Abstract
The utilization of nanotechnology and biotechnology for enhancing the synthesis of plant bioactive chemicals is becoming increasingly common. The hairy root culture technique can be used to increase secondary metabolites such as tropane alkaloids. Agrobacterium was used to induce hairy roots from various explants of Hyoscyamus muticus. The effect of nano-silver particles (AgNPs) at concentrations of 0, 25, 50, 100, and 200 mg/L on tropane alkaloids synthesis, particularly hyoscyamine and scopolamine, was studied in transgenic hairy root cultures. Different types of explants obtained from 10-day-old seedlings of H. muticus were inoculated with two strains of Agrobacterium rhizogenes (15,834 and A4). The antimicrobial activity of an ethanolic extract of AgNPs-induced hairy root cultures of H. muticus was tested. The frequency of hairy roots was higher in hypocotyl, root, leaf, and stem explants treated with A. rhizogenes strain A4 compared to those treated with strain 15,834. In transgenic hairy root cultures, AgNPs application at a concentration of 100 mg/L resulted in the highest total tropane alkaloid production, which exhibited broad-spectrum antimicrobial activity. The study demonstrated the potential of nano-silver as an elicitor for promoting the production of target alkaloids in Hyoscyamus muticus hairy root cultures, which exhibit high biological activity.
Similar content being viewed by others
Introduction
Medicinal plants have been the primary source of life-saving drugs for people around the world since ancient times1. In addition, plant-based medications are essential sources of several pharmaceuticals2. Hyoscyamus muticus L. (H. muticus), an essential medicinal herb belonging to the Solanaceae family3. Scopolamine and hyoscyamine are the most often used chemicals obtained from Hyoscyamus species in traditional medicine. However, due to their complex chemical properties, commercial production of these chemicals is challenging and expensive. Therefore, there is an urgent need to use biotechnology techniques to increase these chemicals in the root cells and aerial parts of the plant. Scopolamine and hyoscyamine are widely used to alleviate spasms and cholinergic symptoms in the parasympathetic nervous system4,5,6.They are also used as narcotic analgesics to treat Parkinson's disease, asthma, and motion sickness7.
According to Zhao et al.8, the production of hyoscyamine and scopolamine begins when ornithine converts into putrescine by the enzyme ornithine decarboxylase (ODC). Moreover, tropane alkaloids may be produced at the highest and quickest rate from transgenic root cultures using Agrobacterium rhizogenes (RI)9. To speed up the accumulation of secondary metabolites in medicinal plants, various methods have been developed, such as high cell product selection, nutrient culture media conditioning, precursor supply, bioreactor system scaling up, hairy root transplantation implementation, plant cell immobilization, and biotransformation10,11.
Tissue culture and genetic modification can be used to increase the production of pharmaceuticals. These techniques make it simpler to extract specific materials from plant tissues rather than from the entire plant. The use of genetic modification can also help to control the process of alkaloid biosynthesis, which is important for the development of medicinal compounds12.
Plant roots may develop hairy roots from the RI (T-DNA) plasmid of RI Agrobacterium. The generated hairy roots are more beneficial than the mother plants because of their genetic and biochemical stability, rapid growth, hormone auto-feeding, low cost of lateral branching needs, and potential for multi-enzyme biosynthesis. In addition, these generated hairy roots have a shorter generation time13,14. Some signaling molecules may serve as catalysts for the induction and production of secondary metabolites in plants15.Signaling components can be utilized as elicitors to trigger the production of targeted secondary metabolites in plant cell cultures. This strategy is effective in inducing the synthesis of secondary metabolites throughout the entire plant using in vivo elicitors.
Nanoparticles (NPs) may act as signaling molecules, starting physiologic and metabolic reactions. The fundamental processes for how NPs could act as signaling molecules to start physiological and metabolic reactions are yet unknown16. Due to their unique properties, NPs increasingly used in many other fields as nanotechnology spreads across the physiology and biochemistry fields17. In addition, this method may supply novel opportunities for study in agriculture and plant sciences. NPs affect seed viability and plant development for potential use in farming. Nanoparticles accelerated germination and plant development in groundnut plants and boosted antioxidant activity18,19.
Moreover, NPs affect plant secondary metabolites through reactive nitrogen/oxygen species, gene expression, and signaling pathways metabolites. Higher plants produce surpass 200,000 secondary metabolites that have various biological activities. Secondary metabolites produced by elicitors are employed in nanomedicines. Furthermore, nanoparticle (NP) routes affect plant secondary metabolism and chemical elicitation20.
