Abstract
Hyperuricemia is reportedly associated with the progression of carotid intima-media thickness (IMT), a surrogate of cardiovascular risks and events. However, factors associated with carotid IMT progression in patients with asymptomatic hyperuricemia are largely unknown. In this post-hoc analysis of the multicenter, randomized PRIZE study, we analyzed data from a total of 326 patients who underwent carotid ultrasonography in a blind manner at baseline and 24 months to evaluate carotid IMT. Mean and maximum IMT at the common carotid artery (CCA) were measured at a central core laboratory. Factors related to the absolute change in mean and maximum IMT from baseline to 24 months were explored. Overall, the adjusted mean [0.0032 (− 0.0214 to 0.0278) mm] and maximum [0.0011 (− 0.0327 to 0.0351) mm] CCA-IMT increased numerically from baseline to 24 months. Multivariable analysis identified higher body mass index, history of atherosclerotic cardiovascular disease (ASCVD), and lower mean CCA-IMT at baseline as significant factors associated with the increase in mean CCA-IMT. In addition, older age and lower mean CCA-IMT at baseline were significant factors for an increased absolute change in the maximum CCA-IMT at 24 months. The present sub-analysis of the PRIZE study showed higher body mass index, history of ASCVD, and older age as significant factors associated with CCA-IMT progression in patients with asymptomatic hyperuricemia. These factors may be considered when identifying the possible risk of atherosclerotic progression in this specific patient population of hyperuricemia.
Trial registration: UMIN000012911 and UMIN000041322.
Similar content being viewed by others
Introduction
Uric acid has significant roles in gout and kidney stone formation. Beyond crystalline arthropathy and urolithiasis, an elevated serum uric acid (SUA) level may be associated with the development of cardiometabolic and cardiovascular diseases, such as hypertension, insulin resistance, chronic kidney disease, heart failure, and coronary artery disease1. Although a causal effect of hyperuricemia on atherosclerotic progression has long been a subject of investigation and debate, an elevated SUA level is at least considered an indicator of cardiovascular events1. In this context, elevated SUA levels are reportedly associated with carotid intima-media thickness (IMT) progression2,3,4, a surrogate marker of cardiovascular risk and events5,6, in healthy individuals and patients with several risk factors. In addition to conventional cardiovascular risk factors, including hypertension, hypercholesteremia, diabetes, and smoking, hyperuricemia has been shown to be an independent predictor of IMT progression7,8,9,10. However, few previous investigations have focused on hyperuricemic patients without gout, and predictors of IMT progression in this specific population are largely unknown. In the present study, we aimed to evaluate factors associated with carotid IMT progression in patients with asymptomatic hyperuricemia.
Methods
Study design and participants
This was a post-hoc analysis of the PRIZE (program of vascular evaluation under uric acid control by xanthine oxidase inhibitor, febuxostat: multicenter, randomized controlled) study, a prospective, open-label, blinded-endpoint clinical trial (University Hospital Medical Information Network Clinical Trial Registry; UMIN000012911 and UMIN000041322). The detailed study protocol and design are available in previous publications11,12,13,14. The study was conducted in accordance with the Declaration of Helsinki, and the study protocol was approved by the Ethics Committee Saga University Hospital (2013-10-02 and 2020-05-R01). Written informed consent for the PRIZE study was obtained from all participants.
Individuals were eligible for the PRIZE study when their SUA level was > 7.0 mg/dl and the maximum IMT of the common carotid artery (CCA) was ≥ 1.1 mm at screening. Patients who received SUA-lowering agents within the 8-week period prior to assessment of eligibility and had gouty tophus or symptoms of gouty arthritis within one year were excluded. Patents with hyperuricemia and not receiving SUA-lowering agents or showing significant carotid plaques were randomly allocated in a 1:1 ratio to receive febuxostat, a xanthine oxidase inhibitor, and appropriate lifestyle modification for hyperuricemia (febuxostat group) or lifestyle modification alone (control group). Randomization was stratified according to age (< 65 or ≥ 65 years), sex, diabetes, SUA level (< 8.0 or ≥ 8.0 mg/dl), and maximum CCA-IMT (< 1.3 or ≥ 1.3 mm). Participants allocated to receive febuxostat were treated with an initial dose of 10 mg daily, followed by an increase to 20 mg daily at one month and an increase to 40 mg daily at two months, if tolerated. Although a dose of 40 mg daily was targeted as the maintenance dose, it could be increased up to 60 mg daily at three months or later. Major exclusion criteria for the present sub-analysis were lack of carotid IMT measurement at baseline and/or 24 months and missing baseline data (Fig. 1). Thus, a total of 326 patients were included in this sub-analysis.
