Abstract
Nicotine delivery and subjective effects are determinants of the ability of potentially less harmful tobacco products such as heated tobacco products (HTPs) to support adult smokers in switching away from cigarettes, and therefore to support tobacco harm reduction. This open-label, randomised, crossover, clinical study in 24 healthy adult smokers study assessed nicotine pharmacokinetics and subjective effects of the Pulze Heated Tobacco System (HTS; Pulze HTP device and three iD stick variants—Intense American Blend, Regular American Blend and Regular Menthol) compared with subjects’ usual brand cigarettes (UBC). Cmax and AUCt were highest for UBC and significantly lower for each Pulze HTS variant. Cmax and AUCt were significantly higher for Intense American Blend compared with Regular American Blend, while AUCt was significantly higher for Intense American Blend compared with Regular Menthol. Median Tmax was lowest (i.e., nicotine delivery was fastest) for subjects’ usual brand cigarettes and similar across the iD stick variants, although no between-product differences were statistically significant. All study products reduced urges to smoke; this effect was greatest for cigarettes although this was not statistically significant. Product evaluation scores for each Pulze HTS variant in the domains of ‘satisfaction’, ‘psychological reward’ and ‘relief’ were similar, and lower than those for UBC. These data demonstrate that the Pulze HTS effectively delivers nicotine and generates positive subjective effects, including satisfaction and reduced urge to smoke. This supports the conclusion that the Pulze HTS may be an acceptable alternative to cigarettes for adult smokers while having a lower abuse liability than cigarettes.
Similar content being viewed by others
Introduction
Cigarette smoking is a cause of serious diseases including lung cancer, heart disease, and emphysema, and is a leading cause of preventable deaths1,2,3,4. Globally, smoking is reported to be responsible for more than 7 million deaths per year4. In Europe, although smoking prevalence is declining smoking still accounts for up to 25% of all-cause mortality5 and leads to approximately 700,000 deaths each year6. While nicotine in cigarette smoke is not harmless, it is not the primary cause of the harmful effects of cigarette smoking7. Instead, smoking-related harms are caused by smokers inhaling chemical toxicants which are formed during the processes of tobacco combustion and pyrolysis8. Around 7000 individual chemicals have been identified in cigarette smoke9 and many of these are linked to cardiovascular disease, respiratory disease, lung cancer and reproductive/developmental toxicity10. Stopping smoking eliminates exposure to associated toxicants and conveys the greatest possible reduction in disease risk for smokers, and is therefore the best course of action smokers can take to improve their health2. However, while large proportions of smokers report intending to quit smoking only a small percentage successfully stop smoking each year11,12,13.
In 2001 the US Institute of Medicine issued the report ‘Clearing the Smoke’, in which it was proposed that ‘For many diseases attributable to tobacco use, reducing risk of disease by reducing exposure to tobacco toxicants is feasible’14,15. This laid the foundation for tobacco harm reduction (THR), which relies on the fundamental principle that both the individual- and population-level health impacts of cigarette smoking can be reduced by the development of, and smoker access to, novel nicotine and tobacco products which deliver nicotine but in the reduced presence, or absence, of the chemicals responsible for smoking-related disease. Particularly aimed at those adult smokers who are either uninterested or unwilling to stop smoking16, support for a toxicant reduction approach to THR is growing. Many public health bodies including the UK Office for Health Improvement and Disparities (formerly Public Health England)17, the UK Royal College of Physicians16, the Government of Canada18, and the New Zealand Ministry of Health19, now advocate for a toxicant reduction approach to THR.
Heated tobacco products (HTPs) in general electrically heat tobacco to temperatures significantly lower than those which cause pyrolysis and combustion in combustible cigarettes20,21,22,23. This electrical heating causes the formation of an inhalable aerosol which contains nicotine24. However, aerosol from HTPs contains significantly fewer and lower levels of harmful chemicals than those found in cigarette smoke25,26,27,28,29. A number of clinical studies examining toxicant exposure have demonstrated significantly reduced exposure to toxicants linked to cardiovascular disease, respiratory disease, and reproductive/developmental toxicity, in smokers who switch to using HTPs30,31,32,33,34,35,36,37,38. Furthermore, these reduced toxicant exposures in switching smokers are associated with favourable changes in biomarkers of potential harm including those indicative of risk of cardiovascular disease, respiratory disease, inflammation/oxidative stress, and lung cancer31,33,39,40,41,42. This provides evidence of the significant harm reduction potential of HTPs and of the role they can play in making a meaningful contribution to THR strategies. However, reduced toxicant exposure is only one attribute that can determine the harm reduction potential of HTPs. Importantly, if the harm reduction potential of a HTP is to be maximised, reduced exposure must be allied with HTP uptake by sufficient numbers of adult smokers who would otherwise continue to smoke cigarettes. It has been suggested for smoking alternatives, such as electronic nicotine delivery systems (ENDS) and HTPs, that nicotine delivery and subjective effects such as satisfaction, liking and reductions in withdrawal symptoms are important factors, alongside their ability to replicate some of the ritualistic/sensorial cues associated with cigarette smoking, in determining their ability to facilitate smokers’ switching away from smoking43,44,45,46,47,48. In support of the association between nicotine delivery, switching, and harm reduction, increasing the e-liquid nicotine concentration of an ENDS is associated both with increased nicotine delivery44 and higher rates of switching away from cigarette smoking49.
Reductions in toxicant exposure and ensuing changes in disease risk in smokers switching to using HTPs have been well described in the literature. However, despite the importance of nicotine delivery, along with positive subjective effects, in providing an acceptable alternative to cigarettes for adult smokers and therefore in supporting THR, much less is known about the delivery of nicotine from commercially available HTPs. The Pulze Heated Tobacco System (HTS), which comprises the Pulze HTP device and iD sticks, is a novel tobacco product which electrically heats a consumable stick that contains reconstituted tobacco to a mean maximum internal temperature of 245 °C, substantially below the point of tobacco combustion. Due to the low operating temperature of the Pulze HTS device, emissions levels of a number of toxicants are significantly lower than those found in cigarette smoke25, although these findings are from an earlier, prototype version of the iD sticks. In this paper, findings are presented from an assessment of nicotine pharmacokinetics and subjective effects of three different variants (Intense American Blend, Regular American Blend and Regular Menthol) of iD sticks used with the Pulze HTS. These data are used to assess whether these attributes contribute to the likely acceptability of the Pulze HTS among adult smokers and therefore to establish the THR potential of the Pulze HTS.
Results
Subject demographics
Brief demographic details of the 24 subjects in the safety population are provided in Table 1, both for each randomisation sequence and overall. Fifty-eight percent of subjects were male, and all subjects were white and not of Hispanic/Latino origin. No major differences in demographics were seen between the randomisation sequence groups although the mean age and number of years smoking of the subjects in one of the product sequence groups was lower than in the other 3 groups (Table 1). Of the 24 subjects, 23 smoked non-menthol cigarettes as their usual brand of cigarette and a single subject smoked menthol cigarettes.
