Abstract
Although Hepatozoon spp. remains the most prevalent intracellular protozoa infecting snakes, it was reported only in a few snake species of the Colubridae family in Türkiye. Moreover, studies on these hemoparasites are not available in venomous nose-horned vipers from Türkiye. In this study, we investigated Hepatozoon spp. in three individual Vipera ammodytes using morphological and molecular methods. Our results were positive for intraerythrocytic Hepatozoon spp. gamonts in all three snakes, exhibiting low parasitemia. The microscopic findings were further confirmed through molecular data. A genus-specific PCR assay targeting the 18S rRNA gene region of Hepatozoon spp., was performed using HemoF/HemoR and Hep300/Hep900 primers. The obtained sequences were concatenated and used in phylogenetic analyses in comparison with different Hepatozoon species. Although our (OP377741) isolate was separated into a different branch, it was clustered with the isolates of H. massardi (KC342526), H. cevapii (KC342525), and H. annulatum (ON262426) from Brazilian snakes. Moreover, gene similarity and pair-wise distance between our isolate and other Hepatozoon species infecting snakes were found to be 89.30–98.63% and 0.009–0.077, respectively. Hence, we reported a new species of Hepatozoon, namely Hepatozoon viperoi sp. nov. infecting V. ammodytes. Since the literature does not indicate the existence of such a Hepatozoon species in V. ammodytes in different countries, our data may contribute to the expanding knowledge of Hepatozoon species in snakes, providing new insights into the biodiversity of the haemogregarine protozoan parasite.
Similar content being viewed by others
Introduction
Haemogregarine protozoa belonging to the Hepatozoon genus (Miller, 1908) (Adeleorina: Hepatozoidae) have a broad host range like birds, lizards, crocodiles, snakes, and mammals, including domestic and wild canids and felids1. These protozoa infect the blood cells of various endothermic and ectothermic intermediate vertebrate hosts and lead an obligatory heteroxenous life. The definitive invertebrate hosts, including ticks, mites, fleas, sucking lice, reduvid bugs, phlebotomine flies, anopheline and culicine mosquitoes, and leeches, play a significant role in maintaining their heteroxenous survival1,2,3. Among haemogregarine parasites, Hepatozoon is the most prevalent genus infecting snakes2,3. Contrary to the mode of transmission in many vector-borne diseases, Hepatozooon infection is usually transmitted horizontally in snakes via oral ingestion of infected invertebrates or intermediate prey4.
In a broad spectrum of animals, particularly mammals and reptiles, as well as birds and amphibians, hepatozoonosis is characterized by moderate to severe symptoms, including anemia, cachexia, fever, lethargy, hyperglobulinemia, weight loss, and anorexia5,6. Although Hepatozoon species are believed not to cause clinical disease due to their excellent host adaptation, many cells (liver, lung, kidney, spleen) can still be damaged during their asexual reproduction. However, in the case of severe infections, hemolytic anemia can also be observed. Furthermore, dehydration, lethargy, open-mouth breathing, and weight loss may be encountered in immune-compromised and aberrant hosts7.
More than 340 Hepatozoon species cause diseases in animals having veterinary importance, including domestic and wild animals8. Previously, new Hepatozoon species or isolates have been detected in different countries in various snake species through advanced molecular diagnostic techniques9,10,11,12,13. However, many of them were related to the Hepatozoon species in colubrid snakes, with restricted information available on viper snakes. Studies are particularly scarce on the snakes belonging to the Vipera genus of the Viperidae family. Also, some studies have not shown the expected results14,15. Although some Hepatozoon species were detected in various animals in Türkiye6, one study in particular reported Hepatozoon infections in colubrid snakes15. However, the investigation of hemogregarine protozoans has not been done at the microscopic or molecular level in nose-horned vipers i.e., Vipera ammodytes in Türkiye.
Thus, the main objective of this study was to detect the presence of Hepatozoon species in nose-horned vipers and determine their phylogenetic relationships with various species or isolates of other snakes and reptiles using morphological examination and molecular techniques.
Material and methods
Study animals
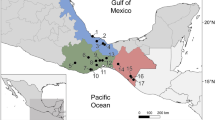
The study consisted of three female nose-horned vipers (Vipera ammodytes Linnaeus, 1758) captured in 2022 from the Göztepe locality (41° 35′ 3.71″ N, 27° 47′ 48.70″ E) of the Vize district of Kırklareli province, Türkiye. Nose-horned vipers are venomous species and generally live in stony and rocky areas. For this study, the vipers were captured using a snake grab stick. Figure 1 shows the locality from where these nose-horned vipers were captured.
The Vize district of Kırklareli province in Türkiye where the nose-horned viper specimens were captured (Satellite image and map were obtained from https://earth.google.com and https://d-maps.com/carte.php?num_car=25320&lang=en, respectively)16.
Blood samples and microscopic examination
The nose-horned vipers were firstly restrained to collect blood. Then, approximately 2 mL of blood was collected through the post-orbital sinus in heparinized hematocrit tubes17,18. Next, the blood samples were transferred to a tube containing ethylenediaminetetraacetic acid (EDTA), and thin blood smears were prepared from each blood sample. The samples were then air dried, fixed in absolute methanol for 5 min, and stained for 20 min using Wright’s staining method19. After smear preparation, the remaining blood samples were stored at − 20 °C for further molecular analysis while the captured horned vipers were released back to their habitats. All smears were examined under a light microscope (Olympus CX 31) at 100× magnification. Relevant literature was used to identify the gamont stages of intraerythrocytic parasites3.
