Abstract
Organellar C-to-U RNA editing in plants occurs in complexes composed of various classes of nuclear-encoded proteins. DYW-deaminases are zinc metalloenzymes that catalyze hydrolytic deamination required for C-to-U modification editing. Solved crystal structures for DYW-deaminase domains display all structural features consistent with a canonical cytidine deamination mechanism. However, some recombinant DYW-deaminases from plants have been associated with ribonuclease activity in vitro. Direct ribonuclease activity by an editing factor is confounding since it is not required for deamination of cytosine, theoretically would be inimical for mRNA editing, and does not have a clear physiological function in vivo. His-tagged recombinant DYW1 from Arabidopsis thaliana (rAtDYW1) was expressed and purified using immobilized metal affinity chromatography (IMAC). Fluorescently labeled RNA oligonucleotides were incubated with recombinant AtDYW1 under different conditions. Percent relative cleavage of RNA probes was recorded at multiple time points from triplicate reactions. The effects of treatment with zinc chelators EDTA and 1, 10-phenanthroline were examined for rAtDYW1. Recombinant His-tagged RNA editing factors AtRIP2, ZmRIP9, AtRIP9, AtOZ1, AtCRR4, and AtORRM1 were expressed in E. coli and purified. Ribonuclease activity was assayed for rAtDYW1 in the presence of different editing factors. Lastly, the effects on nuclease activity in the presence of nucleotides and modified nucleosides were investigated. RNA cleavage observed in this study was linked to the recombinant editing factor rAtDYW1 in vitro. The cleavage reaction is sensitive to high concentrations of zinc chelators, indicating a role for zinc ions for activity. The addition of equal molar concentrations of recombinant RIP/MORF proteins reduced cleavage activity associated with rAtDYW1. However, addition of equal molar concentrations of purified recombinant editing complex proteins AtCRR4, AtORRM1, and AtOZ1 did not strongly inhibit ribonuclease activity on RNAs lacking an AtCRR4 cis-element. Though AtCRR4 inhibited AtDYW1 activity for oligonucleotides with a cognate cis-element. The observation that editing factors limit ribonuclease activity of rAtDYW1 in vitro, suggests that nuclease activities are limited to RNAs in absence of native editing complex partners. Purified rAtDYW1 was associated with the hydrolysis of RNA in vitro, and activity was specifically inhibited by RNA editing factors.
Similar content being viewed by others
Introduction
There are hundreds of genes encoding serial pentatricopeptide repeats (PPRs) in a typical higher plant and this family of genes is essential for various steps of RNA maturation1,2. Many PPR genes in plants also encode a C-terminal domain dubbed the DYW-deaminase domain (Pfam: Pf14432) after the common terminal tripeptide sequence motif aspartate-tyrosine-tryptophan (DYW) and this domain along with upstream regions share common features with characterized nucleotide deaminases3,4,5. The DYW-deaminase domain coordinates two zinc ions per DYW-deaminase subunit4,6 through a conserved [(H/C)xEx(25–30)CxxC] motif common to other nucleotide deaminases and an unique [Hx22Hx6CSC] zinc binding domain. Genetic complementation studies indicate the necessity of zinc binding domains and the proposed catalytic glutamate for RNA editing7,8,9. Cementing the role in editing, expression of moss Physcomitrella patens PPR proteins PPR65 and PPR56 are sufficient for specifically editing their RNA targets in an Escherichia coli expression system10 and purified recombinant PPR65 protein also can edit RNAs in vitro11.
In addition to the clear role for the DYW-deaminase in RNA editing, additional roles in transcript maturation have been proposed. The PPR protein with a C-terminal DYW-deaminase domain called CHLORORESPIRATORY REDUCTION2 (CRR2) is required for the accumulation of processed rps7 and ndhB mRNAs12. Failure of native mRNA termini to accumulate in crr2 knock-out plants results in the inactivity of the NDH complex12. Investigation of RNA sequences in wildtype plants compared to knock-out plants has failed to reveal a direct role in RNA editing for CRR213. However, ribonuclease activity observed in vitro for a recombinant protein with the DYW-deaminase domain of CRR2 was interpreted as evidence for direct endoribonuclease activity in the rps7-ndhB intergenic region14. Complicating a simple direct role in sequence specific cleavage, PPR tracts, like many RNA binding domains, protect RNAs from ribonucleases allowing for the observation of “protected” footprints15. Thus, it can be difficult to discriminate if specific transcripts result from protection from a nuclease acting in trans or from a direct sequence specific cleavage nearby a cis-element. The observation of CRR2 dependent “footprints” at both the 5′ end of ndhB and 3′ end of rps7 transcripts are strongly suggestive of a protective role from nucleases by the CRR2 PPR tract13. Thus, a direct physiologic role for the DYW-deaminase domain associated ribonuclease cleavage remains controversial.
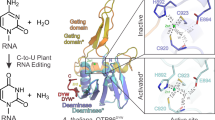
Recently the crystal structure for AtDYW1 has been solved16 and two additional solved crystal structures for the DYW domain of editing factor AtOTP86 have been reported: a tetrameric structure called “active” (PDB https://doi.org/10.2210/pdb7O4F/pdb) and a dimeric structure called “inactive” (PDB https://doi.org/10.2210/pdb7O4E/pdb)6. The “active” conformation has an active site with features consistent with other cytidine deaminases, however the “inactive” conformation likely cannot accommodate a cytidine base substrate due to steric occlusion6. Addition of tetrahydrouridine (THU) during the preparation of a recombinant DYW-deaminase protein was critical for the observation of the “active” conformation but the molecule was not observed in the solved structure6. In addition to THU, the nucleotide ATP was also linked to the “active” conformation6. Additionally, the crystal structure of AtDYW1 suggests interactions with the PPR AtCRR4 form the substrate binding site16. A nuclease-like domain has not been described for the DYW-deaminase in either conformation.