Nicotine pharmacokinetics
Prior to the start of the controlled puffing sessions and following 12 h of abstinence from the use of any tobacco- or nicotine-containing products, the mean uncorrected plasma nicotine concentration was 1.13 ng/ml (standard deviation [SD] 0.773 ng/ml, minimum 0.1 ng/ml, maximum 4.56 ng/ml, median 0.987 ng/ml). During use of any of the study products in the controlled use sessions (puffs taken at 30-s intervals with puffs 3 s in duration), plasma nicotine levels rose rapidly (Fig. 1). On average, during the controlled use session subjects took between 8.3 and 8.5 puffs on each of the iD stick variants and 9.8 puffs on their usual brand cigarettes (Supplementary Table 1).
Baseline-adjusted plasma nicotine concentrations (upper) and urge to smoke (lower) over time following controlled product use in the outcomes population. N = 23–24 in each case. Data points are arithmetic means for each product at each timepoint. Error bars have been removed for clarity; for variability estimates refer to Tables 2 and 4. VAS visual analog scale, HTS heated tobacco system.
The mean maximum plasma nicotine concentration (Cmax) and AUC values were highest for usual brand cigarettes and lower for each of the iD stick variants used with the Pulze HTS (Intense American Blend, Regular American Blend and Regular Menthol; Table 2); statistical analyses showed that Cmax and AUCt for each Pulze HTS variant were significantly lower than for subjects’ usual brand cigarettes (Table 3). Furthermore, Cmax and AUCt were significantly higher for Intense American Blend compared with Regular American Blend, while AUCt (but not Cmax) was significantly higher for Intense American Blend compared with Regular Menthol. No significant differences were seen for either Cmax or AUCt between Regular American Blend and Regular Menthol (Table 3).
Among the Pulze HTS iD stick variants, the median time taken to reach the maximum plasma nicotine concentration (Tmax; Table 2) was highest for Regular American Blend (7.084 min), slightly lower for Regular Menthol (6.942 min), and lowest for Intense American Blend (6.000 min). Median Tmax for subjects’ usual brand cigarettes was 6.250 min (Table 2). Statistical analyses of differences between study products for Tmax values are provided in Supplementary Table 2; differences between products were not statistically significant for any comparisons.
Subjective effects
Urge to smoke a cigarette was assessed prior to study product use and at various timepoints after product use using a single-item questionnaire with responses from subjects to the question ‘How strong is your urge to smoke right now?’ provided on a 100 mm VAS ranging from ‘Not at all’ to ‘Extreme’. Mean baseline (prior to study product use) VAS scores ranged from 57.8 to 64.2 for all products, and descriptive data for mean VAS scores following product use are presented in Table 4. Use of all study products elicited robust reductions in urge to smoke (Fig. 1). The mean maximum change in urge to smoke (Emax) was highest for subjects’ usual brand cigarettes (49.8 ± 30.10) and approximately 20–30% lower for each of the Pulze HTS variants (range 32.9 ± 27.13 to 40.3 ± 25.75). However, statistically significant differences in Emax values between products were only seen for the comparison of Regular American Blend and subjects’ usual brand cigarettes and not for any other comparisons (Table 5). The time of the maximum change in urge to smoke (TEmax) was similar across all study products, while mean AUEC0–240 (area under the effect-time curve from zero to 240 min) was highest for subjects’ usual brand cigarettes, lower for Intense American Blend and lowest for Regular American Blend and Regular Menthol (Table 4). However, no statistically significant differences in AUEC0–240 were seen between the individual study products (Table 5).
PES scores are presented in Table 6. On a scale of 1 to 7, with 1 being “not at all” and 7 being “extremely”, overall mean product evaluation scores for each of the Pulze HTS variants in the domain of ‘satisfaction’ ranged from 2.49 to 3.18, with a slightly higher score of 4.43 for subjects’ usual brand cigarettes. Similarly, in the domains of psychological reward, relief, ease of use, and comfort using in public, mean values were highest for subjects’ usual brand cigarettes and slightly lower, and comparable, for each of the Pulze HTS variants (Table 6). For aversion, mean values were highest for subjects’ usual brand cigarettes and Intense American Blend and lower for both Regular American Blend and Regular Menthol. For dependence concerns, the highest mean value was for usual brand cigarettes and approximately 50% lower, and comparable, for each of the Pulze HTS variants.
Regarding intent to use the product again, 13%, 17%, and 13% of subjects expressed positive likelihood (assessed as a rating on the VAS between the mid-point and ‘definitely would’) of using the Pulze HTS with the iD Intense American Blend stick, the iD Regular American Blend stick, and the iD Regular Menthol stick again, respectively, compared with 48% of subjects who expressed positive likelihood of using their usual brand cigarettes again. Overall, mean raw VAS scores for intent to use the product again ranged from 25.0 to 26.0 (0 = definitely would not and 100 = definitely would) for the Pulze HTS variants, with a higher score of 51.3 for subjects’ usual brand cigarettes.
Safety
There were no SAEs reported in this study and no subjects were discontinued due to AEs. During the product trial period on Day −1, during which subjects were allowed to use a single iD of their choice with the Pulze HTS, one mild AE (dizziness) was reported by one (4%) subject. The Investigator considered this event unrelated to study product use. Overall, AEs were infrequently reported in this study, with six AEs reported by five (21%) subjects after study product randomisation. Catheter site pain was reported three times by three (13%) subjects, and the remaining AEs (constipation, dizziness, and neck pain) were reported by one (4%) subject each. The constipation and dizziness events were moderate in severity, and the catheter site pain and neck pain events were mild. The Investigator considered all AEs to be unlikely related or unrelated to study product.
Mean vital signs (heart rate and blood pressures) remained within normal limits at all study time points, with minimal change from baseline. There were no individual clinically significant vital sign findings in this study.
Discussion
The cornerstone of THR is the principle that the harms associated with cigarette smoking, at both the individual and population levels, can be reduced by providing adult smokers with alternatives to cigarettes which deliver nicotine but with lower levels of the harmful chemicals found in cigarette smoke14,15,16. Acceptability of novel tobacco and nicotine products to adult smokers is an important driver of the likelihood that a given product will support smokers, who would otherwise continue to smoke, in transitioning away from cigarette smoking. Acceptability itself is driven by two main factors; the first factor is nicotine delivery, and it is generally considered that more cigarette-like nicotine delivery is important to uptake of novel tobacco products including ENDS and HTPs, their continued use, and prevention of relapse back to cigarette smoking43,44,45,46,47. Secondly, subjective effects contribute strongly to acceptability. Subjective effects may include positive effects such as satisfaction and liking50,51, as well as relief from negative effects such as urges to smoke/cigarette cravings52 and withdrawal symptoms53. Given the centrality therefore of nicotine delivery and subjective effects in determining the THR potential of non-combustible tobacco products including HTPs, this paper reports a clinical study designed to assess nicotine pharmacokinetics and various subjective effects measures in adult smokers when they used three different variants of iD tobacco sticks with the Pulze HTS. Regarding nicotine pharmacokinetics, use of all three variants of iD sticks used with the Pulze HTS under controlled conditions elicited delivery of nicotine, and there was no difference in nicotine delivery between the comparable menthol and non-menthol (tobacco) iD stick variants. As may be expected, there was evidence of a dose–response effect with greater levels of nicotine in the blood plasma (in terms of both Cmax and AUC) seen when subjects used iD Intense American Blend sticks (which have a higher per-stick aerosol nicotine yield) compared with both iD Regular American Blend sticks and iD Regular Menthol sticks; such a dose-dependent effect has been reported previously for other HTPs54. Nicotine delivery however was statistically significantly different, again in terms of Cmax and AUC, than nicotine delivery from subjects’ usual brand of cigarettes. Median Tmax was lowest (i.e., nicotine delivery was fastest) for subjects’ usual brand cigarettes and similar across the iD stick variants, although no between-product differences were statistically significant. It is notable however that there was a high degree of variability in Tmax across all study products, and also that the median Tmax values were similar across all study products.