Lu et al.21 observed an increase in antioxidant enzyme activities in Brassica juncea seedlings when treated with silver nanoparticles. The elicitation strategy can stimulate the synthesis of specialized metabolites in plant cell cultures by activating genes involved in the secondary metabolism pathways of plants. NP roles in the body are still unknown, but their application as elicitors is growing22.
Microorganisms vary widely in their resistance and cause infectious in humans23. These diseases pose a significant threat to human life and the economy. The usage of antibiotics is restricted because of the resistance of pathogens against many antibiotics. Natural compounds that limit microbial development are thus a specialized research topic24. Essential oils and secondary metabolites found in medicinal plants are valuable resources for the pharmaceutical industry22. Medicinal plants have wide biological and antimicrobial activities and still play an important role in contemporary medicine.
The goal of this study is to investigate the effect of silver nanoparticles (AgNPs) on the synthesis of scopolamine, hyoscyamine, and total alkaloids in the hairy roots of Hyoscyamus muticus L. The study also intends to assess the antibacterial and antifungal capabilities of ethanolic extracts from hairy root cultures against gram-positive and gram-negative bacteria, yeast, and fungus.
Materials and methods
In vitro seeds germination
All plant experiments are conducted in accordance with relevant institutional, national, and international guidelines and legislation. The seeds for the Hyoscyamus muticus study were provided by the Egyptian Ministry of Agriculture's Department of Medicinal and Aromatic Plant Research. The seeds were ready for planting after being surface sterilized in 70% ethanol for 1 min, 25% commercial Clorox (5.25 percent sodium hypochlorite) with a few drops of Tween 20 for 20 min, and three washing in sterile distilled water. Seeds were cultured in a solid (MS, Murashige, and Skoog) basal medium with 30 g/L sucrose25 to stimulate in vitro germination after sterilization25.
Induction of hairy roots
The strains 15,834 and A4 of A. rhizogenes (obtained from the Agriculture Microbiology Department, National Research Centre) were utilized. Each bacterium strain was grown for 2 days in liquid LB medium (Luria Bertani media)26 before being incubated at 28 °C on an orbital shaker at 110 rpm in darkness to achieve an OD of 0.6 at 600 nm.
Inoculated explants
After 10 days of seed growth, Agrobacterium rhizogenes strains 15,834 or A4 were used to inoculate hypocotyl, leaf, stem, and root explants. After 2 days of dark culture in hormone-free MS media having 3% (w/v) sucrose, 7% (w/v) agar, and 0.1 g L−1 Myo-inositol, the inoculated explants were transferred to the same medium supplemented with 200 (mg/L) cefotaxime as an antibiotic. Hairy roots were induced and observed 2 weeks later. The hairy roots were grown in MS medium and subcultured every 10 days after antibiotics were withdrawn for the third time. Every 2 weeks, the colonies were transferred to new 250 (mL) Erlenmeyer flasks containing 30 mL of hormone-free liquid MS media (shaken at 120 rpm at 25 °C in the dark).
Polymerase chain reaction analysis
(See the supplementary file).
Impact of AgNPs on the accumulation and synthesis of alkaloids
AgNP at various concentrations, including 0, 25, 50, 100, and 200 mg/L, were employed in this experiment. The AgNPs were bought from Sigma (Aldrich) under catalogue number 730807. The silver nanoparticles were dissolved in ethanol with polyvinylpyrrolidone (PVP). Silver nanoparticles are described using transmission electron microscopy (TEM). TEM imaging has a significantly higher resolution than light-based imaging techniques due to the fact technique uses electrons rather than light to illuminate the sample.
According to data on AgNP characterization, sodium citrate serves as a stabilizing agent in silver dispersion NPs with a 40 nm particle size (TEM), 0.02 mg/mL in an aqueous buffer. After filtering the AgNPs solution via a micro-filter to sterilize it, the AgNPs were added to an autoclaved, liquid-free MS medium under sterile circumstances at various quantities. The sugar concentration in the in vitro growth medium was set at 30 g/L. After having its pH adjusted to 5.6, the culture medium was autoclaved for 20 min at 121 °C. Separate pieces of hairy roots were transplanted into the growth chamber and kept to grow for 30 days at a constant temperature of 25 degrees Celsius and absolute darkness. The experimental methodology was designed using a totally randomized approach. After (30) days of in vitro cultivation, the fresh and dried weights per jar (in g/jar) were recorded.
Hairy roots fresh and dry weights and measurements
The dry weights of hairy roots were measured 3 weeks after AgNPs were applied. The inoculation was performed with (100 mg/jar). After weighing fresh weight, roots were left in the air for 48 h in the dark at room temperature to dry to keep the thermo-sensitive biological components.