At least three recombinant DYW domain-containing proteins have been linked to ribonuclease activity in vitro: the aforementioned CRR214; At2g0298017 which was later recognized to be a chloroplast RNA editing factor named Organelle Transcript Processing 85 (OTP85)18; and Os05g3071017. The two reports of DYW-associated ribonuclease activity indicate sensitivity to extremely high concentrations (100–200 mM) of the metal chelator EDTA17. Cleavages linked to the DYW domain of AtCRR2 precede adenines (As) in absence of the native PPR tract, which demonstrates some general sequence affinity by the domain alone. Sequence specificity for local bases nearby the targeted cytidine for the RNA editing apparatus has been well documented19,20,21 and recently more precisely shown to relate to the DYW domain22. It is unclear what residues of the DYW domain might be responsible for nuclease activity and the metastable nature of RNA and the simplicity of catalysis yields many possibilities.
In their native environment, PPR proteins with DYW-deaminase domains (PPR-DYWs) have been physically linked to several other proteins that are necessary for RNA editing in Arabidopsis thaliana23 and Zea mays24. RNA editing likely occurs in large heterocomplexes that include PPR proteins, DYW-deaminase domains, RNA editing factor interacting proteins/multiple organellar RNA editing factors RIP/MORFs, organellar zinc-finger (OZ) proteins, and organelle RNA recognition motif (ORRM) proteins23. RIP/MORFs bind PPR proteins25,26 extensively through L-motif interactions of P-L-S motifs27 and have been shown to interact with the E and E+ motifs28 present in the broadly defined enzymatic domain3 that includes the DYW-deaminase domain.
In this study, recombinant AtDYW1 was expressed and purified to investigate nuclease activity associated with an RNA editing enzyme in vitro. The editing factor AtDYW1 is essential for creating the start codon of ndhD transcripts in Arabidopsis29. A ribonuclease activity assay was constructed using Cy-5 labeled oligoribonucleotides with various sequences. In absence of conflict with the established role of AtDYW1 in editing ndhD transcripts in vivo29, RNA cleavage of ndhD sequence containing oligonucleotides in vitro was specifically reduced in the presence of AtCRR4. In oligonucleotides without AtCRR4 cis-elements only RIP family editing complex members could inhibit rAtDYW1 nuclease activity. RIP proteins could not protect RNAs from cleavage catalyzed by RNaseA. Zinc chelators strongly inhibit ribonuclease activity. Nucleotides and modified nucleosides that favor formation of “active” conformations of the DYW-deaminase did not alter ribonuclease activity and editing activity could not be reconstituted in vitro. Therefore, the zinc-dependent nuclease activity associated with the DYW-deaminase is greatly reduced in the context of editing complex member association.
Results
The editing factor DYW1 from Arabidopsis thaliana was examined due to a few unique features that make it amenable for this study versus other DYW-deaminase containing proteins. AtDYW1 does not possess an N-terminal PPR-tract, only a degenerate PPR-like N-terminal region as another editing factor AtCRR4 is required to specifically edit ndhD transcripts in vivo29. Since the AtDYW1 does not have a lengthy PPR-tract, the protein theoretically would not have a strong sequence preference to protect a region of an RNA probe through PPR shielding. All other DYW-deaminase domain containing editing factors in Arabidopsis have N-terminal PPRs. Also, AtDYW1 could be studied as an entire protein not as a C-terminal protein fragment with potentially altered solvent exposure that might affect its tertiary structure. AtDYW1 is not known to be associated with RNA cleavage around the singular ndhD editing target suggesting no coupling between editing and ribonuclease cleavage in vivo.
Recombinant AtDYW1 was expressed in E. coli with a N-terminal 6X-His tag (rAtDYW1) to determine if ribonuclease activity is a common feature for the DYW-deaminase domain. Ample amounts of the protein could be obtained with high purity from a single round of immobilized metal affinity chromatography (Fig. 1A, Fig. S1). Purified proteins resolved by gel filtration combined with a multiple angle light scattering estimated a molecular weight consistent with a monomer (Fig. S2).
Recombinant AtDYW1 has nuclease activity in vitro. (A) An image of a Coomassie-stained SDS-PAGE to assess purity of the IMAC purified rAtDYW1 with 1, 2, and 5 μg loaded per lane. (B) RNA oligonucleotides labeled with a 5′ Cy-5 fluorophore visualized after separation on a 6 M Urea 20% Polyacrylamide gel from assay of nuclease activity. From left to right lanes represent rAtDYW1 and BSA were incubated from < 1 to 60 min followed by a 2.5 μg/mL RNaseA control at 60 min. (C) Relative cleavage \(\left[\frac{(\text{Intensity at }0\text{ min }-\text{ Intesity at timepoint})}{\text{Intensity at }0\text{ min}} \times 100\right]\) was calculated and plotted in an X-ray scatterplot for timepoints 20, 40, and 60 min for rAtDYW1 and an equivalent reaction with addition of 40 U/mL of Proteinase K. Error bars represent 1 standard deviation of the mean for three separate reaction replicates. (D) Ribonuclease activity was assayed in reactions with a titration of rAtDYW1 from 225 to 28:1 molar ratio compared to labeled RNA probe at 15 nM. Dotted trendline is a best fit line using a logarithmic fit. For (B–D) the oligo Zmrps14 was used.