Regarding subjective effects, use of each of the iD sticks used with the Pulze HTS elicited reductions in the urge to smoke cigarettes, which although approximately 20–30% lower in magnitude than that seen when subjects smoked cigarette were only significantly different to the cigarette for the iD Regular American Blend stick variant. The mean time-course (i.e., onset) of urge reductions was also similar across the study products, and similar to the onset of nicotine delivery the iD Intense American Blend stick variant elicited the most rapid reduction in urge to smoke. The composite positive subjective effects of satisfaction, psychological reward and relief on the PES55 were similar across the iD stick variants and slightly lower than those for usual brand cigarettes. Subjects, on average, found the iD Regular American Blend and Regular Menthol stick variants to be less aversive than their usual brand cigarettes, while the iD Intense American Blend stick variant was rated as equally aversive. Importantly, subjects reported much lower concerns that they would become dependent on the iD sticks than on their usual brand cigarettes.
There are few subjective effects assessments of HTPs reported in the literature with which to assess our findings against. However, previous studies46,54,56 have reported, similar to our study, robust reductions in urge to smoke in clinical study subjects using various different types of HTPs. In contrast to our findings though, bearing in mind that direct comparisons are challenging since different questionnaire approaches were used, the difference between product liking scores between cigarettes and HTPs in one of the prior studies54 were far greater than the differences in PES composite satisfaction scores between cigarettes and the Pulze HTS used in this study. This suggests that different HTPs with different heating characteristics and/or different tobacco stick compositions may elicit dissimilar subjective effects and therefore differentially provide support to different smokers switching away from cigarette smoking. Overall though, our findings and those of others in the published literature demonstrate that HTPs in general provide relief from urges to smoke and other withdrawal effects and also generate positive subjective effects, both of which may contribute to their ability to help smokers transition away from cigarette smoking and support THR.
The findings from this study can also be utilised to make an assessment of the relative abuse liability of the Pulze HTS. Abuse liability is a composite measure that can be determined from nicotine pharmacokinetic and subjective effects assessments, which together constitute an assessment of the abuse liability (dependence potential) of a tobacco or nicotine-containing product43,57. It has been proposed that the THR potential, when considering the ability of novel nicotine and tobacco products to reduce harms among adult current smokers, is optimal when appeal and dependence potential (abuse liability) are high and when and toxicity/harmfulness are low7. In this regard, possessing at least some degree of dependence potential is beneficial since it allows the novel product to compete with cigarettes and support switching away from cigarette smoking7,43,58,59. Conversely, too high an abuse liability or dependence potential may lead to the novel product posing an initiation or addiction risk among non-users of nicotine products, particularly among susceptible populations such as youth and young adults58,59. When considering the data presented in this paper, subjects usual brand cigarettes have a high abuse liability since cigarette smoking elicited high blood nicotine levels over a short period of time, as well as inducing strong subjective effects such as satisfaction and psychological reward. This is consistent with the literature which suggests that cigarettes have the highest abuse liability of any tobacco/nicotine product7,60. Each of the iD stick variants also delivered nicotine effectively, and with a speed of onset of nicotine delivery (another determinant of abuse liability)61 comparable to cigarettes, but the degree of delivery was significantly lower than that from cigarettes. Similarly, although the Pulze HTS variants reduced urges to smoke comparably to subjects’ usual brand cigarettes, positive subjective effects were lower in magnitude than those caused by cigarette smoking. Whilst this should not be interpreted that the Pulze HTS is not addictive, taken together, these data suggest that each variant of the Pulze HTS possesses a degree of abuse liability but that this abuse liability is lower than that of cigarettes. Furthermore, since the Cmax was greater and Tmax was shorter for the Pulze HTS compared with those pharmacokinetic parameters reported from studies on nicotine replacement therapy (NRT) gum62,63,64,65 and the Nicorette Inhalator54, likely the abuse liability of the Pulze HTS falls somewhere between cigarettes and NRT. This proposal is in line with the relative risk scale for nicotine-containing products7. In the context of THR, this finding supports that while the Pulze HTS may be an acceptable alternative to cigarette smoking that is satisfying to adult smokers and therefore may support switching, the lower abuse liability compared with cigarettes suggests that the Pulze HTS likely presents less of an initiation/addiction risk among non-users of nicotine than cigarettes. Such a conclusion is supported by survey data which demonstrate that HTP use among never smokers is very uncommon in countries where they are currently marketed66. In summary, the potential generation by the Pulze HTS of an ‘off-ramp’ away from cigarette smoking, while minimising ‘on-ramp’ to initiating tobacco/nicotine use, may serve to enhance the contribution the Pulze HTS can make to THR strategies.
The results presented in this paper should be considered within the context of a number of limitations. Firstly, this study assessed nicotine pharmacokinetics in smokers who used different variants of the Pulze HTS, and the findings were used to make assumptions regarding the potential ability of the Pulze HTS to act as a satisfactory alternative to cigarettes and therefore play a role in supporting THR. While we conclude that the pharmacokinetic and subjective effects profiles of the Pulze HTS will support its use as an acceptable alternative to smoking cigarettes, no data are presented concerning its actual ability to help smokers switch. However, data on other HTPs support our conclusions. For example, a recent study found that in adult smokers asked to switch to using the IQOS HTP, smokers initially substituted IQOS for 59% of their average daily cigarette consumption, and this increased to 87% by at the end of the 3-week study67. Furthermore, in a longer-term study with the IQOS HTP, half of study participants who were asked to switch from cigarette smoking to using IQOS were predominantly using IQOS as their tobacco product at 6 months40. For the glo HTP, biochemically-determined compliance with switching from smoking cigarettes to using glo was seen in 65% of study participants at the end of a 12-month study33. Notably, the nicotine pharmacokinetic parameter Cmax, which is influential in an assessment of abuse liability and of a novel tobacco product’s potential to displace cigarette smoking43,44,45,46,47,61, is similar between the Pulze HTS and other types of HTPs54,68. Taken together with these published data, our findings support the conclusion that the Pulze HTS will likely have a positive impact on THR by supporting either cigarette displacement, resulting in smoking reduction, or complete switching away from cigarette smoking. Studies are underway to ascertain the extent to which the Pulze HTS reduces cigarette consumption in smokers who adopt its use and will be reported in a future manuscript.