Extraction and determination of tropane alkaloids using HPLC
The alkaloids were extracted using the Kamada et al. technique27.To extract tropane alkaloids, hairy root samples (500 mg) were soaked in 10 mL of ethanol containing 2% H2SO4 for (48 h) to extract tropane alkaloids. Adding 14% ammonia at a pH of 8.0 combined with dried, filtered extracts for alkaloid release with using sodium sulfate to remove the water residue. The alkaloids were then re-extracted three times with ether (3 mL), followed by washing with a 10% KCl solution to remove silver ions and salts. Before using high-performance liquid chromatography (HPLC), the filtrate extracts were dried at room temperature, and 2 mL of phosphate buffer was added to each fraction. The HPLC study was carried out in a water system outfitted with an HPLC pump, a UV detector (dual absorbance) at 204 nm, and a UV-absorbance detector (single absorbance). Each sample (20 μL) was injected onto a reversed-phase HPLC column [RP-18, 250, 4.6 (mm), 5-m particle size, Shimadzu, Japan] with NaH2PO4 (0.78 g/L) and H3PO4 (0.2 mL) in water (pH 2.6) as solvent A and acetonitrile as solvent B, with isocratic elution at 40 °C with a ratio of 90%. Using varying quantities of atropine (Sigma Aldrich, USA) as the standard (300, 200, 100, 50, 20, and 10 g/mL) to create a standard calibration curve. The correlation between concentration and peak area of the standard was calculated using the minimum square technique (R2 value).
Antimicrobial activity
Yeast Candida albicans (ATTC90028), fungal strains Aspergillus terreus (SQu14026), Fusarium chlamydosporum (F25), Alternaria alternata (Te19), and Microsporum canis (IFM 45,829), and bacterial strains Staphylococcus aureus (ATCC 29,213), Bacillus subtilis (NBRC:13,719), Pseudomonas aeruginosa (ATTC35639), Escherichia coli (ATTC11775), and Klebsiella pneumonia (ATCC 15,380) were used to evaluate antimicrobial activity of hairy root ethanolic extract by using disc diffusion method28. A final concentration of 200 (µg/mL) was attained by diluting the ethanol extract with dimethyl sulfoxide (DMSO). Twenty microliters of extract were applied to sterile discs. Plates of nutrient agar and Czapek's agar media were seeded with 100 µL of bacterial and fungal inoculum (0.5 Mac Farland29. The Petri dishes containing bacteria were incubated at 37 degrees Celsius for 24 h, while those containing fungi were incubated at 28 °C for 72 h. DMSO-treated discs were used as the negative control, whereas ampicillin and fluconazole were used as positive controls for bacterial and fungal strains, respectively. Antimicrobial activity was evaluated by measuring the diameter of the inhibitory zones.
The statistical analysis
The arrangement for each experiment was completely randomized with three replicates. The data was subjected to Analysis of Variance (ANOVA) as described by Snedecor and Cochran30 followed by Duncan's Multiple Range Test (DMRT)31 at P < 0.05 for the significant differences between treatments using COSTAT package ver. 6.4 (CoHort software, Monterey, USA).
Results
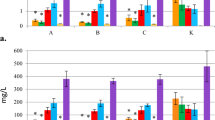
The effect of inoculating different Hyoscyamus muticus L. explants with two strains of A. rhizogenes on the development of hairy roots was investigated. After 10 days of seed culture, the hypocotyl, leaf, stem, and root explants were excised and inoculated with two strains of Agrobacterium rhizogenes: 15,834 and A4. After a period of 10 days following inoculation, the frequency percentage of hairy root formation, as well as the fresh and dried weights (measured in mg per jar), were recorded. Data in (Fig. 1) depicts the highest frequencies (percentages) of hairy root formation in hypocotyl, root, leaf, and stem explants treated with A. rhizogenes strain A4: 73.4%, 53.7%, and 31.5%, 20.2%, respectively. Fresh weights were 16.37, 4.7, 3.2, and 2.4 (mg/jar), respectively. The dry weights of hypocotyl, root, leaf, and stem explants treated with A. rhizogenes strain A4 were 1.07, 0.32, 0.16, and 0.149 mg/jar, respectively (Fig. 2). However, there was no response with A. rhizogenes (strain 15,834), which produced tumorigenic calli and a few hairy roots; thus, only the A4 strain would be employed in the subsequent experiment.
Polymerase chain reaction analysis
The purpose of the PCR analysis was to determine the presence of T-DNA from Agrobacterium rhizogenesis (Ri plasmid). The T-DNA at the 580 bp of (Ri) plasmid was integrated and expressed in the hairy root of H. muticus infected with the two A. rhizogenesis strains utilized (A4 and 15,834) compared to the DNA isolated from normal H. muticus roots, where no T-DNA was detected (Supplementary Fig. S1).