Recombinant AtDYW1 fractions were initially assayed for ribonuclease activity on a 5′ Cy-5 labeled 26-mer RNA oligonucleotide called Zmrps14 with the sequence (5′-Cy-5-UCAUUUGAUUCGUCGAUCCUCAAAAA) derived from the rps14 gene present in the chloroplast genome of Zea mays. The RNA oligo was chosen since it would allow the study of ribonuclease activity on a general RNA sequence. Ribonuclease activity was observed relative to a bovine serum albumin (BSA) negative control with a distinct pattern of cleavage products compared to RNaseA (Fig. 1B). Addition of 1 U/μL Ribolock ribonuclease inhibitor was found to improve the consistency of the assay and was maintained throughout all reactions. Ribonucleases are ubiquitous in the environment and Ribolock was sufficient to reduce background cleavage. Proteinase K was added to 40 U/mL in reactions to investigate if cleavage was due to protein and not free ions, and a general decline in percent relative cleavage was observed (Fig. 1C). Recombinant AtDYW1 was titrated in a series of reactions with molar ratios of rAtDYW1 versus RNA from 225:1 to 28:1 and a logarithmic trend was observed with greater nuclease activity correlating with increased rAtDYW1 concentration (Fig. 1D).
Oligonucleotides with different sequences were incubated with rAtDYW1 to test specificity of nuclease activity through the creation of new RNA species: (1) A 5′ Cy-5 labeled AtndhD (5′-Cy-5-GGUGUAUCUUGUCUUUACCACGAAUG-3′) oligonucleotide has the cis-element for AtCRR4 with a sequence context of 20 nucleotides upstream and 5 nucleotides downstream of the editing site; (2) an oligonucleotide M13 FAM (5′-UCCUGUGUGAAAUUGUUAUC-FAM-3′) with a 3′ fluorescein moiety; (3) A 5′ Cy-5 labelled ZmndhB (5′-Cy-5-UACUUCGAAAGUAGCUGCUUCAGCUU-3′) oligonucleotide with sequence around another editing site in maize; (4) the aforementioned Zmrps14 oligonucleotide; and (5) A 5′ Tetrachlorofluorescein labelled PpccmFC (5′-TET-UGGUUGGUAAGUAGAGAUGUUCCCACA-3′) oligonucleotide with sequence around an editing site in Physcomitrella patens mitochondria. The patterns of generated RNA species after incubation with rAtDYW1 were distinct between the 5 oligonucleotides (Fig. 2, Fig. S3). The projected positions of cleavage did not demonstrate a robust sequence preference but a more general hydrolysis resulting in 8-mers or shorter RNA species.
AtDYW1 was incubated with 5 different RNA oligonucleotides (AtndhD, M13 FAM, ZmndhB, Zmrps14, and PpccmFC) at 28 °C for 20 min in triplicate reactions (lanes A–C) and compared to the oligonucleotide without incubation with DYW1 (lanes O). RNA species from reactions were separated on a 20% polyacrylamide gel in 6 M urea and imaged using an Azure c400 imager using channels for each specific fluorophore. At left of each gel image, letters represent the likely 3′ nucleotide identify. Bands were ranked as highly (***), moderately (**), and slightly (*) increased species compared to the oligonucleotide control.
Prior experiments examining ribonuclease activity of the DYW-deaminase domain suggested a role for metal ions in catalysis based on titration of the metal chelator EDTA14,17. Recombinant AtDYW1 is known to bind two zinc ions per polypeptide chain4. Fractions of purified rAtDYW1 were dialyzed in a buffer with and without divalent metal chelators (25 mM EDTA, 650 μM 1,10-phenanthroline) for three days. Ribonuclease activity was lower in fractions treated with metal chelators versus without, but activity could not be completely eliminated (Fig. 3).
Ribonuclease activity for rAtDYW1 is sensitive to treatment with zinc ion chelators. Fractions of rAtDYW1 were dialyzed for 3 days with and without an inhibitor cocktail of 25 mM EDTA and 0.65 mM 1,10-phenanthroline. Ribonuclease activity was assayed for dialyzed rAtDYW1 fractions. At top, a picture of RNA oligos with the Zmrps14 sequence separated on a 6 M urea 20% PAGE. Lanes from left to right represent an RNA oligo control, triplicate reactions of the RNA oligo subjected to reaction conditions without enzyme, RNA oligonucleotides treated with rAtDYW1 dialyzed 3 days at 4 °C without chelator, and RNA treated with rAtDYW1 dialyzed in a buffer containing EDTA and 1,10-phenanthroline.
Several editing factors were cloned and expressed to investigate their effect on rAtDYW1 linked activities. Editing factors were chosen based on a possible editosome responsible for the creation of the initiation codon of the chloroplast gene ndhD: (1) the PPR AtCRR4 likely binds to the cis-element upstream of the aCg > aUg editing target; (2) AtDYW1 acts as a deaminase enzyme; (3) RIP2 and RIP9 are theorized to bind L-domains of the PPR or E-domains nearby the DYW domain; and (4) AtOZ1 and AtORRM1 act as critical accessory factors.
Recombinant AtRIP2 could be purified in large quantities (Fig. 4A, Fig. S4). Ribonuclease activity was absent when rAtRIP2 was added alone in equivalent molar amounts as rAtDYW1. However, when equimolar amounts of both rAtDYW1 and rAtRIP2 were added together, little hydrolysis of RNA oligonucleotides was observed (Fig. 4B). The reduction in nuclease activity by rAtRIP2 could either be due to direct protection of RNA or a specific interaction with rAtDYW1. RNA hydrolysis was robust after the addition of 2.5 ng/μL of RNaseA to reactions (Fig. 4C). RNaseA catalyzed nuclease activity remained robust after the addition of 5 μM rAtRIP2, however little hydrolysis was observed for equivalent reactions containing 4 U/μL Ribolock RNase Inhibitor (Fig. 4C). The inhibitory effect of rAtRIP2 on nuclease activity was reduced in reactions with molar excess of rAtDYW1 and a 1:1 molar ratio elicited the greatest protection (Fig. 4D).