A further limitation is that this study assessed use of the Pulze HTS in a clinical environment using a controlled puffing regimen, at a single point in time, and with only a very brief familiarisation period. Although it has not been reported in the literature for HTPs, for other inhaled nicotine products such as ENDS, increases in Cmax have been reported over time as users transition from initial use and become experienced users69,70. This suggests, at least for ENDS, that some degree of familiarisation or acclimatisation occurs subsequent to first use, and this may be secondary to users changing their puffing topography over time70,71. It is possible that acclimatisation similarly occurs for HTPs, and therefore that the nicotine pharmacokinetic parameters reported in this paper do not necessarily reflect those that would be seen in an accustomed Pulze HTS user. However, increases in Cmax values over time for ENDS use, when assessed using similar methods as those used in this Pulze HTS study, have been reported as being less than 25%69. Therefore, our assessment of the abuse liability of the Pulze HTS relative to cigarettes would still remain valid even if similar levels of acclimatisation occurred for this product. Additionally, we have used data from a confined clinical study to make an assessment of the abuse liability of the Pulze HTS in users with limited familiarity of using the product and following the prescribed use of a single Pulze iD stick. While this experimental approach allows us to estimate the relative abuse liability of the different tobacco products assessed in the study, absolute abuse liability in the real world may be different than that reported from this clinical study.
In conclusion, our pharmacokinetic and subjective effects assessments of three iD stick variants when used with the Pulze HTS demonstrates effective nicotine delivery, generates positive subjective effects including satisfaction, psychological reward, and relief, and reduces urge to smoke. These findings support the conclusion that the Pulze HTS is likely to be an acceptable and satisfying alternative to cigarettes for adult smokers who would otherwise continue to smoke and support transitioning away from cigarettes, and therefore that the Pulze HTS has the potential to make a meaningful contribution to THR. Furthermore, when our findings are used to generate an abuse liability assessment, we conclude that the Pulze HTS has a lower abuse liability than cigarettes. The potential generation by the Pulze HTS of an ‘off-ramp’ away from cigarette smoking, while minimising an ‘on-ramp’ to initiating tobacco/nicotine use, likely serves to enhance its THR potential.
Methods
Overall study design
This open-label, randomised, crossover, clinical study received favourable opinion (equivalent to ethics approval) from the Office for Research Ethics Committees Northern Ireland (ORECNI) Health and Social Care Research Ethics Committee A (reference number 21/NI/0144) prior to study commencement. The study was performed in accordance with ethical principles set forth in the Declaration of Helsinki and was compliant with the principles and requirements of International Council for Harmonisation Good Clinical Practice (ICH-GCP), the European Union Clinical Trials Directive, and applicable local regulatory requirements. The study was performed at a single clinical site in Belfast, Northern Ireland (UK) and was registered in the ClinicalTrials.gov repository (NCT05459857). Twenty-four (24) male and female healthy adult smokers participated in this study. Subjects attended the clinic site on two separate occasions: a screening visit and a 5-day confinement period. After completing the study, a follow-up telephone call was conducted for all subjects no longer than 1 week after the end of their confinement period. All subjects provided written informed consent prior to the commencement of any study procedures, including screening assessments.
Study subjects
In total, 51 subjects were screened for participation in this study; 27 subjects either failed screening or were not needed for study participation (i.e., they were study reserves but were not required to take part in the study). Of the 24 enrolled subjects, 23 completed the study; one subject withdrew from the study for personal reasons.
Inclusion criteria were that subjects were healthy adults aged 21–65 years inclusive at screening; self-reported smoking at least 10 manufactured combustible (menthol or non-menthol) cigarettes per day (CPD) for at least 12 months prior to screening; had a urine cotinine ≥ 500 ng/mL at screening; had an exhaled carbon monoxide (eCO) level > 10 parts per million (ppm) at screening; if female and of childbearing potential were using at least one approved form of contraception; if female and of non-childbearing potential had undergone a sterilisation procedure at least 6 months prior to check-in or was postmenopausal with amenorrhea (verified by measuring follicle-stimulating hormone (FSH) levels) for at least 1 year prior to check-in; if a non-vasectomised male agreed to use a condom with spermicide or abstain from intercourse for the duration of the study and extending up to 90 days post-study; if male agreed not to donate sperm for duration of the study and extending up to 90 days post-study; was willing comply with the requirements of the study, including a willingness to use the study HTPs; and provided voluntary consent to participate in this study, which was documented by signing of the signed informed consent form.
Subjects were not allowed to enter the study if any exclusion criteria were met. The main exclusion criteria were a history or presence of clinically significant disease or disorder that, in the opinion of the Investigator, would have jeopardised the safety of the subject or impacted the validity of the study results; had a clinically significant abnormal finding on the physical examination, medical history, vital signs, electrocardiogram, or clinical laboratory results; had an acute illness (e.g., upper respiratory tract infection, viral infection) requiring treatment within 14 days prior to Check-in; systolic blood pressure (BP) < 90 mmHg or > 150 mmHg, diastolic BP < 40 mmHg or > 95 mmHg, or heart rate (HR) < 40 bpm or > 99 bpm at screening; estimated creatinine clearance (using the Cockcroft Gault equation) < 70 ml/min at screening; used medications known to interact with cytochrome P450 2A6 within 3 months prior to check in and throughout the study; used inhalers to treat any medical condition within 3 months prior to check in and throughout the study; used prescription or over-the-counter bronchodilator medication (e.g., inhaled or oral β-agonists) for treatment of any illness within 12 months prior to check in and throughout the study; was allergic to or could not tolerate menthol flavouring agents; had used any prescription smoking cessation treatments, including, but not limited to, varenicline (Chantix®) or bupropion (Zyban®) within 3 months prior to check in; was planning to quit smoking during the study or within the next 3 months or was postponing a quit attempt in order to participate in the study; or had donated blood or blood products (including plasma), had significant blood loss, or received whole blood or a blood product transfusion within 90 days prior to check in.
Study products
The Pulze HTS, which comprises the Pulze HTP device and iD sticks, generates a nicotine-containing aerosol by heating consumable sticks containing reconstituted tobacco using an electrically powered heating device as its heating source. This heating creates an inhalable aerosol to deliver nicotine via the lungs. The device can be operated in two different user-selected modes (‘standard’ and ‘eco’ modes) in which the device heats to temperatures of 345 °C and 315 °C, respectively. In this study, subjects only used the Pulze HTP in ‘standard’ mode. Once switched on the Pulze HTS device is operational for 4 min, which allows users to take approximately 8–9 puffs from a single iD stick.
The tobacco sticks contain a portion of reconstituted tobacco and other non-tobacco components. Three test iD consumables (reconstituted tobacco sticks used with the Pulze HTS device) were assessed in this study; Intense American Blend, Regular American Blend, and Regular Menthol. The aerosol from the Intense and Regular American Blend sticks has a tobacco aroma, while the aerosol from the Regular Menthol sticks has a menthol aroma due to the inclusion of a menthol-flavoured monoacetate filter at the mouth-end of the stick. The reconstituted tobacco in each of the Pulze iD consumables used in this study has a target specification of 4.6 mg of nicotine per stick, and different aerosol yields of nicotine are achieved by the use of different filters in each of the different iD consumables. When used under International Organization for Standardization (ISO) intense machine puffing conditions (55 ml bell-shaped puff over 2 s with a 30-s interpuff interval and with filter vents blocked)72, the Intense American Blend variant produces a higher yield of nicotine (mean yield 1.09 mg per stick) than either the Regular American Blend or Regular Menthol variants (mean yields both 0.64 mg per stick).