DNA extraction
Blood samples of each nose-horned viper were used to extract genomic DNA using a commercial DNA extraction kit (E.Z.N.A.® Blood DNA Mini Kit, Omega BIO-TEK, Georgia, USA), which was performed following the manufacturer’s instructions. The concentrations of the eluted DNA were measured using a nanodrop spectrophotometer (Colibri, TITERTEK BERTHOLD) while the genomic DNAs were stored at − 20 °C for further molecular analysis. Since we did not have enough blood for DNA extraction from one of the vipers, we used the blood and DNA samples from the other two vipers to extract genomic DNA and perform further processes.
PCR amplification and pathogen detection
We performed two PCR assays targeting two different regions of the 18S rRNA gene of apicomplexan parasites by using two primer sets. The HemoF/HemoR primers were used to amplify the 900 bp region while Hep300/Hep900 was used to amplify 600 bp of the 18S rRNA gene region of Hepatozoon spp.20,21. PCR was performed by making minor modifications to the PCR thermal cycles. Table 1 lists the oligonucleotide sequences used in the PCR assay to detect Hepatozoon spp. The reaction mixture for each PCR assay consisted of 10 µL of ExPrime Taq Premix (2X, No dye), 0.2 µL of forward primer, 0.2 µL of reverse primer, 4 µL of DNA template, and 5.6 µL of double-distilled water, making up the final volume to 20 µL. BioRad thermocycler was used for amplification. The PCR programs for the two different primer sets were as follows: HemoF/HemoR; 95 °C for 5 min (initial denaturation), 35 cycles of (94 °C for 30 s, 48.8 °C for 30 s, 72 °C for 1 min), and 72 °C for 5 min (final extension), Hep300/Hep900; 94 °C for 3 min (initial denaturation), 35 cycles of (94 °C for 45 s, 56 °C for 1 min, 72 °C for 1 min) and 72 °C for 7 min (final extension). Distilled water was used for the negative control. The amplified DNA samples were electrophoresed on the agarose gel (1%) in TAE buffer. After electrophoresis, the gel was stained with ethidium bromide and photographed using a UV transilluminator (UVP, Upland, CA, USA).
Sanger sequencing and phylogenetic analysis
PCR products were sequenced in both directions by a commercial company. The obtained sequence chromatograms (forward and reverse sequences) were assembled, and each primer sequence (HepF300/900 and HemoF/R) was edited out to obtain a partial 18S rRNA consensus sequence. These sequences were concatenated (~ 1200 bp) using the Geneious version 7.1.3 (Bomatters, “http://www.geneious.comww”)22. Also, the sequences belonging to the haemogregarine group in Genbank were aligned using Geneious version 7.1.3 by the MUSCLE algorithm (Bomatters, “http://www.geneious.comww”)22. Following Úngari et al.’s study, Adelina dimidiata Schneider, 1875, Adelina grylli Butaeva, 1996 and Klossiella equi Baumann, 1946 were selected as outgroups23. Phylogenetic relationships were inferred by the Bayesian inference (BI) and Maximum likelihood (ML) analysis through MrBayes 3.2.2 and RAxML 7.2.8., respectively24,25. For ML method, JModelTest v.2.1.10 was used to identify the best evolutionary model26. Based on Akaike information criterion (AIC) the Transitional model with a discrete Gamma distribution (TVM + G) was chosen27. Phylogenetic analysis was inferred using PhyML with 1000 replicate bootstraps (> 50%)28. The BI analysis was carried out using MrBayes implemented from the computational resource CIPRES29, the best BIC score was the General Time Reversible model (GTR + I + Γ)30. The Markov chain Monte Carlo (MCMC) algorithm was run for 10,000,000 generations, sampling one tree every 1000 generations. On the burn-in, the first 25% of generations were discarded, and the consensus trees were estimated using the remaining trees. Bayesian posterior probabilities (BPP) cut off was considered > 50%. The aligned sequences of haemogregarine species infecting snakes were compared using a pair-wise distance (p-distance) matrix.
Accession number status
We submitted the sequences obtained from two different primer sets (HemoR/F, Hep300/900) targeting different regions of the 18S rRNA gene region to the GenBank, National Center for Biotechnology Information with the following accession numbers: ON619569/OM866261 (VA1), OM866262/OM866263 (VA2) [HemoR/HemoR], and ON673853/ON673854 (VA1), ON629811/ON629812 (VA2) [Hep300/Hep900]. However, since these sequences represented a short part of the target gene region, they were not included in the phylogenetic analyses. Instead, contig sequences amplified using both primer pairs were concatenated to obtain a sequence representing a longer part (~ 1200 bp) of the 18S rRNA gene (OP377741), which was then used in phylogenetic analyses (https://www.ncbi.nlm.nih.gov/nuccore/OP377741.1/).
