol. 10, 17532–17543 (2011).
Mohamed, S. A., El-Badry, M. O., Drees, E. A. & Fahmy, A. S. Properties of a cationic peroxidase from Citrus Jambhiri cv.. Appl. Biochem. Biotechnol. 150, 127–137 (2008).
Abdel-Aty, A. M., Elsayed, A. M., Salah, H. A., Bassuiny, R. I. & Mohamed, S. A. Egyptian chia seeds (Salvia hispanica L.) during germination: Upgrading of phenolic profile, antioxidant, antibacterial properties and relevant enzymes activities. Food Sci. Biotechnol. 30, 723–734 (2021).
Abdel-Aty, A. M. et al. Purification and characterization of peroxidases from garden cress sprouts and their roles in lignification and removal of phenol and p-chlorophenol. J. Food Biochem. 45, e13526 (2021).
Aibibu, N. et al. Cadmium accumulation in vetiveria zizanioides and its effects on growth, physiological and biochemical characters. Bioresour. Technol. 101, 6297–6303 (2010).
Redovniković, I. R. et al. Poplar response to cadmium and lead soil contamination. Ecotoxicol. Environ. Saf. 144, 482–489 (2017).
Garg, N. & Aggarwal, N. Effect of mycorrhizal inoculations on heavy metal uptake and stress alleviation of Cajanus cajan (L.) Millsp. genotypes grown in cadmium and lead contaminated soils. Plant Growth Regul. 66, 9–26 (2012).
Zhang, S., Zhang, H., Qin, R., Jiang, W. & Liu, D. Cadmium induction of lipid peroxidation and effects on root tip cells and antioxidant enzyme activities in Vicia faba L. Ecotoxicology 18, 814–823 (2009).
Anjum, S. A. et al. Cadmium toxicity in Maize (Zea mays L..): Consequences on antioxidative systems, reactive oxygen species, and cadmium accumulation. Environ. Sci. Pollut. Res. 22, 17022–17030 (2015).
Abdulaal, W. H. et al. Investigation of antioxidant and detoxifying capacities of some date cultivars (Phoenix dactylifera L.) irrigated with sewage water. RSC Adv. 7, 12953–12958 (2017).
Fujita, M. & Hossain, M. Z. Molecular cloning of cDNAs for three tau-type glutathione S-transferases in pumpkin (Cucurbita maxima) and their expression properties. Physiol. Plant. 117, 85–92 (2003).
Reddy, A. M., Kumar, S. G., Jyothsnakumari, G., Thimmanaik, S. & Sudhakar, C. Lead induced changes in antioxidant metabolism of horse gram (Macrotyloma uniflorum L.) and bengal gram (Cicer arietinum L.). Chemosphere 60, 97–104 (2005).
Hossain, M.A., da Silva, J.A.T., & Fujita, M. Glyoxalase system and reactive oxygen species detoxification systemin plant abiotic stress response and tolerance: an intimaterelationship,” In Abiotic Stress in Plants-Mechanisms and Adaptations, A. K. Shanker and B. Venkateswarlu, Eds., INTECH-Open Access Publisher, Rijeka, Croatia, pp. 235–266 (2011).
Nouairi, I. et al. Antioxidant defense system in leaves of Indian mustard (Brassica juncea) and rape (Brassica napus) under cadmium stress. Acta Physiol. Plant 31, 237–247 (2009).
Aravind, P. & Prasad, M. N. V. Cadmium-Zinc interactions in a hydroponic system using Ceratophyllumdemersum L.: adaptive ecophysiology, biochemistry and molecular toxicology. Braz. J. Plant Physiol. 17, 3–20 (2005).
Hossain, M. A., Hasanuzzaman, M. & Fujita, M. Up-regulation of antioxidant and glyoxalase systems by exogenous glycinebetaine and proline in mung bean confer tolerance to cadmium stress. Physiol. Mol. Biol. Plants 16, 259–272 (2010).
Raza, A. et al. Phytoremediation of cadmium: physiological, biochemical, and molecular mechanisms. Biology 9, 177 (2020).
Ulusu, Y., Oztürk, L. & Elmasta, S. M. Antioxidant capacity and cadmium accumulation in parsley seedlings exposed to cadmium stress. Russ. J. Plant Physiol. 64, 883–888 (2017).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
S.A.M. and A.M.A. designed the research; A.M.E., A.M.G., and A.Z.B. conducted the research; S.A.M., A.M.A., and A.Z.B. analyzed the data; S.A.M. and A.M.A. wrote the paper. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdel-Aty, A.M., Elsayed, A.M., Gad, A.A.M. et al. Antioxidant system of garden cress sprouts for using in bio-monitor of cadmium and lead contamination. Sci Rep 13, 10445 (2023). https://doi.org/10.1038/s41598-023-37430-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-37430-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.