Recombinant AtRIP2 inhibits ribonuclease activity of rAtDYW1. (A) The Coomassie stained SDS-PAGE image indicates the purity of rAtRIP2 fractions after one round of Ni-NTA affinity chromatography and one round of size exclusion chromatography. (B) Ribonuclease activity on RNA oligonucleotides was assayed at 0 min, 20 min, 40 min and 60 min in triplicate reactions (representative image from one time course shown) in the presence of rAtDYW1, rAtRIP2, and rAtDYW1 mixed with rAtRIP2 in an equimolar ratio (1:1). At top a representative image is shown of the set of the 6 M urea 20% PAGE used to quantify percent relative cleavage. Below a graph displays % relative cleavage of all triplicate reactions across 4 timepoints. (C) Ribonuclease activity was assayed for triplicate reactions containing 2.5 μg/mL RNaseA, 2.5 ng/μL RNaseA + 5 μM rAtRIP2, and 2.5 μg/mL RNaseA + 4 U/μL Ribonuclease inhibitor (RNaseIN). At top a representative image of one time-course reaction is shown and below a X–Y scatterplot displays % relative cleavage as a function of time for triplicate reactions. (D) Activity was measured in triplicate reactions with various stoichiometric ratios of rAtRIP2 versus rAtDYW1 from (1:1 to 1:200). Representative gel images are shown at left and % relative cleavage for triplicate reactions are represented in a X–Y scatterplot at right. (B–D) Error bars represent 1 standard deviation from the mean for triplicate reaction replicates. (B–D) used the oligo Zmrps14.
Several attempts at expression and purification of the RIP/MORF family member rAtRIP9 led to small protein yields with many degraded proteins present in enriched fractions (Fig. S5). Addition of approximately equimolar quantities of rAtRIP9 to rAtDYW1 led to reduced hydrolysis of RNA oligonucleotides compared to equivalent reactions with only rAtDYW1 (Fig. S5b). Expression and purification of the Zea mays ortholog rZmRIP9 yielded large quantities of stable protein (Fig. 5A, Fig. S6). When rZmRIP9 was added to nuclease reactions in increasing molar ratios with a static concentration of rAtDYW1 decreasing amounts of relative cleavage was observed (Fig. 5B).
Recombinant ZmRIP9 progressively inhibits ribonuclease activity of rAtDYW1 at increasing relative molar ratios. (A) Recombinant ZmRIP9 was purified using Ni-NTA affinity followed by size exclusion chromatography steps. An image of a representative Coomassie SDS-PAGE indicates purity. (B) At top, ribonuclease activity was assayed for triplicate reactions at 0, 20, 40, and 60 min timepoints containing rZmRIP9 mixed with rAtDYW1 with stoichiometric ratios of 5:1, 2:1, 1:1, and 1:2. Below, a X–Y scatterplot displays % relative cleavage at 4 timepoints. Error bars represent 1 standard deviation from the mean for triplicate reactions. (B) Nuclease reaction use the Zmrps14 oligo.
Other editing factors were expressed and purified to examine their effects on rAtDYW1 linked RNA hydrolysis. The zinc finger protein rAtOZ1 could be purified (Fig. 6A, Fig. S7) and despite some hydrolysis in the absence of rAtDYW1, extensive hydrolysis was observed in reactions with equimolar concentrations with rAtDYW1 (Fig. 6B). Recombinant rAtORRM1 could be purified in low amounts in the presence of degradation products (Fig. S7). There was no difference in nuclease activity of reactions with around equimolar amounts of rAtORRM1 and rAtDYW1 versus rAtDYW1 alone (Fig. S7c).
The editing factor rAtOZ1 does not strongly inhibit rAtDYW1 at an equimolar ratio. (A) Recombinant AtOZ1 was purified using a single round of IMAC and an SDS-PAGE image is shown to evaluate protein purity. (B) Ribonuclease activity was assayed for triplicate reactions containing rAtDYW1, rAtOZ1, and rAtDYW1 + rAtOZ1 mixed in an equimolar ratio on the oligonucleotide Zmrps14. A representative image of a 6 M urea 20% PAGE is shown at top and the data from the triplicate reactions is represented in the X–Y scatterplot below. (B) Error bars represent 1 standard deviation from the mean.
The binding partner with AtDYW1 called AtCRR4 was expressed and purified (Fig. 7A; Fig. S7). The PPR protein AtCRR4 does not possess a DYW-deaminase domain and acts as a specificity factor for AtDYW1 in an editing complex on AtndhD transcripts. Hydrolysis of the Zmrps14 RNA probe lacking the AtCRR4 cis-element was minimal after 60 min in the presence of recombinant AtCRR4 (Fig. 7B). Percent relative cleavage of RNA in reactions with equimolar amounts of rAtCRR4 and rAtDYW1 were comparable to reactions with rAtDYW1 alone (Fig. 7B).
Recombinant AtDYW1 ribonuclease activity was reduced on oligonucleotides with AtndhD sequences when incubated with rAtCRR4 but not affected by not affected by addition of nucleotides or tetrahydrouridine. (A) Recombinant AtCRR4 was purified by IMAC followed by gel filtration and the SDS-PAGE image relates purity. (B) Hydrolysis of RNA Zmrps14 oligonucleotides was assayed in triplicate reactions with rAtDYW1, rAtDYW1 + rAtCRR4 mixed in an equimolar ratio, and rAtCRR4. At top a representative image of RNA oligonucleotides separated on a 6 M urea 20% PAGE. Below a scatterplot represents % relative cleavage at 4 time points for the triplicate reactions. (C) Images of 20% PAGE in 6 M urea show RNA species created through out a 0, 20, 40, 60 min time course from incubation of AtndhD oligonucleotides with rAtDYW1 alone (top), rAtDYW1 with equimolar rAtCRR4 (middle), and rAtDYW1 with equimolar rAtCRR4 in the presence of THU. Reactions were run in triplicate and lanes are labeled for each reaction with (A–C). (D) % relative cleavage was plotted versus time calculated from the intensity of bands from the gel using ImageJ. Error bars represent one standard deviation from the mean for triplicate reactions. (B,D) Error bars represent 1 standard deviation from the mean.