Statistical analysis
The experimental data were analyzed using the SPSS 25 program (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.). Data were evaluated after controlling for normal distribution prerequisites (Kolmogorov–Smirnov test). Since the differences between the two independent groups did not meet the parametric test prerequisites, the Mann–Whitney U test was used. Variables were expressed as median (min/max) values. The values of p < 0.05, p < 0.01, and p < 0.001 were accepted as the significance levels of the tests.
Ethics approval
All experimental protocols were approved by Ethics Committee of Çanakkale Onsekiz Mart University (Approval number: HADYEK, 2021/10–05). All methods were carried out in accordance with relevant guidelines and regulations and in compliance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. After collecting blood samples, horned vipers were released back into the areas from which they were captured.
Results
We found that all three nose-horned vipers (V. ammodytes) in the Thrace Region of Türkiye were infected with the Hepatozoon species. The infected vipers were adult females with low parasitemia. We observed mature gamonts of Hepatozoon in the erythrocytes of the hosts. Since we did not perform histopathological examinations, we could not evaluate other developmental stages of the parasite. The molecular and phylogenetic analysis verified the species as an undescribed Hepatozoon spp. in V. ammodytes.
Species description
Phylum: Apicomplexa Levine, 1970.
Class: Conoidasida Levine, 1988.
Subclass: Coccidia Leucart, 1879.
Order: Eucoccidiorida Leger, 1911.
Suborder: Adeleorina Leger 1911.
Family: Hepatozoidae Wenyon, 1926.
Genus: Hepatozoon Miller, 1908.
Species: Hepatozoon viperoi Ceylan, Ungari and Sevinc sp. nov.
Taxonomic summary
Type-host: nose-horned viper Vipera ammodytes Linnaeus, 1758 (Serpentes: Viperidae: Viperinae).
Type-locality: Göztepe locality (41° 35′ 3.71″ N, 27° 47′ 48.70″ E) in Vize district of Kırklareli province in Türkiye.
Site of infection: erythrocytes (blood).
Vector: unknown.
Etymology: considering the genus name of the infected host (Vipera ammodytes), the protozoan was named Hepatozoon viperoi sp. nov. This is the first Hepatozoon species reported in the nose-horned viper belonging to the Vipera genus.
Parasitemia: 0.1–0.4%.
Deposited materials: blood smears and DNA samples from V. ammodytes. These materials are deposited in Selcuk University, Faculty of Veterinary Medicine, Department of Veterinary Parasitology.
Gene sequence: the 18S rRNA concatenated gene sequence obtained from the blood of V. ammodytes was submitted to GenBank under the accession number (OP377741).
Microscopy
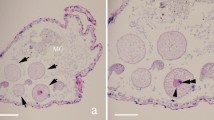
We found that the nose-horned viper V. ammodytes was infected with a Hepatozoon species. In this study, gamonts of Hepatozoon spp. were detected microscopically in thin blood smears of all captured horned vipers. Figure 2 shows different microscopic images of gamonts in each snake, separately. Although the parasitemia level was quite low (< 0.1%) in VA1 and VA3 samples, it was slightly higher in VA2 (0.4%) sample. Overall, our morphological and morphometric data along with the molecular and phylogenetic analysis revealed a new Hepatozoon species.
Photomicrographs of intraerythrocytic stages of Hepatozoon viperoi sp. nov. Miller, 1908 found in blood samples of Vipera ammodytes; intraerythrocytic gamonts of Hepatozoon viperoi sp. nov. showing the centric nucleus and no vacuoles [A: VA1 (×100 magnification); B: VA2 (×40 magnification); C: VA3 (×100 magnification)].
Morphological and morphometric analysis of gamonts
Gamonts of the Hepatozoon viperoi sp. nov. were detected only in the erythrocytes of V. ammodytes, with no other developmental stages being observed in the thin blood smears. Gamonts were enclosed in a thin parasitophorous vacuole. They were elongated and had one end more tapered while the cytoplasm was stained whitish pink. Sausage-shaped gamonts were found near the nucleus of the infected erythrocytes, exhibiting a uniform cytoplasm. Furthermore, gamonts displaced the nucleus of the host erythrocytes. Table 2 presents the morphometric parameters including the length and width of the erythrocytes and the length and width of the nucleus of the gamonts. The pinkish-purple gamont nuclei were centrally located and were mostly quadrangular in shape. The length, width, and area of the gamonts (mean ± standard deviation) were 14.05 ± 0.44 µm, 5.83 ± 0.42 µm (n = 15), and 64.96 ± 6.64 µm2, respectively. The length, width, and area of the nucleus of the parasite were 6.35 ± 0.65 µm, 5.20 ± 0.47 µm, and 22.91 ± 3.25 µm2 (n = 20), respectively.
The nucleus of the infected erythrocyte had an elongated appearance and was compressed between the gamont and the membrane of the erythrocyte. The infected host erythrocytes showed significant hypertrophy (P < 0.05) along with higher mean length (P < 0.001) and width (P < 0.05). Although the areas of infected erythrocytes were significantly higher than the areas of non-infected erythrocytes (P < 0.001), the area of their nuclei remained smaller (P < 0.05). The length, width, and area of the infected erythrocytes were 18.31 ± 0.71 µm, 11.91 ± 0.88 µm (n = 15), and 165.07 ± 11.17 µm2 (n = 20), respectively, while the length, width, and area of the uninfected erythrocytes were 15.60 ± 0.69 µm, 11.22 ± 0.66 µm (n = 15), and 144.83 ± 17.11 µm2 (n = 20), respectively. Table 2 indicates the detailed measurement values of the morphometric parameters.