Carotid IMT measurement
According to the standard protocol, the carotid IMT images were recorded by a dedicated sonographer using high-resolution carotid ultrasonography with a > 7.5-MHz liner transducer at each study site and were read by an experienced analyzer at a central core laboratory in a blinded manner. IMT measurement was performed in accordance with the Japan Society of Ultrasonics in Medicine and the Japan Academy of Neurosonology using an automated IMT measurement software program (Vascular Research Tools 5, Medical Imaging Applications LLC, Coralville, USA)15. Longitudinal B-mode images of each CCA were obtained, and mean CCA-IMT was determined within a 10-mm region proximal to the origin of the bulb to obtain the averaged thickness of carotid plaque. Maximum CCA-IMT was also evaluated within the region. The mean and maximum IMT values on the left and right sides were averaged at baseline and at 24 months. The absolute change from baseline to 24 months was calculated.
Definitions
In the present analysis, hypertension was defined as having a previous diagnosis of hypertension (office systolic blood pressure ≥ 140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg) or previous antihypertensive medications, according to the guidelines16. Diabetes was defined as a previous diagnosis of diabetes (glycated hemoglobin ≥ 6.5%, fasting plasma glucose ≥ 126 mg/dl, or 2-h plasma glucose after 75-g oral glucose tolerance test ≥ 200 mg/dl, or a random plasma glucose ≥ 200 mg/dl) or previous glucose-lowering medications, based on the guidelines17. Dyslipidemia was defined as a previous diagnosis of dyslipidemia or previous lipid-lowering medications. Atherosclerotic cardiovascular disease (ASCVD) included coronary artery disease (a composite of a previous diagnosis of angina pectoris and myocardial infarction or a history of percutaneous coronary intervention and coronary artery bypass grafting) and cerebrovascular disease (a previous diagnosis of ischemic stroke and transient ischemic attack). Patients were divided into two groups according to age (≥ 75 vs. < 75 years), body mass index (BMI) (≥ 25 vs. < 25), and the presence or absence of ASCVD.
Study endpoint and statistical analysis
The primary endpoint of this sub-analysis was the absolute change in mean CCA-IMT from baseline to 24 months. The absolute change in maximum CCA-IMT from baseline to 24 months was also evaluated. Factors associated with IMT progression in patients with hyperuricemia were explored.
Statistical analysis was performed using R statistical software version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria). All continuous data are expressed as median [interquartile range], and categorical data are shown as frequency (%). Multivariable analysis was performed using a multiple linear regression model for identifying factors associated with the absolute change in mean and maximum CCA-IMT from baseline to 24 months. Non-standardized and standardized regression coefficients were evaluated, and an adjusted change in CCA-IMT from baseline to 24 months with a 95% confidence interval was determined. Twelve factors reportedly associated with IMT progression, including age, sex, BMI, hypertension, diabetes, dyslipidemia, smoking, a history of ASCVD, renal function (assessed with estimated glomerular filtration rate), SUA level, high-sensitivity C-reactive protein level, and baseline mean CCA-IMT were included into the multivariable model in addition to the study allocation3,4,18,19,20,21,22,23,24,25,26,27. Because the C-reactive protein level was not normally distributed, it was log-transformed in the multivariable analysis. A p-value of < 0.05 was considered statistically significant.
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki, and the study protocol was approved by the local institutional review boards and independent ethic committees at all sites. Written informed consent for the PRIZE study was obtained from all participants.
Results
From May 2014 to June 2016, a total of 514 patients were enrolled in the PRIZE study and were randomized to either the febuxostat group (n = 257) or the control group (n = 257), among whom 326 (63.4%) were included in the present analysis. Baseline characteristics are shown in Table 1. Median age was 70 [63, 76] years, men accounted for more than 80% of the analysis population, and cardiovascular risk factors were prevalent. More than one-third of the population had a history of ASCVD, and patients were well treated with medications, including antiplatelet and lipid-lowering agents (Table 1). During the study period, 3 of 326 (0.9%) patients developed gout, all of whom were in the control group.