Effects of AgNPs on the development of hairy roots
The different concentrations of silver nanoparticles (0, 25, 50, 100, and 200 (mg/L), were applied to transgenic hairy root cultures (100 mg/jar) treated with the Agrobacterium strain (A4). The dry weights of hairy root cultures derived from hypocotyl, root, leaf, and stem explants were 0.76, 0.676, 0.63, and 0.487 (g/jar), respectively (Fig. 3). Furthermore, the control had the highest dry weight, followed by treatments containing 25, 50, 100, and 200 (mg/L) of AgNPs for all hairy roots generated from the various explants employed. However, increasing the concentration of AgNPs decreased the growth of hairy roots while increasing the alkaloids content. This indicates that the application of AgNPs acts as an elicitor/stressor, depending on the applied concentration.
AgNPs treatment affects total alkaloids, hyoscine, and hyoscyamine accumulation and synthesis
Table 1 shows that the highest total alkaloids (3.57, 3.45, 3.43, 3.32, and 3.25%) were recovered from hairy root cultures coming from root explants treated with 100, 50, 200, 25, and 0 mg/L AgNPs, respectively. Root, leaf, stem, and hypocotyl explants recorded a high accumulation rate of total alkaloids, respectively. In addition, a high accumulation rate of hyoscine, 0.037, 0031, 0.029, 0.027, and 0.023% were recorded with hairy root cultures produced from root explants treated with 100, 50, 200, 25, 0 (control) mg/L of AgNPs, respectively. The maximum value of hyoscine responded from hairy root cultures produced from root, leaf, stem, and hypocotyl explants. Moreover, the maximum value of hyoscyamine was 0.046% in the extract of hairy roots derived from root explants exposed to AgNPs at (100 mg/L). The extracted hairy root cultures of root, leaf, stem, and root explants showed the best results of hyoscyamine accumulation, respectively.
Antimicrobial activity
An inhibitory zone assay was used to determine the antimicrobial activity of an ethanolic extract of hypocotyl explants grown in an H. muticus L hairy root culture (Table 2 and Fig. 4). Ethanolic extract inhibited the growth of several bacterial and fungal strains tested. The inhibitory diameter values range from 9.0 to 20.3 (mm) among different bacterial species. The ethanolic extract exhibited strong antibacterial activity against B. subtilis (NBRC: 13,719) and S. aureus (ATCC 29,213), but only had a weak effect against E. coli (ATTC11775). However, there was no observable effect against P. aeruginosa (ATTC35639) and K. pneumoniae (ATTC35639). In yeast tests, the ethanolic extract suppresses the development of Candida albicans. Furthermore, it was effective against Aspergillus terreus (SQu14026) and Microsporum canis (IFM 45,829) but ineffective against Fusarium chlamydosporum (F25) and Alternaria alternata.
Discussion
Defense Secondary metabolites are activated by biological or non-biological elicitors. In hairy root cultures, Agrobacterium rhizogenesis, as a biological elicitor, stimulates alkaloid synthesis in H. muticus, including hyoscine and hyoscyamine. Tepfer32 demonstrated that Agrobacterium may transfer large plasmids carrying T-DNA into the genome of host plant cells. Many species with hairy rootstocks have been propagated using Agrobacterium rhizogenes. Plants generated from different rootstock cultures have piqued the interest of researchers due to their genetic and metabolic stability, high growth rates, and ability to generate by-products in proportions comparable to wild type roots.
Nanoparticles as nano-biological elicitors have emerged as novel plant biosynthesis triggers in recent years33,34. Due to their chemical and physical properties, NPs with a high surface-to-volume ratio, the capacity to influence electron exchange, and strong surface contact capabilities are potential candidates for modifying the redox state and impacting plant growth, performance, and quality35.
Application of Ag-NPs increased artemisinin by 3.9-fold in 20-day-old hairy root cultures of Artemisia annua L.36 Hydroponically grown Bacopa monnieri L. treated with copper-based NPs (Cu) boosted saponin, alkaloid, flavonoid, and phenol content, as well as antioxidant activity37. Celastrol, a therapeutic phytochemical38, was enhanced after AgNPs were applied to adventitious and hairy root cultures of Celastrus paniculatus. Recently, cell suspension cultures of Hypericum perforatum L. treated with metal (Ag, Au, Cu, Pd) and metal oxide (CeO2, CuO, TiO2, ZnO) NPs enhanced the production of a variety of bioactive secondary metabolites39.