An oligonucleotide with the sequence containing the cis-element for AtCRR4 and the native editing target for AtDYW1 was assayed for hydrolysis by rAtDYW1. Hydrolysis was observed in the presence of rAtDYW1 but was reduced in the presence of rAtCRR4 (Fig. 7C,D). The pattern of resulting RNA species produced by cleavage changed only slightly with the addition of rAtCRR4, which resulted in increased stability of full-length oligonucleotides and RNAs ending in A24-G22 (Fig. S9). Based on the crystal structure of the AtDYW1 it has been hypothesized that a AtDYW1–AtCRR4 complex creates the substrate binding site16. The addition of 2 mM THU promoted the “active” substrate binding site for the DYW domain from OTP866. Addition of 2 mM THU to nuclease reactions containing both rAtDYW1 and rAtCRR4 did not affect the rate of cleavage nor the resulting RNA species profile (Fig. S9). Additionally, RNA hydrolysis was equivalent in reactions with rAtDYW1 alone under standard conditions with 2 mM THU, ATP, and CMP (Fig. S10a).
Since most of the components of the theoretical editing complex were at hand, an attempt was made to reconstruct an active editosome in vitro by combining all purified editing factors. Unfortunately, conversion of an RNA with ndhD sequences was not observed in vitro (Fig. S10b).
Discussion
DYW-deaminases domains are most likely not exclusively either a C-to-U editase or a ribonuclease enzyme
It has been posited that the DYW-deaminase domain might be adapted to either RNA editing or RNA cleavage functions14. This was largely based on initial findings that the PPR protein with the DYW-deaminase domain called AtCRR2 had been found to be necessary for transcript maturation without any known editing function, and at the time, no editing factors were known to be directly linked to ribonuclease activity14,17. Conflicting with this hypothesis, one PPR-DYW protein found to have ribonuclease activity At2g0298017 was later named OTP85 and found necessary for an RNA editing site in ndhD transcripts18. Our results add additional support against the hypothesis of distinct molecular functions for the DYW-deaminase as AtDYW1 is clearly an editing factor29 with ribonuclease activity in vitro (Fig. 1, Fig. S1). There is no conflict with the C-to-U editing enzyme function in vivo based on the cleavage activity observed in vitro as the contexts are fundamentally different. Native editing complexes are known to contain many different proteins in unknown stoichiometries. Despite the potentially artifactual nature of the ribonuclease activity observed for the DYW-deaminase in vitro, ribonuclease activity has been observed for at least 4 recombinant DYW-deaminase proteins. Specific features of the domain likely lead to these observations as equivalently expressed and purified proteins did not possess a similar activity.
The DYW-deaminase as a bifunctional enzymatic domain?
It is attractive to posit that based on evidence of nuclease activity in vitro and confirmed editing activity in vivo that AtDYW1 is a bifunctional enzyme. However, in this study, equimolar concentrations of AtDYW1 with editing factors of the RIP family almost completely abrogate nuclease activity. Also, if the RNA substrate has a cis-element for AtCRR4 than nuclease activity is reduced in the presence of rAtCRR4. Since RIP family proteins have been found to tightly bind PPR proteins23, probably through PPR L-motifs27 and E/E+ motifs28, it is likely that in organelles PPR-DYW proteins are commonly associated with RIP proteins. Additional evidence is required to directly link the DYW-deaminase domain with ribonuclease cleavage in vivo.
As improved structural information becomes available for the domain, perhaps the biochemical mechanism responsible for nuclease activity might become apparent. The solved crystal structures for the DYW-deaminase domain indicate “active” and “inactive” conformations6. The active conformation has all the features consistent with a nucleotide deaminase enzyme confirming the results from several biochemical studies7,8,9,10,11. No function has been ascribed to the “inactive” conformation. Equipped with a model of the DYW-deaminase bound to an RNA ligand, it might be possible to identify possible interactions associated with nuclease activity.
This study did not investigate the physiological function of the ribonuclease activity linked to AtDYW1. It is unlikely to relate to editing of ndhD transcripts. The nuclease activity does not require ndhD sequences or PPR specificity factors. There is some controversy over the physiological function of the DYW-deaminase domain of AtCRR2 where RNA cleavage dependent on the PPR-DYW was first postulated12. Subsequently, ribonuclease activity was demonstrated by the DYW-deaminase domain17. A recent study that focused on transcripts bound to AtCRR2 was dismissive of direct nuclease activity in vivo largely due to the observation that both native 5’ and 3’ transcript termini contain the AtCRR2 cis-element13. Since RIP/MORF proteins reduce cleavage of at least one DYW-deaminase in vitro, the discrepancy between absence of specific cleavage observed in vivo and general cleavage in vitro for AtCRR2 might be explained by the relative presence in vivo or absence in vitro of the PPR tract and RIP/MORF proteins.
Speculative potential roles of DYW-deaminase linked nuclease activity
Though our findings are not able to indicate a specific biological function for DYW-deaminase linked ribonuclease activity, the activity has been observed in vitro. This could be due to an artifact in nonnative conditions or a hidden biological function. Perhaps DYW-domains estranged from editing complexes might participate in RNA degradation instead of deamination. Degradation of RNAs outside of the editosome might confer some additional protection from unwanted off-target C-to-U changes due to promiscuous deaminase activity. This speculative biological activity might be difficult to separate from general nuclease activity as it is an interaction likely outside of an appropriate editing complex/substrate. Presently any potential physiological role for the DYW-deaminase linked nuclease activity in vivo remains speculative.
Conclusions
This study supports previous experiments that have demonstrated the DYW-deaminase domain can be linked to ribonuclease activity in vitro. Ribonuclease activity was extended to include DYW-deaminases known to be RNA editing enzymes. This activity was greatly reduced in the presence of equimolar ratios of editing factors that are known to associate in native editing complexes. Thus, robust DYW-deaminase associated ribonuclease activity could only be observed in isolation from RIP proteins or rAtCRR4 with cognate cis-elements in vitro, limiting the context of possible physiological functions in vivo.