Remarks
Although the literature review reported on the presence of Hepatozoon spp. in some snakes of Türkiye, no Hepatozoon species have been formally described (using morphological or a combined approach of morphology and molecular) in snakes so far. Tome et al. detected Hepatozoon spp. at the molecular level in some colubrid snakes, including Dolichophis caspius, Elaphe sauromates, and Natrix tessellata, however, the isolates were not identified at the species level15. This said, no morphological or morphometric data on Hepatozoon species are available in snakes in Türkiye. The morphological and morphometric characteristics of Hepatozoon viperoi sp. nov. determined in our study can be compared to Hepatozoon species found in some Brazilian snakes, which may show a phylogenetic relationship. To date, although no studies have reported the occurrence of Hepatozoon species in V. ammodytes, some new species including H. cuestensis, H. cevapii, and H. massardi have been described in rattlesnakes (Crotalus durissus terrificus). These rattlesnakes belong to the genus Crotalus, subfamily Crotalinae, and family Viperidae. Among these, H. cevapii and H. massardi showed phylogenetic proximity to H. viperoi sp. nov. described in our study. Therefore, we used these two species for the morphological and morphometric comparison. Moreover, we included a recently described new species H. annulatum, found in a Brazilian colubrid snake Leptodeira annulata, in our comparison due to its phylogenetic proximity to H. viperoi sp. nov.
The morphology of gamonts of all aforementioned Hepatozoon species, including H. viperoi sp. nov., caused the displacement of erythrocyte nuclei. All species of gamonts appeared to be elongated, with one end slightly more tapered than the other. The cytoplasmic structures of the H. cevapii and H. massardi gamonts showed basophilic and granulous cytoplasm. However, the cytoplasm of H. viperoi sp. nov. gamonts showed uniformity. Differentiating H. viperoi sp. nov. from the other species based on microscopic examination may be difficult due to their similar morphology. However, at this point, morphometric measurements can act as a guide.
The length, width, and area of the H. cevapii gamonts found in the colubrid snake species Oxyrhopus rhombifer in Brazil were 14.81 ± 0.99 µm, 5.02 ± 0.76 µm, and 64.38 ± 4.70 µm2, respectively, while the length, width, and area of the parasites’ nucleus were 4.35 ± 0.28 µm, 3.99 ± 0.98 µm, and 14.01 ± 1.87 µm2 (n = 30), respectively13. The area of the H. cevapii gamonts was similar to that of the H. viperoi sp. nov., with minor differences in other morphometric measurements. However, significant morphometric differences were observed between the gamonts’ nuclei. The values of the nuclei length, width, and area of H. viperoi sp. nov. gamonts were higher than that of H. cevapii.
The length, width, and area of the H. massardi gamonts found in Crotalus durissus terrificus species in Brazil were 17.4 ± 0.7 µm, 3.0 ± 0.3 µm, and 38.9 ± 3.7 µm2, respectively, while the length, width, and the area of the parasites’ nucleus were 4.7 ± 0.3 µm, 2.3 ± 0.2 µm, and 9.1 ± 0.8 µm2, respectively9. Although the gamont length of H. viperoi sp. nov. was shorter than that of H. massardi, the other morphometric measurements including the gamont width and area and the nucleus length, width, and area were higher in the gamonts of H. viperoi sp. nov.
The length, width, and area of H. annulatum gamonts described in Leptodeira annulata, a colubrid snake species in Brazil, were 14.25 ± 0.54 µm, 5.34 ± 0.26 µm, and 64.32 ± 5.90 µm2, respectively, while the length, width, and the area of the parasites’ nucleus were 3.91 ± 0.63 µm, 4.13 ± 0.29 µm, and 16.95 ± 2.01 µm2, respectively13. The morphometric measurements of the H. viperoi sp. nov. including the gamont’s length, width, and area were found to be very similar to that of H. annulatum. However, the measurements of the nucleus of the gamont showed significant differences between both species.
Hepatozoon. viperoi sp. nov. detected in our study showed phylogenetic similarity with H. cevapii, H. massardi, and H. annulatum species found in various Brazilian snakes. However, these three Hepatozoon species have not been detected in Türkiye yet. The absence of morphological-morphometric studies of Hepatozoon spp. in the snakes of Türkiye led us to compare our findings with those found in the snakes of other countries. Although morphological examination plays a significant role in the differential diagnosis, it is still difficult to predict the Hepatozoon species without morphometric measurements, which requires further molecular confirmation.
Molecular data, sequencing, and phylogenetic analysis
Genomic DNAs were amplified using two sets of primers (HemoF/HemoR, Hep300/Hep900) targeting different regions of the 18S rRNA gene of Hepatozoon. Amplified products were sent for sequencing, and the obtained sequences were submitted to the GenBank database of the National Center for Biotechnology Information. The contig sequences obtained using the pair of primers HemoF and HemoR were further aligned and compared using the Geneious version 7.1.3 (Bomatters, www.geneious.comww)22. Since these sequences were 100% similar, another pair of primers (HepF300/900) was used to amplify another part of the 18S rRNA gene, which was then concatenated with the sequence amplified using HemoF/HemoR resulting in a ~ 1200 bp sequence (OP377741).