Agrobacterium rhizogenes-mediated technique is effective in inducing H. muticus hairy roots and secondary metabolic products. Two Agrobacterium strains were examined for their potential to stimulate rhizogenesis in our study, and A4 was shown to be the most virulent, resulting in the rapid formation of hairy roots at the fastest growth rates. The most common strain, 15,834, demonstrated great virulence only in the production of neoplastic callus but not in the propagation of infection via rhizomes.
These results are in agreement with the study conducted by Vanhala et al.40, who observed that the Agrobacterium strains (LBA9402, A4, 15,834, and C58CI) had an impact on the growth rate, maturation, and synthesis of hyoscyamine in transformed H. muticus. Specifically, the C58C1 strain resulted in the highest level of alkaloids, indicating its effectiveness. Similarly, Nicoll et al.41 found that, the 15,834 strain was the least effective in promoting the development of hairy roots in H. muticus, which aligns with our findings. Additionally, Yoshimatsu and Shimamura42 reported that A. niger infection in opium poppy hypocotyls reduced its production. They noted that unlike the 15,834 strain that produced hairy roots, A. rhizogenes MAFF 03–01,724 and an unknown strain induced Calli.
Williams and Ellis43 reported that 15,834 strain was the most potent and efficient in promoting the development of hair roots in Catharanthus roseus G. Don, as shown by Brillanceau et al.44. Additionally, Vanhala et al.40 found that the Agrobacterium strains had an impact on the maturation, expansion, and hyoscyamine production of H. muticus mutant root cultures.
The enhanced growth and yield of Avena sativa resulting from nanoparticle treatment may be attributed to the enhancement of essential element concentrations in plant tissues, as well as the elevation of peroxidase, catalase, and nitrite reductase activities in such tissues45.
Furthermore, AgNPs have been found to affect the production of total alkaloids, including hyoscyamine and hyoscine, in H. muticus. Shakeran et al.46 reported that nano-silver treatment caused a 2.4-fold increase in atropine concentration in hairy root cultures of D. metel after elicitation, which is consistent with our findings. Sharafi et al.11 also observed that NPs play a crucial role in elevating artemisinin production in A. annua. H. reticulatus treated with SiO2 NPs 100 and 200 (mg/L) for 24 h accumulated the most total phenolic and flavonoids contents as reported by Ahad et al.47. Also, HPLC analysis showed that after 24 h of treatment with 100 (mg/L) SiO2 NPs, H. reticulatus transformed roots accumulated much more hyoscyamine 140.15 (mg/g Fresh Weight) and scopolamine 67.71 (mg g−1 Fresh Weight) than non-treated roots, by 1212% and 272%, respectively. Moreover, optimal doses and exposure times were found to increase hyoscyamine and scopolamine synthesis in H. reticulatus hairy root cultures treated with iron oxide NPs40. The results also showed that hyoscyamine and scopolamine concentrations in the treated hairy root cultures were higher than in the control group.
Elicitation of H. muticus hairy root culture with sliver nanoparticles might be due to the activation of enzymatic reactions involved in the synthesis pathways of tropane alkaloids. Sliver nanoparticles are a novel elicitor of the hairy root culture of H. muticus.
The ethanolic extract derived from the hairy root culture of H. muticus L. exhibited potent antibacterial activity against B. subtilis (NBRC:13,719) and S. aureus (ATCC 29,213), but it only displayed weak activity against E. coli (ATTC11775), and it did not show any activity against P. aeruginosa (ATTC35639) and K. pneumoniae (ATTC35639). However, the ethanolic extract demonstrated strong anti-Candida albicans (C. albicans) activity in yeast tests. Additionally, it was highly effective against A. terreus (SQu14026) and M. canis (IFM 45,829), but it had no effect against F. chlamydiosporum (F25) and A. alternata.
According to Almalki48, the methanol extract of H. muticus hairy root cultures demonstrated mild antimicrobial activity against various bacteria, including B. subtilis (MTCC441), E. faecalis (ATCC 29,212), S. aureus (ATCC 25,923), S. epidermidis (MTCC 3615), E. coli (ATCC25923), K. pneumoniae (ATCC 15,380), and P. aeroginosa (ATCC 27,853). However, it did not exhibit any activity against A. niger but was effective against several other fungi. In contrast, Elsharkawy et al.49 found that the methanolic extract of H. muticus aerial parts was successful in reducing the growth of S. aureus and Bacillus cereus. They also observed an inhibitory effect on E. coli, K. pneumoniae, and P. aeruginosa. However, Kebaili et al.50 demonstrated that the H. muticus hydroalcoholic extract was effective against Escherichia coli and Staphylococcus aureus but not against Pseudomonas aeruginosa. The antimicrobial activity of a methanol extract of H. muticus leaves showed mild effectiveness against Escherichia coli, Pseudomonas aeruginosa, and Listeria monocytogenes, and weak activity against Staphylococcus aureus, while showing no effectiveness against fungal growth51.