Methods
Expression of recombinant proteins
Recombinant proteins AtDYW1, AtRIP2, AtRIP9, ZmRIP9, AtORRM1, AtCRR4 and AtOZ1 were expressed from pET21a plasmids encoding each respective amino acid sequence downstream of N-terminal Hex-His tag. Plasmids used to express rAtDYW1 were used from a prior study4. Plasmids encoding non-DYW editing factors were constructed through traditional restriction-based cloning. Phusion DNA polymerase (ThermoScientific) and oligonucleotide primers (IDT) with the sequences AtRIP2_ForEcoRI: CAAGGAATTCATGGCTTTGCCTTTGTCTG, AtRIP 2_RevPstI: GCAACTGCAGTCATCTTGTGTTTTCTCTGCGG, AtRIP9_ForBamHI: GCAAGGATCCATGGCTTCCTTCACACAAC, AtRIP9_RevHinDIII: CACAAAGCTTTTAAGAGGAATCAGAGGCTGC, ZmRIP9_ForBamHI: GCATGGATCCGCCGCCGCCTTCCCTGC, ZmRIP9_RevSalI: GCATGTCGACTCACGAAGACGCGGACTCGG, AtORRM1_ForBamHI: GCATGGATCCTCTTCTGCAATTTCCGCACC, AtORMM1_RevXhoI: GCATCTCGAGCTAGAGCCCGAAACTTGGTTG, AtCRR4ForEcoRI: GCATGAATTCGCTTTTGCCTCTTCTCGAC, AtCRR4RevHinDIII: GCATAAGCTTCTACAATGTACTGGAAACTTCAATGC, AtOZ1_BamHIF: GCATGGATCCATGAACAACTCCACCAGACTC, and AtOZ1_SallR: GCATGTCGACTCATTTATCTCCTTTACCAGTGGG were used to create amplicons containing the genes for each editing factor using cDNA as template. All amplicons were cut using the appropriate restriction enzymes and directly cloned into pET21a, except in the case of AtRIP2 which was initially cloned into pBluescriptII and then subcloned into pET21a.
Plasmids were transferred to E. coli Rosetta2 DE3 pLys hosts. Each strain was grown in LB broth at 37 °C to an OD600 of 0.6 and cooled by transfer to an 18 °C incubator for 30 min. Protein expression in bacterial cultures was induced by the addition to a final concentration of 1 mM IPTG and incubated at 18 °C with shaking for 4 h. Cells were harvested by centrifugation at 5000xg for 20 min and stored at − 80 °C.
Protein purification
Cell pellets containing rAtRIP2, rAtRIP9, rZmRIP9 and rAtOZ1 were resuspended in a chilled lysis buffer with 50 mM HEPES pH 7.0, 250 mM NaCl, 10% glycerol, 0.01% (w/v) CHAPS, and 10 mM imidazole. Similarly, cell pellets containing rAtDYW1 and AtORRM1 were resuspended in a chilled lysis buffer with 20 mM tris–HCl pH 7.3, 150 mM NaCl, 10% glycerol, 0.01% (w/v) CHAPS, and 10 mM imidazole. Cell pellets for rAtCRR4 were resuspended in 50 mM tris–HCl pH 7.3 @ RT, 250 mM NaCl, 1% glycerol, 0.01% (w/v) CHAPS, and 10 mM imidazole. PMSF suspended in 2-proponol was added to a final concentration of 1 mM before cell lysis. Cell suspensions were sonicated 6 times at 80% of the maximum amplitude for 20 s, each with 1 min of rest in between bursts. Insoluble cellular debris was removed through pelleting in the centrifuge at 12,000×g for 30 min. The N-terminal his-tagged proteins rAtDYW1, rAtRIP2, rAtRIP9, ZmRIP9, rAtORRM1, rAtCRR4, and rAtOZ1 were purified using IMAC with His-PURE NiNTA resin. Proteins were assessed for purity using Coomassie stained SDS-PAGE. Size exclusion chromatography was performed with a Superdex S200 10/300 GL column (GE Life Sciences). The column was equilibrated and AtCRR4, AtORRM1, AtRIP2m ZmRIP9 proteins were purified using a running buffer composed of 20 mM tris–HCl pH 7.3, 150 mM NaCl, 10% glycerol at a flow rate of 0.25 mL/min.
Immunoblotting
The samples were mixed with TruPAGE LDS Sample Buffer (Sigma-Aldrich) and electrophoresed in a 4–20% TruPAGE SDS PAGE Gel (Sigma-Aldrich). Proteins were transferred to 0.45 µm PVDF membranes by electroblotting overnight. After the transfer was completed, the membranes were incubated in 1X TBST with 5% (w/v) nonfat dry milk for an hour at room temp. Primary antibody dilutions were made in 1xTBST containing 5% milk as follows: anti-RIP924 1:5000, anti-6× His HRP Conjugate (Invitrogen) 1:2500, anti-MBP (Invitrogen) 1:2500. Membranes were incubated with each respective antibody dilution on a plate shaker at 4 °C overnight, followed by three, 10-min washes with 1× TBST at room temperature. Membranes were incubated with Peroxidase Conjugated Goat anti-Rabbit (H + L) antibody (Invitrogen) for one hour at room temperature followed by three additional 10-min washes with 1X TBST. The ProtoGlow ECL Detection Kit (National Diagnostics) and an Azure c400 gel imager (Azure Biosystems Inc.) was used to visualize the blots.