For use as a comparator product, all subjects provided their usual brand of cigarette.
Randomisation
Subjects who completed the study screening assessments were assigned a unique randomisation identification number. Subsequently, each subject, based on the identification number, were assigned to use the study products according to one of four product sequences, which were prepared by Celerion, Inc. These sequences were ABCD, BDAC, CADB and DCBA where A is the Pulze HTS with iD Intense American Blend sticks, B is the Pulze HTS with iD Regular American Blend sticks, C is the Pulze HTS with iD Regular Menthol sticks, and D is subjects’ usual brand cigarettes.
Study procedures
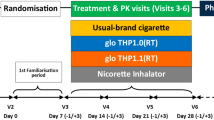
This study was a randomised, open label, crossover, confinement study in 24 male and female cigarette smokers. The study assessed 3 test products and a cigarette comparator and evaluated nicotine pharmacokinetics, subjective effects, puff topography and product safety. An overview of the study design is presented in Fig. 2.
At Visit 1 (screening), which took place within 27 days prior to study procedures on Day −1, subjects underwent numerous assessments to check their eligibility to participate in the study, to review their health status, and to assess their nicotine consumption habits. Screening procedures included a physical examination (including oral cavity and oropharynx), vital signs, electrocardiogram, body mass index (BMI), clinical laboratory tests (hematology, serum chemistry, and urinalysis), serology, urine/saliva drug, urine/breath alcohol, cotinine screen, eCO, and pregnancy and FSH tests (for females as appropriate). If requested, subjects were offered smoking cessation advice and contact information for a smoking cessation support service.
Visit 2 was a 5-day confinement period; subjects who successfully completed the screening procedures and met all the inclusion criteria and none of the exclusion criteria were eligible to check in to the clinical site for the confinement period. Subjects checked into the clinic on Day −1 and remained at the clinic until Day 4 for daily study product use, nicotine pharmacokinetics sampling, subjective questionnaire assessments, puff topography evaluation, and safety assessments. On Day −1, following eligibility confirmation, subjects undertook a familiarisation session with the study HTPs and the questionnaires. The site’s clinical team explained how the Pulze HTS was to be used. Subjects had the opportunity to see the products/devices and packaging and participated in a product trial in which they consumed one iD stick, of a flavour chosen by each subject, using the Pulze HTS. An explanation of how the questionnaires were to be administered to the subjects was given. After the familiarisation session and completion of check-in procedures, subjects were allowed to smoke their own cigarettes ad libitum but abstained from the use of any tobacco- or nicotine-containing products for at least 12 h prior to the start of the controlled product use session on the morning of Day 1. In the morning of Day 1, after pre-use assessments and confirmation of eligibility, the subjects were randomised to 1 of 4 product sequences and then provided a single product of the study product in the sequence to which they had been randomised. On Days 1 through 4, subjects used the assigned study product under controlled conditions (i.e., completely used a single iD tobacco consumable HTP stick or smoked a single cigarette), with puffs taken at 30-s intervals and puffs 3 s in duration. Blood samples for nicotine assessment were collected 5 min prior to initiating product use and at 2, 4, 6, 8, 10, 15, 30, 45, 60, 120, and 240 min following the start of study product use. Subjective effects questionnaires were administered to the subjects at defined intervals throughout the day, and safety was also monitored throughout the day. For all subjects, meals and snacks were provided at the appropriate times during confinement at the clinic site. Each meal and/or snack served at the site was standardised, was similar in caloric content and composition, and was taken at approximately the same time on each day. When confined at the clinic site, subjects were required to fast from all food and drink except water between meals and snacks.
On Days 1 through 4, following the 4-h pharmacokinetic blood collection, subjects started a 4-h ad libitum product use session (no limits on cigarette or HTP consumption) with the same study product as that used during the morning controlled use session. Puff topography assessments were also made in the study but are not reported in this manuscript, and no blood samples were taken in this period for nicotine pharmacokinetic analyses. After completion of the ad libitum use session, subjects were allowed to smoke their own cigarettes ad libitum until at least 12 h prior to the start of the morning controlled product use session scheduled on the following day. On Day 4, following completion of study assessments, subjects were allowed to smoke their own cigarettes and left the clinic after completing all final check-out requirements.
A follow-up telephone call (Visit 3) was made by the clinic in an attempt to contact all subjects who used at least one study product (including subjects who terminated the study early) using their standard procedures approximately 7 days after the final product use to determine if any adverse events (AEs) had occurred since the last study visit.
Nicotine pharmacokinetics
To determine blood plasma nicotine concentrations during and after use of the study products, blood samples (approximately 4 ml) were collected through an indwelling venous catheter at the time-points described above. Blood samples were drawn into dipotassium ethylenediaminetetraacetic acid (K2EDTA) vacutainer tubes via an intravenous catheter port and the plasma fraction separated off by centrifugation and pipetting. Plasma nicotine was analysed by liquid chromatography-tandem mass spectrometry (LC–MS/MS) at Celerion Bioanalytical Services (Lincoln, Nebraska, USA) using a validated analytical method with appropriate quality controls according to the Food and Drug Administration (FDA) Guidance for Industry (Title 21 Code of Federal Regulations Part 58). Processing of samples was completed by a non-tobacco user. The lower limit of quantification of plasma nicotine using the analytical method was 0.2 ng/ml.
Subjective effects assessments
The Intent to Use [100 mm visual analog scale (VAS)], Urge to Smoke (VAS), and Product Evaluation Scale (7-point scale55) questionnaires were completed using a computerised tablet device. All relevant software specific to the electronic questionnaires were provided by IVR Clinical Concepts (IVRCC; Saratoga Springs, New York, USA). The Urge to Smoke questionnaire was completed at Time 0 (pre-product use) and at 4, 8, 15, 45, 60, 120, and 240 min relative to the start of product use on Days 1, 2, 3, and 4. The Intent to Use questionnaire was completed at 240 min following the start of study product use on Days 1, 2, 3, and 4. The 21-item Production Evaluation Scale (PES) questionnaire was completed at 240 min following the start of study product use on each of Days 1, 2, 3, and 4. PES subscale scores in the domains of satisfaction (items 1, 2, 3, and 12), psychological reward (items 4, 5, 6, 7, and 8), aversion (items 9, 10, 16, and 18), and relief (items 11, 13, 14, 15, and reversed for 20), were generated from the individual items as described previously55.
Statistical analyses
The safety population comprised all subjects who had successfully completed eligibility requirements after checking in to the clinic site and used at least one study product. The outcomes population was a subset of the safety population and consisted of subjects who used a study product and had evaluable nicotine pharmacokinetics or subjective effects data. This population was used in the summary and analysis of all data presented in this paper.