Conclusion
The results revealed that Agrobacterium strain A4 was more effective in promoting hairy root formation compared to strain 15,834. However, the highest dry weight of hairy roots was obtained from hypocotyl explants, and hairy roots derived from root explants showed significant accumulation and production of secondary metabolites such as total tropane alkaloids, hyoscine, and hyoscyamine. Moreover, treatment with AgNPs at 100 mg/L resulted in the highest production of total tropane alkaloids, including hyoscine and hyoscyamine, in hairy roots obtained from hypocotyl explants. Additionally, the ethanol extracts of hairy roots obtained from hypocotyl explants exhibited high to mild antimicrobial activity against tested gram-positive bacteria, gram-negative bacteria, and fungi. These findings demonstrate that nano-silver can be a promising elicitor for enhancing the production of secondary metabolites in Hyoscyamus muticus hairy root cultures, which has potential economic benefits.
Data availability
The data in this study are available upon request from the corresponding author.
References
Bertrand, I. et al. The impact of temperature on the inactivation of enteric viruses in food and water: A review. J. Appl. Microbiol. 112, 1059–1074 (2012).
Gayathri & Kiruba, V. Study of biochemical parameters of four different medicinal plants. (2014).
Madani, H., Hosseini, B., Dehghan, E. & Rezaei-chiyaneh, E. Enhanced production of scopolamine in induced autotetraploid plants of Hyoscyamus reticulatus L.. Acta Physiol. Plant. 37, 1–11 (2015).
Pitta-Alvarez, S. I., Spollansky, T. C. & Giulietti, A. M. The influence of different biotic and abiotic elicitors on the production and profile of tropane alkaloids in hairy root cultures of Brugmansia candida. Enzyme Microb. Technol. 26, 252–258 (2000).
Wan, F., Du, X., Liu, H., He, X. & Zeng, Y. Protective effect of anisodamine hydrobromide on lipopolysaccharide-induced acute kidney injury. Biosci. Rep. 40, 20201812 (2020).
Palazón, J., Navarro-Ocaña, A., Hernandez-Vazquez, L. & Mirjalili, M. H. Application of metabolic engineering to the production of scopolamine. Molecules 13, 1722–1742 (2008).
Ghorbanpour, M., Hatami, M. & Hatami, M. Activating antioxidant enzymes, hyoscyamine and scopolamine biosynthesis of Hyoscyamus niger L. Plants with nano-sized titanium dioxide and bulk application. Acta Agric. Slov. 105, 23–32 (2015).
Zhao, T. et al. Engineering tropane alkaloid production based on metabolic characterization of ornithine decarboxylase in Atropa belladonna. ACS Synth. Biol. 9, 437–448 (2020).
Harfi, B., Khelifi, L., Khelifi-Slaoui, M., Assaf-Ducrocq, C. & Gontier, E. Tropane alkaloids GC/MS analysis and low dose elicitors’ effects on hyoscyamine biosynthetic pathway in hairy roots of Algerian Datura species. Sci. Rep. https://doi.org/10.1038/s41598-018-36625-4 (2018).
Sharafi, A. et al. Enhanced morphinan alkaloid production in hairy root cultures of Papaver bracteatum by over-expression of salutaridinol 7-o-acetyltransferase gene via Agrobacterium rhizogenes mediated transformation. World J. Microbiol. Biotechnol. 29, 2125–2131 (2013).
Mirzaee, H., Sharafi, A. & Hashemi Sohi, H. In vitro regeneration and transient expression of recombinant sesquiterpene cyclase (SQC) in Artemisia annua L.. South African J. Bot. 104, 225–231 (2016).
Valimehr, S., Sanjarian, F., Sohi, H. H., Sharafi, A. & Sabouni, F. A reliable and efficient protocol for inducing genetically transformed roots in medicinal plant Nepeta pogonosperma. Physiol. Mol. Biol. Plants 20, 351–356 (2014).
Giri, A. & Narasu, M. L. Transgenic hairy roots: Recent trends and applications. Biotechnol. Adv. 18, 1–22 (2000).
Banerjee, S., Singh, S. & Rahman, L. U. Biotransformation studies using hairy root cultures—A review. Biotechnol. Adv. 30, 461–468 (2012).
Zhao, J., Davis, L. C. & Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 23, 283–333 (2005).
Hatami, M. & Ghorbanpour, M. Defense enzyme activities and biochemical variations of Pelargonium zonale in response to nanosilver application and dark storage. Turkish J. Biol. 38, 130–139 (2014).