RNA nuclease assay
Recombinant AtDYW1 was added to a final concentration of 3.4 μM with a 5′ Cy-5 fluorescently labeled RNA at a final concentration of 15 nM under final reaction conditions of 13 mM Tris–HCl pH 7.9, 22.5 mM NaCl, 30 mM KCl, 6 mM MgCl2, 2 mM DTT, 10 U Ribolock RNase Inhibitor I, 37.5 mM imidazole, 25 mM EDTA, 1.5% w/v glycerol. Reactions were incubated at 28 °C for 1 h and stopped at timepoints by transfer to a − 80 °C freezer. Reaction aliquots (10 μL) were diluted with 5 µL of 3× Stop Buffer (95% (v/v) formamide, 20 mM EDTA, 0.05% (w/v) bromophenol blue, and 0.05% SDS) and loaded onto 20% denaturing polyacrylamide gel containing 6 M urea. Gels were imaged using an Azure c400 gel imager (Azure Biosystems) and band intensity calculated using ImageJ30.
RNA substrate preparation for RNA editing analysis
Editing reactions were performed under standard conditions previously developed for plant organelle C-to-U RNA editing31. The RNA editing substrate for rAtDYW1 contained the ndhD C2 editing site and sequences − 100 nt upstream/+ 5 nt downstream of the editing sites. Adapter SK and KS sequences were then added to the ends to ensure specific amplification of the RNA substrate. A PCR amplicon template to make the substrate was constructed by PCR amplification with primers AtndhDC2_SK_FOR: CGCTCTAGAACTAGTGGATCTTATTGACAAGTACTCGTACTC and AtndhDC2_KS_Rev: TCGAGGTCGACGGTATCCATTCGTGGTAAAGACAAGATAC using Arabidopsis thaliana gDNA as template. A second round of PCR added a 5′ T7 promoter sequence used to create the substrate using the TranscriptAid T7 High Yield Transcription Kit (ThermoScientific). The transcription product was treated with DNase I (ThermoScientific) to remove DNA while the remaining RNA was purified with an RNA Clean and Concentrator Kit (Zymo Research). Editing assays were performed in reaction mixtures (12.5 μL) that contained 30 mM HEPES–KOH (pH 7.7), 10 mM tris–HCl, 3 mM magnesium acetate, 75 mM NaCl, 45 mM potassium acetate, 30 mM ammonium acetate, 1 mM ATP, 5 mM dithiothreitol, 1% Polyethylene 16 Glycol 6000, 10% glycerol, 0.005% (w/v) CHAPS, 20 mM imidazole, 30 U of RiboLockTM RNase inhibitor (ThermoScientific), 1× Complete proteinase inhibitor mixture (Boehringer Mannheim), 1 fmol of mRNA substrate, and 6.25 μl purified protein cocktail. Reactions with 6 RNA editing factors included final concentrations of 2 μM rAtDYW1, 0.3 μM rAtCRR4, 1 μM rAtRIP2, 2 μM rAtRIP9, 2.7 μM rAtOZ1, and 0.7 μM rAtORRM. Reactions without rAtDYW1 but containing other editing factors included final concentrations of 0.4 μM rAtCRR4, 1.2 μM rAtRIP9, 3.2 μM rAtOZ1, and 0.8 μM rAtORRM. Editing was assayed using an adapted quantitative poisoned primer extension assay32 modified by the use of fluorescent instead of 32P radiolabeled oligonucleotides.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Abbreviations
- PPR:
-
Pentatricopeptide repeat
- RIP/MORF:
-
RNA editing factor interacting proteins/multiple organellar RNA editing factors
- ORRM:
-
Organelle RNA recognition motif
- OZ:
-
Organellar zinc-finger
- CRR:
-
Chlororespiratory reduction
- THU:
-
Tetrahydrouridine
- OTP85:
-
Organelle transcript processing 85
- EDTA:
-
Ethylenediaminetetraacetic acid
- PMSF:
-
Phenylmethanesulfonyl fluoride
- ATP:
-
Adenosine 5′-triphosphate
- CMP:
-
Cytidine 5′-monophosphate
- PCR:
-
Polymerase chain reaction
- ndhD :
-
NADH dehydrogenase subunit 4
- rps14 :
-
Ribosomal protein S14
- ndhB :
-
NADH dehydrogenase subunit 2
- rps7 :
-
Ribosomal protein S7
- Ni-NTA:
-
Nickel-nitrilotriacetic acid
- IMAC:
-
Immobilized metal affinity chromatography
- CHAPS:
-
3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate
References
Lurin, C. et al. Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell 16(8), 2089–2103 (2004).
Schmitz-Linneweber, C. & Small, I. Pentatricopeptide repeat proteins: A socket set for organelle gene expression. Trends Plant Sci. 13(12), 663–670 (2008).
Cheng, S. F. et al. Redefining the structural motifs that determine RNA binding and RNA editing by pentatricopeptide repeat proteins in land plants. Plant J. 85(4), 532–547 (2016).
Hayes, M. L., Giang, K., Berhane, B. & Mulligan, R. M. Identification of two pentatricopeptide repeat genes required for RNA editing and zinc binding by C-terminal cytidine deaminase-like domains. J. Biol. Chem. 288(51), 36519–36529 (2013).
Iyer, L. M., Zhang, D., Rogozin, I. B. & Aravind, L. Evolution of the deaminase fold and multiple origins of eukaryotic editing and mutagenic nucleic acid deaminases from bacterial toxin systems. Nucleic Acids Res. 39(22), 9473–9497 (2011).
Takenaka, M. et al. DYW domain structures imply an unusual regulation principle in plant organellar RNA editing catalysis. Nat. Catal. 4(6), 510–522 (2021).
Wagoner, J. A., Sun, T., Lin, L. & Hanson, M. R. Cytidine deaminase motifs within the DYW domain of two pentatricopeptide repeat-containing proteins are required for site-specific chloroplast RNA editing. J. Biol. Chem. 290(5), 2957–2968 (2015).
Hayes, M. L., Dang, K. N., Diaz, M. F. & Mulligan, R. M. A conserved glutamate residue in the C-terminal deaminase domain of pentatricopeptide repeat proteins is required for RNA editing activity. J. Biol. Chem. 290(16), 10136–10142 (2015).