Both Bayesian Inference (BI) and the Maximum Likelihood (ML) phylogenetic analyses resulted in identical tree topologies (Fig. 3). The phylogenetic analyses included isolates of adeleorinid parasites (Haemogregarinidae, Hepatozoidae, Karyolysidae, and Dactylosomatidae) available in GenBank. Hepatozoon spp. belong to polyphyletic groups that form two separate clades according to their vertebrate host species. Hepatozoon spp. isolated from large mammals form a sister clade with the Karyolysus genus while the isolates obtained in our study clustered with the sequences of the reptile and anuran hosts belonging to a large Hepatozoidae clade. Although our isolate clustered with the isolates obtained from Brazilian snakes, including Hepatozoon massardi O´Dwyer, Moço, Paduan, Spenassatto, Silva and Ribolla 2013 (KC342526), Hepatozoon cevapii O´Dwyer, Moço, Paduan, Spenassatto, Silva and Ribolla 2013 (KC342525/ON236891) and Hepatozoon annulatum Úngari, Netherlands, Silva and O´Dwyer 2022 (ON262426), it was clustered on a different branch. The gene similarity and pair-wise distance are summarized in Table 3.
Consensus phylogram of haemogregarines based on 18S rDNA sequences. The topology trees with Bayesian inference (BI) and Maximum likelihood (ML) analyses were identical (represented by the BI tree). The values associtaed with the branches are related to the bootstrap values (BI/ML). The scale bar represents 0.02 nucleotide substitutions per site. Adelina dimidiata (DQ096835), Adelina grylli (DQ096836) and Klossia equi (MH211602) were used as outgroups.
Discussion
The snake species Vipera ammodytes (Linnaeus, 1758) is characterized by its prominent fleshy horn at the tip of the snout. As one of the most venomous vipers in Eurasia, V. ammodytes are widely distributed between Austria and Italy, and across the Balkan Peninsula to Türkiye and Caucasus31,32. These vipers live in mesophytic and xerophytic forests, dry cliffs, mosaic meadows, screes, scrubs, and even on artificial stone walls. They commonly prey on lizards and small mammals by subduing them with their venom32,33. Telford stated that the infection of the snakes with Hepatozoon species may be based on the predation of infected vertebrate hosts or the ingestion of the invertebrate vectors3. Therefore, in this study, we investigated the existence of Hepatozoon spp. in vipers. The microscopic findings revealed the intraerythrocytic sausage-shaped gamonts. However, since microscopy was inadequate for species identification, we further diagnosed it molecularly34.
A wide variety of pathogens can infect reptiles, with some groups of parasites receiving little attention. As a result, their biodiversity awaits clarification. Systematics of parasites commonly rely on some features, including the morphological and lifecycle characteristics of parasites, some of which are difficult to evaluate. However, molecular techniques have eliminated these difficulties, in turn, increasing the knowledge of their biology, biodiversity, and phylogeny34,35,36. In this study, we determined the presence of Hepatozoon viperoi sp. nov. in Vipera ammodytes both microscopically and molecularly. Although many such species have been identified in snakes, our study may further contribute to the information on biodiversity and phylogeny of the Hepatozoon genus, which is believed to have not received enough attention. Studies on new Hepatozoon species or isolates in snakes are contributing to the study of haemogregarine protozoan fauna9,10,11,37. A literature review indicated the inadequacy of studies on haemoprotozoan infections in reptiles in Türkiye, especially snakes. A study conducted by Tome et al. investigated Hepatozoon species in some colubrid snakes, namely Coronella austriaca (Smooth snake), Dolichophis caspius (Caspian whipsnake), Eirenis modestus (Dwarf snake), Elaphe sauromates (Blotched rat snake), Hemorrhois nummifer (Coin-marked snake), and Natrix tessellata (Tessellated water snake)15. Additionally, Hepatozoon spp. were detected in D. caspius (KJ408513) in Kırklareli, E. sauromates (KJ408514) in Erzurum, and N. tessellata (KJ408526) in Karakose. Vipera genus along with colubrid snakes is an essential member of the Turkish herpetofauna, especially in the Thrace region and the parts of Eastern Anatolia close to Georgia and Armenia31. The available literature does not have data on the morphological or molecular detection of Hepatozoon spp. in snakes belonging to the Viperidae family in Türkiye. Therefore, the originality of the present study is based on the lack of studies detecting Hepatozoon spp. in Vipera species of different countries.