Overall, the mean [from 0.81 (0.73, 0.93) mm to 0.81 (0.72, 0.93) mm] and maximum [from 1.01 (0.91, 1.16) mm to 1.01 (0.89, 1.17) mm] CCA-IMT did not changed significantly from baseline to 24 months. Overall, the adjusted mean [0.0032 (− 0.0214 to 0.0278) mm] and maximum [0.0011 (− 0.0327 to 0.0351) mm] CCA-IMT increased numerically from baseline to 24 months.
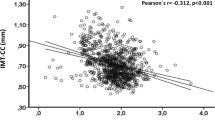
Multivariable analysis identified higher BMI, a history of ASCVD, and a lower mean CCA-IMT at baseline as significant factors associated with an increased absolute change in mean CCA-IMT from baseline to 24 months (Table 2 and Table S1). In addition, older age and a lower mean CCA-IMT at baseline were significant risk factors for an increased absolute change in maximum CCA-IMT (Table 3 and Table S2). In the multivariable analysis for the increase in maximum CCA-IMT, higher BMI and a history of ASCVD were not significantly associated with IMT progression (Table 3 and Table S2). Figure 2 displays the correlations of mean and maximum CCA-IMT with age and BMI. The adjusted absolute difference of change in mean and maximum CCA-IMT in age, BMI, and ASCVD categories are shown in Table S3 and S4. Patients aged ≥ 75 years with BMI ≥ 25 and ASCVD (n = 23) had an increase in mean CCA-IMT at 24 months [0.053 (0.009 to 0.097) mm], while mean CCA-IMT was not significantly changed during the follow-up period in those aged < 75 years with BMI < 25 and no ASCVD (n = 86) [0.003 (− 0.032 to 0.038) mm], with a significant between-group difference [0.050 (0.013 to 0.087) mm, p = 0.008].
Discussion
In the multicenter, prospective, randomized, controlled PRIZE study, mean and maximum CCA-IMT were analyzed in patients with asymptomatic hyperuricemia in a blinded manner at a central core laboratory at baseline and 24 months. In the present post-hoc analysis, higher BMI, a history of ASCVD, and older age were identified as factors related to IMT progression, confirming the importance of conventional risk markers in this specific population. To our knowledge, this is the first study to investigate factors associated with the development of carotid atherosclerosis in asymptomatic hyperuricemic patients.
Carotid IMT, the thickness of the intimal and medial layer of the carotid artery wall, can be non-invasively measured by ultrasound imaging and has been used as a surrogate marker of early-stage atherosclerosis in daily practice and clinical studies5. In general, among numerous previous studies, mean CCA-IMT progression reportedly ranges from 0 to 30 μm per year25,27. The European guidelines do not recommend systematic use of carotid IMT to improve risk assessment because of the lack of methodological standardization and the uncertainty of the added value of IMT in predicting future cardiovascular events28. However, a recent meta-analysis of 119 randomized, controlled trials including 100,667 patients showed that carotid IMT progression was significantly associated with an increased risk of cardiovascular disease, and effects of interventions on carotid IMT regression or delayed progression improved outcomes5, supporting the usefulness of IMT on carotid ultrasonography as a surrogate marker in clinical trials, as in the PRIZE study. Previous studies have indicated that conventional cardiovascular risk factors, such as hypertension, diabetes, dyslipidemia, current smoking, and obesity, are related to carotid IMT progression in various populations5,27,29, and elevated SUA levels may be associated with the development of carotid atherosclerosis3,4,7,8,9,10. However, no studies have focused on patients with an elevated SUA level in investigating IMT progression.
A meta-analysis of 15 cross-sectional studies including 11,382 participants showed that SUA levels correlated positively with carotid IMT4, but the present analysis and the original PRIZE study did not demonstrate a significant relationship between baseline SUA level and SUA-lowering therapy with febuxostat and carotid IMT progression. In a previous prospective longitudinal study that included patients with multiple cardiovascular risk factors in which core laboratory analysis was performed on carotid ultrasonography, faster IMT progression was observed in the group with elevated SUA levels (> 4.8 mg/dl for women and > 5.7 mg/dl for men) compared to their counterparts3. Given that the present analysis included only patients with elevated SUA levels (i.e. > 7.0 mg/dl), the results may be reasonable. In a previous single-center, cross-sectional study, the presence of hyperuricemia was significantly associated with the increase in carotid IMT in patients with essential hypertension, particularly in women30. A meta-analysis showed that there was a significant correlation between carotid-femoral pulse wave velocity and SUA levels in hypertensive women but not in men31. Because men accounted for more than 80%, and approximately 90% of patients had hypertension in the present study, the association between elevated levels of SUA and IMT progression may have been unclear. In addition, in a healthy population, the relation of SUA to cardiovascular organ damage including carotid plaque formation remains to be established32.