Due to this being the first study assessing nicotine pharmacokinetics and subjective effects of the Pulze HTS, no sample size calculations could be performed. However, a sample size of 24 subjects was deemed adequate to meet the study objectives and this is in line with similar study designs in the literature46,56,68.
Demographics
Descriptive statistics are reported for continuous variables (age, weight, height, and BMI) and frequency counts were tabulated for categorical demographics variables (sex, ethnicity, and race). Descriptive statistics are also provided for smoking history variables (cigarettes smoked per day and number of years smoking).
Nicotine pharmacokinetics
Unadjusted plasma nicotine concentrations that were below the limit of quantitation (BLQ) were set to one-half of the lower limit of quantitation (LLOQ) for the calculation of descriptive statistics. Individual plasma nicotine concentrations were adjusted for baseline nicotine levels (“baseline-adjusted”) and all pharmacokinetic parameters were calculated based on the adjusted concentrations. Baseline adjustment was performed by subtraction of the pre-existing nicotine concentration from each nicotine concentration obtained after test product administration in that period/day for each subject using the following equation:
where Ct is the adjusted concentration at time t, Ct unadjusted is the observed concentration at time t, C0 is the pre-product use concentration (−5 min), Kel = ln(2)/t½, t½ is 2 h (approximate nicotine half-life), t is the actual sampling time since product administration, and t1 is the actual sampling time since the time of the pre-product use sample. After correction for pre-product use values, negative values were assigned a value of zero and all other values obtained were reported as is even if these values were BLQ.
SAS® software (Version 9.4) was used for data presentation and summarisation including descriptive statistics, statistical analyses, summary tables, graphs, and data listings. Descriptive statistics were generated for plasma nicotine concentrations and nicotine pharmacokinetic parameters by study product for all subjects, including sample size (n), arithmetic mean (mean), SD, coefficient of variation (CV%), standard error of the mean (SEM), minimum, median, and maximum at each nominal time-point. In addition, geometric mean, and geometric CV%, are provided for the Cmax (maximum plasma nicotine concentration) and AUC (area under the plasma nicotine concentration–time curve) parameters. Mean concentration–time profiles are presented on linear scales. Missing data were treated as missing, and no imputation was conducted.
A linear mixed-effects model for analysis of variance (ANOVA) was performed on the natural log-transformed pharmacokinetic parameters Cmax and AUCt following the morning product use session on each of Days 1, 2, 3, and 4. The model included sequence, product, and study period as fixed effects and subject-nested-within-sequence as a random effect. Geometric least-squares means (LSM), and 95% confidence intervals (CIs), are provided for the pharmacokinetic parameters Cmax and AUCt by study product. Geometric LSM ratios, 95% CIs of the geometric LSM ratios, and p-values are provided for the product comparisons of Cmax and AUCt. The comparisons of interest included each of the products compared to each other. The above statistical analyses were performed using SAS® PROC MIXED.
A non-parametric analysis (Wilcoxon Signed Rank test) was performed for the comparisons of Tmax (time to maximum plasma nicotine concentration) between each of the study products. The median difference and 95% CI of the difference are presented for each comparison. The CIs were constructed using Walsh Averages and the appropriate quantile of the Wilcoxon Signed Rank Test statistic.
Subjective effects
Urge to smoke
The derived parameters Emax (maximum reduction in urge to smoke), TEmax (time of the maximum reduction in urge to smoke), and AUEC0–240 (area under the time-effect curve between time zero and 240 min), are listed by subject and summarised by product using descriptive statistics, including n, mean, SD, CV%, SEM, minimum, Q1, median, Q3, maximum, and 95% CI. A linear mixed-effects model for ANOVA was used to compare urge to smoke parameters without data transformation; the mixed model includes product sequence, period, and product as fixed effects and subject nested within product sequence as a random effect. LSM and 95% CIs are provided for the Emax and AUEC0–240 by product. LSM difference, 95% CIs of the LSM difference, and p-values are provided for the product comparisons for Emax and AUEC0–240. The comparisons of interest included each of the products compared to each other.
Product evaluation scale
Descriptive statistics for the composite (satisfaction, psychological reward, aversion and relief) and individual (ease of use, comfort using in public and dependence concerns) PES factor scores55 are provided by product.
Intent to use
Descriptive statistics for the VAS raw score and bipolar score are summarised.
Safety assessments
Safety was monitored through physical examination (symptom-driven), vital signs measurements, electrocardiograms, and clinical laboratory tests (serum chemistry, hematology, and urinalysis). Adverse event (AE) information was also collected throughout the study. AEs [including serious AEs (SAEs)] were recorded from the start of the first product used until the end-of-study telephone call. Severity/intensity were graded as mild, moderate, or severe, and AEs were also assessed as unlikely, possibly, or probably related to the study product by the investigator.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
International Agency for Research on Cancer. IARC Handbooks of Cancer Prevention. Reversal of Risk After Quitting Smoking Vol. 11 (IARC, 2007).
US Department of Health and Human Services. The health consequences of smoking: 50 years of progress: A report of the surgeon general. atlanta: department of health and human services. (Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014). https://www.surgeongeneral.gov/library/reports/50-years-of-progress/.
World Health Organization. WHO Report on the Global Tobacco Epidemic: Warning About the Dangers of Tobacco (World Health Organization, 2011).
World Health Organization. Tobacco. https://www.who.int/news-room/fact-sheets/detail/tobacco. Accessed 9 Oct 2022
Global Disease Burden 2019 Collaborators. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet 397, 2337–2360 (2021).
Organisation for Economic Co-operation and Development. Health at a Glance: Europe 2020: State of Health in the EU Cycle. (2020). https://www.oecd-ilibrary.org/sites/1c429c01-en/index.html?itemId=/content/component/1c429c01-en. Accessed 16 Nov 2022.
Abrams, D. B. et al. Managing nicotine without smoke to save lives now: Evidence for harm minimization. Prev. Med. 117, 88–97 (2018).
Baker, R. R., Massey, E. D. & Smith, G. An overview of the effects of tobacco ingredients on smoke chemistry and toxicity. Food Chem. Toxicol. 42(Suppl), S53-83 (2004).
Perfetti, T. & Rodgman, A. The complexity of tobacco and tobacco smoke. Beitr. Tabakforsch. Inte. 24, 215–232 (2011).
Food and Drug Administration. Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke; Established List. Docket No. FDA–2012–N–0143, in Federal Register, (Food and Drug Administration, 2012).
Bandi, P. et al. Changes in smoking cessation-related behaviors among US adults during the COVID-19 pandemic. JAMA Netw. Open. 5, e2225149 (2022).
Pesce, G. et al. Time and age trends in smoking cessation in Europe. PLoS ONE 14, e0211976 (2019).
Babb, S., Malarcher, A., Schauer, G., Asman, K. & Jamal, A. Quitting smoking among adults: United States, 2000–2015. MMWR Morb. Mortal Wkly. Rep. 65, 1457–1464 (2017).
Institute of Medicine. Clearing the Smoke: Assessing the Science Base for Tobacco Harm Reduction (The National Academies Press, 2001).