A study conducted by Tome et al. in Portugal reported no causative agent in snub-nosed vipers, Vipera latastei (Bosca) (n = 22)15. Similarly, even in Poland, Hepatozoon spp. was not detected in the European adder Vipera berus (n = 48)14. Nasiri et al. conducted a study on parasites in Iranian snakes and investigated three species from the Vipera genus but found no blood parasites in the zigzag mountain vipers (Vipera albicornuta) and Transcaucasian meadow vipers (Vipera ursinii eriwanensis)38. Although they observed intraerythrocytic gametocytes of hemoparasites in West-Asian blunt-nosed vipers (Vipera lebetina obtusa), they did not perform any molecular confirmation at the species or genus level. The data remains insufficient on the molecular or microscopic prevalence of Hepatozoon spp. in vipers belonging to the genus Vipera, however, these parasites were detected in several viper species belonging to other genera of the Viperidae family9,14,15,37,38,39,40,41,42. Previously, in Brazil, O’Dwyer et al. detected Hepatozoon cevapii sp. nov., Hepatozoon cuestensis sp. nov., and Hepatozoon massardi sp. nov. in highly venomous pit vipers and Crotalus durissus terrificus (rattlesnake)9. In a similar study, Ungari et al. reported H. musa and H. cuestensis in Crotalus durissus of Brazil37. In Morocco and Mauritania, Tome et al. detected Hepatozoon spp. in Saharan horned vipers (Cerastes cerastes) while Medrano-Tupiza et al. detected H. seurati in the same snake species in Egypt15,41. In Saudi Arabia, Hepatozoon bashtari n. sp. was detected in Echis coloratus, which is a painted saw-scaled viper42, while in Pakistan and Egypt, Hepatozoon echisi and H. mehlhorni were both reported in the species Echis carinatus, respectively43,44. In this study, for the first time, the presence of Hepatozoon spp. was investigated in V. ammodytes, and a new Hepatozoon species, Hepatozoon viperoi sp. nov., was identified both microscopically and molecularly. Sequencing and phylogenetic analysis revealed that our concatenated sequence (OP377741) clustered with sequences from the reptile and anuran hosts belonging to a large Hepatozoidae clade. Hepatozoon viperoi sp. nov. clustered with other isolates, including Hepatozoon massardi O´Dwyer, Moço, Paduan, Spenassatto, Silva and Ribolla 2013 (KC342526), Hepatozoon cevapii O´Dwyer, Moço, Paduan, Spenassatto, Silva and Ribolla 2013 (KC342525/ON236891), and Hepatozoon annulatum Úngari, Netherlands, Silva and O´Dwyer 2022 (ON262426) obtained from Brazilian snakes but on a different branch in the phylogenetic tree. Blastn analysis revealed gene similarity between Hepatozoon viperoi sp. nov. and the isolates obtained from snakes of Brazil (93.18–98.40%, PD: 0.009–0.077), Australia (89.30%, PD: 0.061), Canada (92.27%, PD: 0.074), China (98.40%, PD: 0.012), and South Africa (92.50–92.95%, PD: 0.072–0.077). Clustering of our concatenated sequence with the sequences from the reptile and anuran host revealed a relationship between the similarities in the detected nucleotides and the feeding or hunting patterns of nose-horned vipers. Tome et al. stated that the genetic lineages infecting the prey may also parasitize snakes4. Our study findings also supported this view, however, further studies encompassing a wider sample of vipers may be required to establish clearer genetic proximities between snakes and prey.
Vipera ammodytes are among the strictly protected fauna species mentioned in Annex II of the Bern Convention on the Conservation of European Wildlife and Natural Habitats45. Also, recently, it has been listed in the Least Concern (LC) category according to the International Union for Conservation of Nature (IUCN) criteria46. Detailed investigations are necessary on whether Hepatozoon species or other infectious disease agents could affect various physiological activities of vipers along with exploring the extent of the parasitic effect on them since vipers hold an important place in terms of the biodiversity of Türkiye's herpetofauna. Because diseases occurring in natural populations can adversely affect their chances of survival, mainly the reproductive functioning of living beings, it is crucial to take necessary protective actions. One of the main constraints in studying parasites remains the limited comparative genetic data of different host species, especially, wild populations belonging to various geographical areas. Our study reported a new Hepatozoon species in nose-horned viper V. ammodytes, which has not been studied previously. Hence, this study can contribute to the lack of literature in this regard.
Wildlife species include unique clues allowing early detection of changes in the ecosystem, including habitat fragmentation, environmental pollution, and the presence of pathogens. Wildlife has been frequently considered as sentinels for pathogen emergence and persistence47,48,49,50. Therefore, studies regarding the haemoparasites of snakes, a significant part of wildlife, becomes important. In conclusion, we used morphological and molecular techniques to describe a new Hepatozoon species (Hepatozoon viperoi sp. nov.) infecting V. ammodytes found in the Thrace region of Türkiye. To the best of our knowledge, this is the first study reporting a haemoprotozoan parasite in nose-horned viper in Türkiye. The identification of a new Hepatozoon species may increase the knowledge of parasitic fauna infecting nose-horned viper and also their biodiversity, which has been poorly studied. Therefore, we believe that this study may help identify unknown threat factors, which in turn, may be required for generating conservation strategies for nose-horned viper.
Data availability
All data generated or analyzed during the current study are included in this article. The concatenated sequence has been deposited in the NCBI GenBank database under the accession number OP377741 (https://www.ncbi.nlm.nih.gov/nuccore/OP377741.1/).
References
Al-Qurarishy, S., Abdel-Ghaffar, F., Dkhil, M. A. & Abdel-Gaber, R. Haemogregarines and criteria for identification. Animals 11, 170 (2021).