Boussardon, C. et al. The cytidine deaminase signature HxE(x)n CxxC of DYW1 binds zinc and is necessary for RNA editing of ndhD-1. New Phytol. 203(4), 1090–1095 (2014).
Oldenkott, B., Yang, Y., Lesch, E. & Knoop, V. Schallenberg-Rüdinger: Plant-type pentatricopeptide repeat proteins with a DYW domain drive C-to-U RNA editing in Escherichia coli. Commun. Biol. 2, 85 (2019).
Hayes, M. L. & Santibanez, P. I. A plant pentatricopeptide repeat protein with a DYW-deaminase domain is sufficient for catalyzing C-to-U RNA editing in vitro. J. Biol. Chem. 295(11), 3497–3505 (2020).
Hashimoto, M., Endo, T., Peltier, G., Tasaka, M. & Shikanai, T. A nucleus-encoded factor, CRR2, is essential for the expression of chloroplast ndhB in Arabidopsis. Plant J. 36(4), 541–549 (2003).
Ruwe, H., Gutmann, B., Schmitz-Linneweber, C., Small, I. & Kindgren, P. The E domain of CRR2 participates in sequence-specific recognition of RNA in plastids. New Phytol. 222(1), 218–229 (2019).
Okuda, K. et al. Pentatricopeptide repeat proteins with the DYW motif have distinct molecular functions in RNA editing and RNA cleavage in Arabidopsis chloroplasts. Plant Cell 21(1), 146–156 (2009).
Pfalz, J., Bayraktar, O. A., Prikryl, J. & Barkan, A. Site-specific binding of a PPR protein defines and stabilizes 5’ and 3’ mRNA termini in chloroplasts. EMBO J. 28(14), 2042–2052 (2009).
Toma-Fukai, S. et al. Structural insight into the activation of an Arabidopsis organellar C-to-U RNA editing enzyme by active site complementation. Plant Cell 35, 1888 (2022).
Nakamura, T. & Sugita, M. A conserved DYW domain of the pentatricopeptide repeat protein possesses a novel endoribonuclease activity. FEBS Lett. 582(30), 4163–4168 (2008).
Hammani, K. et al. A study of new Arabidopsis chloroplast RNA editing mutants reveals general features of editing factors and their target sites. Plant Cell 21(11), 3686–3699 (2009).
Hayes, M. L. & Hanson, M. R. Identification of a sequence motif critical for editing of a tobacco chloroplast transcript. RNA 13(2), 281–288 (2007).
Miyamoto, T., Obokata, J. & Sugiura, M. A site-specific factor interacts directly with its cognate RNA editing site in chloroplast transcripts. Proc. Natl. Acad. Sci. U.S.A. 101(1), 48–52 (2004).
Neuwirt, J., Takenaka, M., Van der Merwe, J. A. & Brennicke, A. An in vitro RNA editing system from cauliflower mitochondria: Editing site recognition parameters can vary in different plant species. RNA 11(10), 1563–1570 (2005).
Maeda, A. et al. DYW deaminase domain has a distinct preference for neighboring nucleotides of the target RNA editing sites. Plant J. 111(3), 756–767 (2022).
Sun, T., Bentolila, S. & Hanson, M. R. The unexpected diversity of plant organelle RNA editosomes. Trends Plant Sci. 21(11), 962–973 (2016).
Sandoval, R. et al. Stable native RIP9 complexes associate with C-to-U RNA editing activity, PPRs, RIPs, OZ1, ORRM1 and ISE2. Plant J. 99(6), 1116–1126 (2019).
Bentolila, S., Oh, J., Hanson, M. R. & Bukowski, R. Comprehensive high-resolution analysis of the role of an Arabidopsis gene family in RNA editing. PLoS Genet. 9(6), e1003584 (2013).
Takenaka, M. et al. Multiple organellar RNA editing factor (MORF) family proteins are required for RNA editing in mitochondria and plastids of plants. Proc. Natl. Acad. Sci. U.S.A. 109(13), 5104–5109 (2012).
Yan, J. et al. MORF9 increases the RNA-binding activity of PLS-type pentatricopeptide repeat protein in plastid RNA editing. Nat. Plants 3, 17037 (2017).
Bayer-Csaszar, E. et al. The conserved domain in MORF proteins has distinct affinities to the PPR and E elements in PPR RNA editing factors. BBA Gene Regul. Mech. 1860(8), 813–828 (2017).
Boussardon, C. et al. Two interacting proteins are necessary for the editing of the NdhD-1 site in Arabidopsis plastids. Plant Cell 24(9), 3684–3694 (2012).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9(7), 671–675 (2012).
Hayes, M. L. & Hanson, M. R. Assay of editing of exogenous RNAs in chloroplast extracts of Arabidopsis, maize, pea, and tobacco. Methods Enzymol. 424, 459–482 (2007).
Peeters, N. M. & Hanson, M. R. Transcript abundance supercedes editing efficiency as a factor in developmental variation of chloroplast gene expression. RNA 8(4), 497–511 (2002).
Acknowledgements
Thanks for the contributions of Lloyd Greiner, Lily Nguyen, Trami Pham, Alfredo Jimenez-Salinas, Saul De La Pena, and Bao Tran for the purification of various proteins, condition optimization, and titration.
Funding
The work was funded by the NIH SCORE SC2 GM122718, and NIH R15 G00371 awarded to Michael L. Hayes. Robert D. Boyd was supported by NSF Grant # HRD-1700556.
Author information
Authors and Affiliations
Contributions
R.B. and M.H. came up with initial hypothesis and performed experiments. M.H. wrote the main manuscript text and prepared all figures. R.B. and M.H. reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boyd, R.D., Hayes, M.L. A ribonuclease activity linked to DYW1 in vitro is inhibited by RIP/MORF proteins. Sci Rep 13, 10723 (2023). https://doi.org/10.1038/s41598-023-36969-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-36969-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.