Selvakesavan, R. K., Kruszka, D., Shakya, P., Mondal, D. & Franklin, G. Impact of nanomaterials on plant secondary metabolism. In Nanomaterial Interactions with Plant Cellular Mechanisms and Macromolecules and Agricultural Implications (eds Al-Khayri, J. M. et al.) 133–170 (Springer International Publishing, 2023). https://doi.org/10.1007/978-3-031-20878-2_6.
Hussein, H. A. A., Darwesh, O. M. & Mekki, B. B. Environmentally friendly nano-selenium to improve antioxidant system and growth of groundnut cultivars under sandy soil conditions. Biocatal. Agric. Biotechnol. 18, 101080 (2019).
Hussein, H. A. A., Darwesh, O. M., Mekki, B. B. & El-Hallouty, S. M. Evaluation of cytotoxicity, biochemical profile and yield components of groundnut plants treated with nano-selenium. Biotechnol. Reports 24, e00377 (2019).
Zhang, Y. et al. Effects of metal nanoparticles and other preparative materials in the environment on plants: From the perspective of improving secondary metabolites. J. Agric. Food Chem. 70, 916–933 (2022).
Sharma, P. et al. Silver nanoparticle-mediated enhancement in growth and Antioxidant status of Brassica juncea. Appl. Biochem. Biotechnol. 167, 2225–2233 (2012).
Akbaş, P., Uslu, H., Uslu, G. A. & Alkan, H. Hyoscyamus reticulatus L. tohum ekstraktinin antimikrobiyal ve apoptotik etkinliğinin araştirilmasi. Süleyman Demirel Üniv. Fen Bilim. Enstitüsü Derg. 24(2), 317–322 (2020).
Prestinaci, F., Pezzotti, P. & Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathogens Global Health 109, 309–318 (2015).
Tabassam, Q. et al. GC-MS metabolomics profiling and HR-APCI-MS characterization of potential anticancer compounds and antimicrobial activities of extracts from Picrorhiza kurroa roots. J. Appl. Biomed. 19, 26–39 (2021).
Murashige, T. & Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15, 473–497 (1962).
Lennox, E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology 1, 190–206 (1955).
Kamada, H., Okamura, N., Satake, M., Harada, H. & Shimomura, K. Alkaloid production by hairy root cultures in Atropa belladonna. Plant Cell Rep. 5, 239–242 (1986).
Hayes, A. J. & Markovic, B. Toxicity of Australian essential oil Backhousia citriodora (Lemon myrtle). Part 1. Antimicrobial activity and in vitro cytotoxicity. Food Chem. Toxicol. 40, 535–543 (2002).
Wade, D. et al. Hematological and antifungal properties of temporin A and a cecropin A-temporin A hybrid. Acta Biochim. Pol. 48, 1185–1189 (2001).
Snedecor, G. W. & Cochran, W. G. Statistical Methods (The Iowa State University Press, U.S.A, 1989).
Duncan, D. B. Multiple range and multiple F tests. Biometrics 11, 1 (1955).
Tepfer, D. Ri T-DNA from Agrobacterium rhizogenes: A source of genes having applications in rhizosphere biology and plant development, ecology and evolution. Plant-Microbe Interact. 242–342 (1989).
Shakya, P., Marslin, G., Siram, K., Beerhues, L. & Franklin, G. Elicitation as a tool to improve the profiles of high-value secondary metabolites and pharmacological properties of Hypericum perforatum. J. Pharm. Pharmacol. 71, 70–82 (2019).
de Jesus, S., Rivero-Montejo, M.V.-H. & Torres-Pacheco, I. Nanoparticles as novel elicitors to improve bioactive compounds in plants. Agriculture 11(2), 134. https://doi.org/10.3390/agriculture11020134 (2021).
Moharrami, F., Hosseini, B., Sharafi, A. & Farjaminezhad, M. Enhanced production of hyoscyamine and scopolamine from genetically transformed root culture of Hyoscyamus reticulatus L. elicited by iron oxide nanoparticles. Vitr. Cell. Dev. Biol. Plant 53, 104–111 (2017).
Zhang, B., Zheng, L. P., Yi Li, W. & Wen Wang, J. Stimulation of artemisinin production in Artemisia annua hairy roots by Ag–SiO2 core-shell nanoparticles. Curr Nanosci. 9(363), 370 (2013).
Lala, S. Enhancement of secondary metabolites in Bacopa monnieri (L.) Pennell plants treated with copper-based nanoparticles in vivo. IET Nanobiotechnol. 14, 78–85 (2020).