Nevertheless, because patients with hyperuricemia are at a high risk of cardiovascular events1, the identification of factors associated with the development of atherosclerosis is essential. In the present analysis, higher BMI, a history of ASCVD, and older age were identified as factors associated with IMT progression, while cardiovascular risk factors such as hypertension, diabetes, and dyslipidemia were not. It is important that the present study indicated higher BMI as a predictor of IMT progression, because obesity and hyperuricemia are closely associated based on both genetic and environmental factors33. Given that obesity is a modifiable risk factor and weight loss ameliorates cardiovascular risk, lifestyle intervention should be considered in patients with hyperuricemia to delay atherosclerosis34,35. In contrast to BMI, a history of ASCVD and older age are not modifiable. However, the identification of patients at high risk for IMT progression and future cardiovascular events may lead to intensive therapy, presumably resulting in improved clinical outcomes36,37. Interestingly, in addition to those three predictors, lower IMT at baseline was counterintuitively associated with IMT progression in the present study38, which may be explained by the fact that our study cohort had a relatively thin carotid plaques. Further studies are needed to clarify the impact of higher BMI, a history of ASCVD, and older age on carotid IMT progression and cardiovascular events in this specific population of patients with hyperuricemia.
The present analysis has several limitations. The PRIZE study was a prospective, randomized, controlled study, but the present analysis was conducted in a post-hoc fashion. Because most of participants in this study were Japanese, caution is warranted to interpret the results given the possible racial difference in the progression of carotid IMT39. A certain proportion of patients did not have IMT data at 24 months, resulting in the relatively small sample size. Nonetheless, standardized analysis of carotid ultrasonography at a central core laboratory was a study strength. Despite the entry criterion of CCA-IMT ≥ 1.1 mm, which may have affected the results and was measured at each participating hospital, the median maximum CCA-IMT at baseline evaluated by a core laboratory was < 1.1 mm, reinforcing the importance of organized and standardized IMT measurement. Since all patients included in the present study were asymptomatically hyperuricemic and none of them had a normal level of SUA at baseline, whether our results can be applied to populations with a normal SUA level is uncertain.
Conclusions
The present sub-analysis of the PRIZE study identified higher BMI, a history of ASCVD, and older age as factors associated with carotid IMT progression in patients with asymptomatic hyperuricemia. These factors may be considered when identifying the possible risk of atherosclerotic progression in this specific patient population of hyperuricemia.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- ASCVD:
-
Atherosclerotic cardiovascular disease
- BMI:
-
Body mass index
- CCA:
-
Common carotid artery
- IMT:
-
Intima-media thickness
- SUA:
-
Serum uric acid
References
Saito, Y., Tanaka, A., Node, K. & Kobayashi, Y. Uric acid and cardiovascular disease: A clinical review. J Cardiol. 78(1), 51–57 (2021).
Tavil, Y. et al. Uric acid level and its association with carotid intima-media thickness in patients with hypertension. Atherosclerosis 197(1), 159–163 (2008).
Mannarino, M. R. et al. Association between uric acid, carotid intima-media thickness, and cardiovascular events: Prospective results from the IMPROVE study. J. Am. Heart Assoc. 10(11), e020419 (2021).
Ma, M. et al. Meta-analysis of the correlation between serum uric acid level and carotid intima-media thickness. PLoS ONE 16(2), e0246416 (2021).
Willeit, P. et al. Carotid intima-media thickness progression as surrogate marker for cardiovascular risk: Meta-analysis of 119 clinical trials involving 100 667 patients. Circulation 142(7), 621–642 (2020).
Katakami, N. et al. Clinical utility of carotid ultrasonography in the prediction of cardiovascular events in patients with diabetes: A combined analysis of data obtained in five longitudinal studies. J. Atheroscler. Thromb. 25(10), 1053–1066 (2018).