Stratton, K., Shetty, P., Wallace, R. & Bondurant, S. Clearing the smoke: The science base for tobacco harm reduction–executive summary. Tob Control. 10, 189–195 (2001).
Royal College of Physicians. Nicotine without Smoke. Tobacco Harm Reduction. A Report by the Tobacco Advisory Group of the Royal College of Physicians (Royal College of Physicians, 2016).
Office for Health Improvement and Disparities. Nicotine Vaping in England: An Evidence Update Including Health Risks and Perceptions (2022). https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1107701/Nicotine-vaping-in-England-2022-report.pdf. Accessed 16 Nov 2022.
Health Canada. Vaping and Quitting Smoking. https://www.canada.ca/en/health-canada/services/smoking-tobacco/vaping/smokers.html. Accessed 16 Nov 2022.
New Zealand Ministry of Health. Position Statement on Vaping. https://www.health.govt.nz/our-work/preventative-health-wellness/tobacco-control/vaping-smokefree-environments-and-regulated-products/position-statement-vaping. Accessed 9 Oct 2022.
Eaton, D. et al. Assessment of tobacco heating product THP1.0 Part 2: Product design, operation and thermophysical characterisation. Regul. Toxicol. Pharmacol. 93, 4–13 (2018).
Kärkelä, T., Tapper, U. & Kajolinna, T. Comparison of 3R4F cigarette smoke and IQOS heated tobacco product aerosol emissions. Environ. Sci. Pollut. Res. Int. 29, 27051–27069 (2022).
Schaller, J. P., Pijnenburg, J. P. M., Ajithkumar, A. & Tricker, A. R. Evaluation of the Tobacco Heating System 2.2. Part 3: Influence of the tobacco blend on the formation of harmful and potentially harmful constituents of the tobacco heating system 2.2 aerosol. Regul. Toxicol. Pharmacol. 81(Suppl 2), S48-s58 (2016).
Smith, M. R. et al. Evaluation of the tobacco heating system 2.2. Part 1: Description of the system and the scientific assessment program. Regul. Toxicol. Pharmacol. 81(Suppl 2), S17-s26 (2016).
Jaccard, G. et al. Comparative assessment of HPHC yields in the tobacco heating system THS2.2 and commercial cigarettes. Regul. Toxicol. Pharmacol. 90, 1–8 (2017).
Chapman, F. et al. Multiple endpoint in vitro toxicity assessment of a prototype heated tobacco product indicates substantially reduced effects compared to those of combustible cigarette. Toxicol. Vitro. 86, 105510 (2022).
Forster, M. et al. Assessment of novel tobacco heating product THP1.0. Part 3: Comprehensive chemical characterisation of harmful and potentially harmful aerosol emissions. Regul. Toxicol. Pharmacol. 93, 14–33 (2018).
Malt, L. et al. The product science of electrically heated tobacco products: A narrative review of the scientific literature. F1000 Res. 11, 121 (2022).
Poynton, S. et al. A novel hybrid tobacco product that delivers a tobacco flavour note with vapour aerosol (Part 1): Product operation and preliminary aerosol chemistry assessment. Food Chem. Toxicol. 106, 522–532 (2017).
Szparaga, M., Świercz, R. & Stępnik, M. Review of data on chemical content in an aerosol resulting from heating a tobacco or a solution used in e-cigarettes and in the smoke generated from the reference cigarettes. Toxicol. Mech. Methods. 31, 323–333 (2021).
Gale, N. et al. Changes in biomarkers of exposure on switching from a conventional cigarette to the glo tobacco heating product: A randomized controlled ambulatory study. Nicotine Tob. Res. 23, 584–591 (2021).
Gale, N. et al. Changes in biomarkers after 180 days of tobacco heating product use: A randomised trial. Intern. Emerg. Med. 16, 2201–2212 (2021).
Gale, N. et al. Changes in biomarkers of exposure on switching from a conventional cigarette to tobacco heating products: A randomized, controlled study in healthy japanese subjects. Nicotine Tob. Res. 21, 1220–1227 (2019).
Gale, N., McEwan, M., Hardie, G., Proctor, C. J. & Murphy, J. Changes in biomarkers of exposure and biomarkers of potential harm after 360 days in smokers who either continue to smoke, switch to a tobacco heating product or quit smoking. Intern. Emerg. Med. 17, 2017–2030 (2022).
Haziza, C. et al. Reduction in exposure to selected harmful and potentially harmful constituents approaching those observed upon smoking abstinence in smokers switching to the menthol tobacco heating system 2.2 for 3 months (part 1). Nicotine Tob. Res. 22, 539–548 (2020).
Haziza, C. et al. Evaluation of the Tobacco Heating System 2.2. Part 8: 5-day randomized reduced exposure clinical study in Poland. Regul. Toxicol. Pharmacol. 81(Suppl 2), S139–S150 (2016).
Haziza, C. et al. Biomarker of exposure level data set in smokers switching from conventional cigarettes to tobacco heating system 2.2, continuing smoking or abstaining from smoking for 5 days. Data Brief. 10, 283–293 (2017).
Lüdicke, F. et al. Effects of switching to the tobacco heating system 2.2 menthol, smoking abstinence, or continued cigarette smoking on biomarkers of exposure: A randomized, controlled, open-label, multicenter study in sequential confinement and ambulatory settings (part 1). Nicotine Tob. Res. 20, 161–172 (2018).
McEwan, M. et al. A randomized controlled study in healthy participants to explore the exposure continuum when smokers switch to a tobacco heating product or an E-cigarette relative to cessation. Toxicol. Rep. 8, 994–1001 (2021).
Haziza, C. et al. Favorable changes in biomarkers of potential harm to reduce the adverse health effects of smoking in smokers switching to the menthol tobacco heating system 2.2 for 3 months (part 2). Nicotine Tob. Res. 22, 549–559 (2020).
Lüdicke, F. et al. Effects of switching to a heat-not-burn tobacco product on biologically relevant biomarkers to assess a candidate modified risk tobacco product: A randomized trial. Cancer Epidemiol. Biomark. Prev. 28, 1934–1943 (2019).
Lüdicke, F. et al. Effects of switching to the menthol tobacco heating system 2.2, smoking abstinence, or continued cigarette smoking on clinically relevant risk markers: A randomized, controlled, open-label, multicenter study in sequential confinement and ambulatory settings (part 2). Nicotine Tob. Res. 20, 173–182 (2018).
Sakaguchi, C., Nagata, Y., Kikuchi, A., Takeshige, Y. & Minami, N. Differences in levels of biomarkers of potential harm among users of a heat-not-burn tobacco product, cigarette smokers, and never-smokers in Japan: A post-marketing observational study. Nicotine Tob. Res. 23, 1143–1152 (2021).
Fearon, I. M. Human abuse liability assessment of e-cigarettes: Why, what and how?. Drug Test Anal. https://doi.org/10.1002/dta.3251 (2022).
Goldenson, N. I., Fearon, I. M., Buchhalter, A. R. & Henningfield, J. E. An open-label, randomized, controlled, crossover study to assess nicotine pharmacokinetics and subjective effects of the JUUL system with three nicotine concentrations relative to combustible cigarettes in adult smokers. Nicotine Tob. Res. 23, 947–955. https://doi.org/10.1093/ntr/ntab001 (2021).