Moola, A. K., Senthil Kumar, T. & Ranjitha Kumari, B. D. Enhancement of Celastrol compound by silver nanoparticles and acetosyringone in Celastrus paniculatus Willd. through adventitious and hairy root culture. J. Plant Biochem. Biotechnol. 31, 429–434 (2022).
Kruszka, D., Selvakesavan, R. K., Kachlicki, P. & Franklin, G. Untargeted metabolomics analysis reveals the elicitation of important secondary metabolites upon treatment with various metal and metal oxide nanoparticles in Hypericum perforatum L. cell suspension cultures. Ind. Crops Prod. 178, 114561 (2022).
Vanhala, L., Hiltunen, R. & Oksman-Caldentey, K. M. Virulence of different Agrobacterium strains on hairy root formation of Hyoscyamus muticus. Plant Cell Rep. 14, 236–240 (1995).
Monique Nicoll, S., Brigham, L. A., Wen, F. & Hawes, M. C. Expression of transferred genes during hairy root development in pea. Plant Cell. Tissue Organ. Cult. 42, 57–66 (1995).
Yoshimatsu, K. & Shimomura, K. Transformation of opium poppy (Papaver somniferum L.) with Agrobacterium rhizogenes MAFF 03–01724. Plant Cell Rep. 11, 132–136 (1992).
Williams, R. D. & Ellis, B. E. Alkaloids from Agrobacterium rhizogenes-transformed Papaver somniferum cultures. Phytochemistry 32, 719–723 (1993).
Brillanceau, M. H., David, C. & Tempé, J. Genetic transformation of Catharanthus roseus G. Don by Agrobacterium rhizogenes. Plant Cell Rep. 8, 63–66 (1989).
Hrubý, M., Cígler, P. & Kuzel, S. Contribution to understanding the mechanism of titanium action in plant. J. Plant Nutr. 25, 577–598 (2002).
Shakeran, Z., Keyhanfar, M., Asghari, G. & Ghanadian, M. Improvement of atropine production by different biotic and abiotic elicitors in hairy root cultures of Datura metel. Turkish J. Biol. 39, 111–118 (2015).
Hedayati, A., Hosseini, B., Palazon, J. & Maleki, R. Improved tropane alkaloid production and changes in gene expression in hairy root cultures of two Hyoscyamus species elicited by silicon dioxide nanoparticles. Plant Physiol. Biochem. 155, 416–428 (2020).
Almalki, M. A. In vitro antibacterial, antifungal and other medical properties of endangered medicinal plant seeds. Pharmacol. Pharm. 08, 189–204 (2017).
Elsharkawy, E. R., Abdelaziz, E., Emad, M. A. & Ahmed, M. H. A. Antioxidant, antimicrobial and antifeedant activity of phenolic compounds accumulated in Hyoscyamus muticus L.. African J. Biotechnol. 17, 311–321 (2018).
Kebaili, Z. et al. Assessment of alkaloid content and antibacterial activity of Hyoscyamus Albus and Hyoscyamus Muticus collected in two different climatic regions in Algeria. J. Biochem. Technol. 10, 1–6 (2019).
Ayari-Guentri, S. et al. Hyoscyamus muticus L. subsp. falezlez methanolic extract: Phytochemical composition and biological activities. Eur. J. Biol. Res. 12, 190–206 (2022).
Acknowledgements
The authors are thankful to the Botany and Microbiology Department, Faculty of Science (Girls Branch), Al-Azhar University. The authors would also like to thank the Department of Botany, National Research Centre.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
A.M.A.: designed the study, methodology , manuscript writing and review; S.O.A.: designed the study ,methodology, manuscript writing and original draft preparation; H.A.H., designed the study, methodology, manuscript writing and original draft preparation; I.M.M.A. designed the study, methodology, manuscript writing and data analysis; E.S.A.: designed the study ,preparation of figures and tables methodology, and manuscript writing; A.M.R.: designed the study, manuscript writing, methodology, review and data analysis; S.K.M.K.: designed the study, methodology, writing and manuscript editing and review; D.A.M.M.: designed the study, visualization, sample preparation, methodology and manuscript writing; NNA: designed the study, original draft preparation, methodology and manuscript review. S.S.H.: designed the study, original draft preparation, methodology and manuscript review; A.M. designed the study, original draft preparation, methodology and manuscript review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdelkawy, A.M., Alshammari, S.O., Hussein, HA.A. et al. Effect of silver nanoparticles on tropane alkaloid production of transgenic hairy root cultures of Hyoscyamus muticus L. and their antimicrobial activity. Sci Rep 13, 10397 (2023). https://doi.org/10.1038/s41598-023-36198-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-36198-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.