Peng, L. H. et al. Carotid intima-media thickness in patients with hyperuricemia: A systematic review and meta-analysis. Aging Clin. Exp. Res. 33(11), 2967–2977 (2021).
Wu, T. W. et al. Associations of cardiovascular risk factors with carotid intima-media thickness in middle-age adults and elders. J. Atheroscler. Thromb. 24(7), 677–686 (2017).
Qu, B. & Qu, T. Causes of changes in carotid intima-media thickness: A literature review. Cardiovasc. Ultrasound. 13, 46 (2015).
Herder, M., Johnsen, S. H., Arntzen, K. A. & Mathiesen, E. B. Risk factors for progression of carotid intima-media thickness and total plaque area: A 13-year follow-up study: The Tromso Study. Stroke 43(7), 1818–1823 (2012).
Tanaka, A. et al. Febuxostat does not delay progression of carotid atherosclerosis in patients with asymptomatic hyperuricemia: A randomized, controlled trial. PLoS Med. 17(4), e1003095 (2020).
Oyama, J. et al. Rationale and design of a multicenter randomized study for evaluating vascular function under uric acid control using the xanthine oxidase inhibitor, febuxostat: The PRIZE study. Cardiovasc. Diabetol. 15, 87 (2016).
Kusunose, K. et al. Effect of febuxostat on left ventricular diastolic function in patients with asymptomatic hyperuricemia: A sub analysis of the PRIZE Study. Hypertens. Res. 45(1), 106–115 (2022).
Tanaka, A. et al. Association between serum urate level and carotid atherosclerosis: An insight from a post hoc analysis of the PRIZE randomised clinical trial. RMD Open 8(1), e002226 (2022).
Kinoshita, M. et al. Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017. J. Atheroscler. Thromb. 25(9), 846–984 (2018).
Shimamoto, K. et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2014). Hypertens. Res. 37(4), 253–390 (2014).
American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care 37(Suppl 1), S14-80 (2014).
Chambless, L. E. et al. Risk factors for progression of common carotid atherosclerosis: The Atherosclerosis Risk in Communities Study, 1987–1998. Am. J. Epidemiol. 155(1), 38–47 (2002).
van der Meer, I.M., Iglesias del Sol, A., Hak, A.E., Bots, M.L., Hofman, A., & Witteman, J.C. Risk factors for progression of atherosclerosis measured at multiple sites in the arterial tree: The Rotterdam Study. Stroke 34(10), 2374–2379 (2003).
Tattersall, M. C. et al. Predictors of carotid thickness and plaque progression during a decade: The Multi-Ethnic Study of Atherosclerosis. Stroke 45(11), 3257–3262 (2014).
Rosvall, M. et al. Risk factors for the progression of carotid intima-media thickness over a 16-year follow-up period: The Malmö Diet and Cancer Study. Atherosclerosis 239(2), 615–621 (2015).
Sturlaugsdottir, R. et al. Predictors of carotid plaque progression over a 4-year follow-up in the Reykjavik REFINE-study. Atherosclerosis 269, 57–62 (2018).
Gracia, M. et al. Predictors of subclinical atheromatosis progression over 2 years in patients with different stages of CKD. Clin. J. Am. Soc. Nephrol. 11(2), 287–296 (2016).
Eltoft, A. et al. C-reactive protein in atherosclerosis—A risk marker but not a causal factor? A 13-year population-based longitudinal study: The Tromsø study. Atherosclerosis 263, 293–300 (2017).
Willeit, P. et al. Inflammatory markers and extent and progression of early atherosclerosis: Meta-analysis of individual-participant-data from 20 prospective studies of the PROG-IMT collaboration. Eur. J. Prev. Cardiol. 23(2), 194–205 (2016).
Tschiderer, L., Klingenschmid, G., Seekircher, L. & Willeit, P. Carotid intima-media thickness predicts carotid plaque development: Meta-analysis of seven studies involving 9341 participants. Eur. J. Clin. Invest. 50(4), e13217 (2020).
Nezu, T., Hosomi, N., Aoki, S. & Matsumoto, M. Carotid intima-media thickness for atherosclerosis. J. Atheroscler. Thromb. 23(1), 18–31 (2016).
Visseren, F. L. J. et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 42(34), 3227–3337 (2021).