Smith, T. G. The genus Hepatozoon (Apicomplexa: Adeleina). J. Parasitol. 82, 565–585 (1996).
Telford, J. S. R. Hemoparasites of the Reptilia: Color Atlas and Text (CRC Press, 2009).
Tomé, B., Maia, J. P. & Harris, D. J. Hepatozoon infection prevalence in four snake genera: Influence of diet, prey parasitemia levels, or parasite type?. J. Parasitol. 98, 913–917 (2012).
Baneth, G. Perspectives on canine and feline hepatozoonosis. Vet. Parasitol. 181, 3–11 (2011).
Ceylan, O., Sevinc, F. & Xuan, X. Primary tick-borne protozoan and rickettsial infections of animals in Turkey. Pathogens 10, 231 (2021).
Nardini, G., Leopardi, S. & Bielli, M. Clinical hematology in reptilian species. Vet. Clin. Exot. Anim. 16, 1–30 (2013).
Dantas-Torres, F. & Otrando, D. Hepatozoonosis. In Arthropod borne diseases (ed. Marcondes, C. B.) 363–368 (Springer, 2017).
O’Dwyer, L. H. et al. Description of three new species of Hepatozoon (Apicomplexa, Hepatozoidae) from rattlesnakes (Crotalus durissus terrificus) based on molecular, morphometric and morphologic characters. Exp. Parasitol. 135, 200–207 (2013).
Borges-Nojosa, D. M. et al. A new species of Hepatozoon Miller, 1908 (Apicomplexa: Adelerina) from the snake Philodryas nattereri Steindachner (Squamata: Dipsadidae) in northeastern Brazil. Syst. Parasitol. 94, 65–72 (2017).
Cook, C. A., Netherlands, E. C., van As, J. & Smit, N. J. Two new species of Hepatozoon (Apicomplexa: Hepatozoidae) parasitising species of Philothamnus (Ophidia: Colubridae) from South Africa. Folia Parasitol. 65, 004 (2018).
Ungari, L. P. et al. Description of a new species Hepatozoon quagliattus sp. nov. (Apicomplexa: Adeleorina: Hepatozoidae), infecting the sleep snake, Dipsas mikanii (Squamata: Colubridae: Dipsadinae) from Goias State, Brazil. Acta Parasitol. 66, 871–880 (2021).
Ungari, L. P. et al. Diversity of haemogregarine parasites infecting Brazilian snakes from the Midwest and Southeast regions with a description of two new species of Hepatozoon (Apicomplexa: Adeleorina: Hepatozoidae). Parasitol. Int. 89, 102587 (2022).
Haklova, B. et al. Phylogenetic relationship of Hepatozoon blood parasites found in snakes from Africa, America and Asia. Parasitology 141, 389–398 (2014).
Tomé, B. et al. Patterns of genetic diversity in Hepatozoon spp. infecting snakes from North Africa and the Mediterranean Basin. Syst. Parasitol. 87, 249–258 (2014).
Google Earth V 9.186.0.0. (accessed 30 September 2022); https://earth.google.com.
MacLean, G. S., Lee, S. K. & Wilson, K. F. A simple method of obtaining blood from lizards. Copeia 2, 338–339 (1973).
Samour, J. & Hart, M. Hawkey’s Atlas of Wild and Exotic Animal Haematology 1st edn. (CRC Press, 2020).
Başoğlu, M. & Öktem, N. Zoofizyoloji Pratikumu (Ege Univ. Fen Fak. Kitaplar Serisi, 1984) (in Turkish).
Perkins, S. L. & Keller, A. K. Phylogeny of nuclear small subunit rRNA genes of hemogregarines amplified with specific primers. J. Parasitol. 87, 870–876 (2001).
Ujvari, B., Madsen, T. & Olsson, M. High prevalence of Hepatozoon spp. (Apicomplexa: Hepatozoidae) infection in water pythons (Liasis fuscus) from tropical Australia. J. Parasitol. 90, 670–672 (2004).
Kearse, M. et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28, 1647–1649 (2012).
Ungari, L. P. et al. A new species, Dactylosoma piperis n. sp. (Apicomplexa, Dactylosomatidae), from the pepper frog Leptodactylus labyrinthicus (Anura, Leptodactylidae) from Mato Grosso State, Brazil. Parasite 27, 73 (2020).
Huelsenbeck, J. P. & Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17, 754–755 (2001).
Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313 (2014).
Darriba, D., Toboada, G. L., Doallo, R. & Posada, D. JModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 9, 772 (2012).
Posada, D. Using Modeltest and Paup* to select a model of nucleotide substitution. Curr. Protoc. Bioinf. 6, 6.5.1-6.5.14 (2003).
Guindon, S. & Gascuel, O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52, 696–704 (2003).
Miller, M. A., Pfiffer, W. & Schwartz, T. Creating the CIPRES Science Gateway for Inference of Large Phylogenetic Trees. 2010 Gateway Computing Environments workshop (2010).
Tavaré, D. Some probabilistic and statistical problems in the analysis of DNA sequences. Lect. Math. Life Sci. 17, 57–86 (1986).
Tomovic, L. Systematics of the nose-horned viper (Vipera ammodytes, Linnaeus, 1758). Herpetol. J. 16, 191–201 (2006).