Hajek, P. et al. Nicotine delivery and users’ reactions to Juul compared with cigarettes and other e-cigarette products. Addiction 115, 1141–1148 (2020).
Phillips-Waller, A., Przulj, D., Pesola, F., Smith, K. M. & Hajek, P. Nicotine delivery and user ratings of IQOS heated tobacco system compared with cigarettes, juul, and refillable e-cigarettes. Nicotine Tob. Res. 23, 1889–1894 (2021).
Phillips-Waller, A., Przulj, D., Smith, K. M., Pesola, F. & Hajek, P. Nicotine delivery and user reactions to Juul EU (20 mg/ml) compared with Juul US (59 mg/ml), cigarettes and other e-cigarette products. Psychopharmacology 238, 825–831 (2021).
Gades, M. S. et al. The role of subjective responses in electronic cigarette uptake and substitution in adult smokers. Drug Alcohol Depend. 212, 107999 (2020).
Goldenson, N. I. et al. Differences in switching away from smoking among adult smokers using JUUL products in regions with different maximum nicotine concentrations: North America and the United Kingdom. Nicotine Tob. Res. 23, 1821–1830 (2021).
Shiffman, S. & Kirchner, T. R. Cigarette-by-cigarette satisfaction during ad libitum smoking. J. Abnorm. Psychol. 118, 348–359 (2009).
West, R. Tobacco smoking: Health impact, prevalence, correlates and interventions. Psychol. Health 32, 1018–1036 (2017).
Lechner, W. V. et al. Effects of negative affect, urge to smoke, and working memory performance (n-back) on nicotine dependence. Subst. Use Misuse. 53, 1177–1183 (2018).
Hughes, J. R. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob. Res. 9, 315–327 (2007).
Hardie, G. et al. An abuse liability assessment of the glo tobacco heating product in comparison to combustible cigarettes and nicotine replacement therapy. Sci. Rep. 12, 14701 (2022).
Hatsukami, D. K., Zhang, Y., O’Connor, R. J. & Severson, H. H. Subjective responses to oral tobacco products: scale validation. Nicotine Tob. Res. 15, 1259–1264 (2013).
Maloney, S. et al. Acute effects of JUUL and IQOS in cigarette smokers. Tob. Control. 30, 449–452 (2020).
Vansickel, A., Baxter, S., Sherwood, N., Kong, M. & Campbell, L. Human abuse liability assessment of tobacco and nicotine products: Approaches for meeting current regulatory recommendations. Nicotine Tob. Res. 24, 295–305 (2022).
Ashley, D. L., Spears, C. A., Weaver, S. R., Huang, J. & Eriksen, M. P. E-cigarettes: How can they help smokers quit without addicting a new generation?. Prev. Med. 140, 106145 (2020).
Cahn, Z. et al. Applying the population health standard to the regulation of electronic nicotine delivery systems. Nicotine Tob. Res. 23, 780–789 (2021).
Gottlieb, S. & Zeller, M. A nicotine-focused framework for public health. N. Engl. J. Med. 377, 1111–1114 (2017).
Henningfield, J. E. & Keenan, R. M. Nicotine delivery kinetics and abuse liability. J. Consult. Clin. Psychol. 61, 743–750 (1993).
Goldenson, N. I., Buchhalter, A. R., Augustson, E. M., Rubinstein, M. L. & Henningfield, J. E. Abuse liability assessment of the JUUL system in four flavors relative to combustible cigarette, nicotine gum and a comparator electronic nicotine delivery system among adult smokers. Drug Alcohol Depend. 217, 108395 (2020).
Hansson, A., Rasmussen, T. & Kraiczi, H. Single-dose and multiple-dose pharmacokinetics of nicotine 6 mg gum. Nicotine Tob. Res. 19, 477–483 (2017).
Stiles, M. F. et al. Pharmacodynamic and pharmacokinetic assessment of electronic cigarettes, combustible cigarettes, and nicotine gum: Implications for abuse liability. Psychopharmacology 234, 2643–2655 (2017).
Stiles, M. F. et al. Assessment of the abuse liability of three menthol Vuse Solo electronic cigarettes relative to combustible cigarettes and nicotine gum. Psychopharmacology 235, 2077–2086 (2018).
Adamson, J. et al. Results from a 2018 cross-sectional survey in Tokyo, Osaka and Sendai to assess tobacco and nicotine product usage after the introduction of heated tobacco products (HTPs) in Japan. Harm. Reduct. J. 17, 32 (2020).
Stone, M. D., DeAtley, T., Pianin, S., Strasser, A. A. & Audrain-McGovern, J. Switching from cigarettes to IQOS: A pilot examination of IQOS-associated reward, reinforcement, and abstinence relief. Drug Alcohol Depend. 238, 109569 (2022).
Picavet, P., Haziza, C., Lama, N., Weitkunat, R. & Lüdicke, F. Comparison of the pharmacokinetics of nicotine following single and ad libitum use of a tobacco heating system or combustible cigarettes. Nicotine Tob. Res. 18, 557–563 (2016).
Hajek, P. et al. Nicotine intake from electronic cigarettes on initial use and after 4 weeks of regular use. Nicotine Tob. Res. 17, 175–179 (2015).
Farsalinos, K. E. et al. Nicotine absorption from electronic cigarette use: Comparison between experienced consumers (vapers) and naïve users (smokers). Sci. Rep. 5, 11269 (2015).
Kimber, C. F., Soar, K. & Dawkins, L. E. Changes in puffing topography and subjective effects over a 2-week period in e-cigarette naïve smokers: Effects of device type and nicotine concentrations. Addict. Behav. 118, 106909 (2021).
International Organization for Standardization. ISO 20778:2018. Cigarettes—Routine Analytical Cigarette Smoking Machine—Definitions and Standard Conditions with an Intense Smoking Regime. (2018).
Acknowledgements
The authors would like to thank Celerion (Belfast, UK), for conducting the study and the internal Imperial Brands PLC Reading Committee for their critical review of the manuscript.
Author information
Authors and Affiliations
Contributions
S.Mc.D., T.N., K.R., E.M. and G.O’.C. designed and oversaw the conduct of the study and data analyses/reporting. I.M.F. interpreted and contextualised the data and wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
This study was funded by Imperial Brands PLC, the manufacturer of the Pulze Heated Tobacco System examined in this study. SMcD, TN, EM, KR and GO’C were employees of Imperial Brands PLC at the time the study was designed and conducted, and hold shares in Imperial Brands PLC. IMF is an independent consultant to e-cigarette/tobacco product manufacturers, including Imperial Brands PLC, to provide scientific support for clinical and behavioural studies and general regulatory support. IMF is also a retained Scientific Advisory Board member of Qnovia, LLC. IMF holds shares in British American Tobacco.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
McDermott, S., Reichmann, K., Mason, E. et al. An assessment of nicotine pharmacokinetics and subjective effects of the pulze heated tobacco system compared with cigarettes. Sci Rep 13, 9037 (2023). https://doi.org/10.1038/s41598-023-36259-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-36259-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.