Raitakari, O. T. et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: The cardiovascular risk in young Finns study. JAMA 290(17), 2277–2283 (2003).
Maloberti, A. et al. Sex-related relationships between uric acid and target organ damage in hypertension. J. Clin. Hypertens. (Greenwich). 20(1), 193–200 (2018).
Rebora, P. et al. Association between uric acid and pulse wave velocity in hypertensive patients and in the general population: A systematic review and meta-analysis. Blood Press. 29(4), 220–231 (2020).
Maloberti, A. et al. Hyperuricemia prevalence in healthy subjects and its relationship with cardiovascular target organ damage. Nutr. Metab. Cardiovasc. Dis. 31(1), 178–185 (2021).
Gong, M. et al. Converging relationships of obesity and hyperuricemia with special reference to metabolic disorders and plausible therapeutic implications. Diabetes Metab. Syndr. Obes. 13, 943–962 (2020).
Powell-Wiley, T. M. et al. Obesity and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 143(21), e984–e1010 (2021).
Gregg, E. W. et al. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: A post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 4(11), 913–921 (2016).
Huang, R. et al. Comparative effects of lipid lowering, hypoglycemic, antihypertensive and antiplatelet medications on carotid artery intima-media thickness progression: A network meta-analysis. Cardiovasc. Diabetol. 18(1), 14 (2019).
Nathan, D. M. et al. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N. Engl. J. Med. 348(23), 2294–2303 (2003).
Salonen, R., Nyyssönen, K., Porkkala, E., Rummukainen, J., Belder, R., Park, J.S. et al. Kuopio Atherosclerosis Prevention Study (KAPS). A population-based primary preventive trial of the effect of LDL lowering on atherosclerotic progression in carotid and femoral arteries. Circulation 92(7), 1758–1764 (1995).
Lutsey, P. L. et al. Associations of acculturation and socioeconomic status with subclinical cardiovascular disease in the multi-ethnic study of atherosclerosis. Am. J. Public Health 98(11), 1963–1970 (2008).
Funding
The PRIZE study was supported by Teijin Pharma Limited, Japan. The funding body had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
Y.S.: Conceptualization, Methodology, Writing-original draft, Visualization. A.T.: Conceptualization, Methodology, Writing-review & editing, Investigation, Data curation, Project administration. T.I.: Writing-review & editing, Investigation, Software. H.Y.: Writing-review & editing, Formal analysis. Y.K.: Writing-review & editing, Investigation. M.N.: Writing-review & editing, Investigation. M.M.: Writing-review & editing, Investigation. Y.O.: Writing-review & editing, Investigation. Y.K.: Writing-review & editing, Investigation, Supervision. K.N.: Conceptualization, Methodology, Writing-review & editing, Investigation, Data curation, Funding acquisition, Resources, Project administration. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
AT received honoraria from Boehringer Ingelheim and research funding from GlaxoSmithKline and Takeda. MM has received honoraria from Sanofi, Takeda Pharmaceutical Company Ltd., Eli Lilly Japan, Mitsubishi Tanabe Pharma Corporation, Astellas Pharma Inc., Novo Nordisk Pharma Ltd., Sumitomo Dainippon Pharmaceutical Company and MSD; research funding from Sysmex Corporation and Nissui; and subsidies or donations from Novartis Pharma, Sanofi, and Novo Nordisk Pharma Ltd. KN has received honoraria from MSD, Astella, AstraZeneca, Novartis Pharma, Ono Pharmaceutical, Daiichi Sankyo, Mitsubishi Tanabe Pharma, Eli Lilly Japan, Boehringer Ingelheim Japan, Takeda Pharmaceutical; Research grant from Asahi Kasei, Astellas, Mitsubishi Tanabe Pharma, Teijin Pharma, Terumo, Boehringer Ingelheim Japan, Eli Lilly and Company, Mochida Pharmaceutical, Fuji Yakuhin; Scholarship from Daiichi Sankyo Healthcare, Teijin Pharma, Medtronic, Bayer Yakuhin. All other authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saito, Y., Tanaka, A., Ishizu, T. et al. Factors associated with carotid intima-media thickness progression in patients with asymptomatic hyperuricemia: insights from the PRIZE study. Sci Rep 13, 10927 (2023). https://doi.org/10.1038/s41598-023-37183-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-37183-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.