Lakusic, M., Bjelica, V. & Tomovic, L. Record size for the nosed-horned viper, Vipera ammodytes (Linnaeus, 1758) from Serbia. Hepetol. Notes 14, 605–607 (2021).
Tomovic, L. Vipera ammodytes. In: Red Book of Fauna of Serbia II—Reptiles. 233–239 pp. Tomović et al. (Eds.). University of Belgrade-Faculty of Biology & Institute for Nature Conservation of Serbia (2015).
O’Donoghue, P. Haemoprotozoa: making biological sense of molecular phylogenies. Int. J. Parasitol. Parasites Widl. 6, 241–256 (2017).
Morrison, D. A. Evolution of the Apicomplexa: Where are we now?. Trends Parasitol. 25, 375–382 (2009).
Ghai, R. R. & Chapman, C. A. Meet the parasites: Genetic approaches uncover new insights in parasitology. Taprobanica 4, 60–64 (2012).
Ungari, L. P. et al. Molecular characterization and identification of Hepatozoon species Miller, 1908 (Apicomplexa: Adeleina: Hepatozoidae) in captive snakes from Brazil. Parasitol. Res. 117, 3857–3865 (2018).
Nasiri, V. et al. A description of parasites from Iranian snakes. Exp. Parasitol. 147, 7–15 (2014).
Moço, T. C. et al. Morphological, morphometric and molecular characterization of Hepatozoon spp. (Apicomplexa, Hepatozoidae) from naturally infected Caudisona durissa terrifica (Serpentes, Viperidae). Parasitol. Res. 110, 1393–1401 (2012).
Morsy, K. et al. Developmental stages of Hepatozoon seurati (Laveran and Pettit 1911) comb. Nov., a parasite of the corned viper Cerastes cerastes and the mosquito Culex pipiens from Egypt. Parasitol. Res. 112, 2533–2542 (2013).
Medrano-Tupiza, E., Morales-Arciniega, S., Santander-Parra, S., Nunez-Naranjo, L. & Puga-Torres, B. Absence of haemoparasites in wildlife snakes, located in the ecological reserves Cota 70, Cotacachi-Cayapas and Sumaco-Napo-Galeras in Ecuador. Res. Zool. 7, 7–10 (2017).
Abdel-Baki, A. S., Mansour, L., Al-Malki, E. S., Al-Quraishy, S. & Abdel-Haleem, H. M. Morphometric and molecular characterisation of Hepatozoon bashtari n. sp. in painted saw-scaled viper, Echis coloratus (Ophidia, Viperidae). Parasitol. Res. 11, 3793–3801 (2020).
Mohiuddin, A., Pal, R. A. & Warsi, A. A. Haemogregarina echisi n. sp. from the saw-scaled viper Echis carinatus of the Sind Region of West Pakistan. J. Protozool. 14, 255–259 (1967).
Bashtar, A. R., Abdel-Ghaffar, F. A. & Shazly, M. A. Life cycle of Hepatozoon mehlhorni sp. Nov. in the viper Echis carinatus and the mosquito Culex pipiens. Parasitol. Res. 77, 402–410 (1991).
Anonymous. Convention on the conservation of European wildlife and natural habitats. Council of Europe. CETS no, p. 104 (1982).
IUCN. The IUCN Red List of Threatened Species. Version 2021–3. https://www.iucnredlist.org (2022).
Johnstone, C. P., Lill, A. & Reina, R. D. Does habitat fragmentation cause stress in the agile antechinus? A haematological approach. J. Comp. Physiol. B. 182, 139–155 (2012).
Carson, C. et al. Potential wildlife sentinels for monitoring the endemic spread of human Buruli ulcer in South-East Australia. PLOS Negl. Trop. Dis. 8, e2668 (2014).
Boonyapisitsopa, S. et al. Sentinel model for influenza A virus monitoring in free-grazing ducks in Thailand. Vet. Microbiol. 182, 35–43 (2016).
Lloyd, T. C. et al. Modeling hematologic and biochemical parameters with spatiotemporal analysis for the free-ranging eastern box turtle (Terrapene carolina carolina) in Illinois and Tennessee, a potential biosentinel. EcoHealth 13, 467–479 (2016).
Acknowledgements
The authors would like to thank Prof. Bilal Dik, Prof. Hasan Hüseyin Dönmez, and Prof. Emrah Sur for their contribution to photographing and measuring the gamonts and Assist. Prof. Merve Ider for statistical analysis.
Funding
This study did not receive financial support from any organization.
Author information
Authors and Affiliations
Contributions
O.C., C.G., and F.S. conceptualized the study. C.G., B.B., and M.T. carried out the field studies and collected the materials. O.C., G.S., and C.C. conducted laboratory analysis. L.P.Ú. and L.H.O. performed phylogenetic analysis. O.C. wrote the original draft of the manuscript. All authors have read and agreed to the submitted version of the manuscript. All authors have agreed to this final version of the manuscript and give their consent for its publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ceylan, O., Úngari, L.P., Sönmez, G. et al. Discovery of a new Hepatozoon species namely Hepatozoon viperoi sp. nov. in nose-horned vipers in Türkiye. Sci Rep 13, 9677 (2023). https://doi.org/10.1038/s41598-023-36814-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-36814-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.