Abstract
A series of novel Er3+-doped bismuth-germanate glasses containing different tungsten concentrations with a molar composition of 97.5[(75 − x)GeO2–25Bi2O3–(x)WO3]–2Sb2O3–0.5Er2O3 (x = 5, 10, 15, 20, and 25 mol%) were fabricated. Their thermal properties are measured by differential scanning calorimetry. A structural investigation by Raman spectroscopy suggested that changes occurred in the glass network by WO3 incorporation. By laser excitation at 980 nm, a strong emission from Er3+ ions at 1532 nm is observed, while the WO3 addition caused changes in the emission spectra. The emission cross-section spectra of Er3+ are calculated by both McCumber and Füchtbauer–Ladenburg theories and their comparison showed these theories yielded slightly different results, but in both cases, the calculations showed that a gain signal in L-band can be achieved when 30% of the Er3+ ions are at the excited state. This study proves that the Er3+-doped bismuth-germanate glasses are suitable for optical fiber amplifier applications operating at C- and L-band.
Similar content being viewed by others
Introduction
Rare-earth-doped fibers are attracting a lot of attention for their appropriate properties to amplify optical signals. Silica-based Er3+-doped fibers have been used in optical communication systems for many years. However, Er3+ ions have low solubility and narrow fluorescence spectrum in silica, which results in Er3+-doped silica fibers working relatively inefficiently in the L-band1,2. The high demand for information transmission on the communication networks has made it urgent to employ erbium-doped fiber amplifiers (EDFA) in the L-band3. Wavelength division multiplexing (WDM) systems are widely used in EDFAs and improving the capacity of WDMs by broadening their operating wavelength range would increase the efficiency of EDFAs in return4. There is a lot of research devoted to achieving EDFAs with broad gain bandwidth and high gain in new glass host materials such as silicate, germanate, and tellurite glasses5,6,7.
Germanate-based heavy metal oxide (HMO) glasses and fibers, especially lead-germanate, have been extensively studied and they are shown to be efficient in amplifying optical signals in the L-band8,9,10. Germanate-based HMO glasses yield medium and long-range structural disorder as a characteristic of a vitreous system that improves the thermal stability of the glass and solubility of rare-earth ions (REI), giving great potential for applications in optical communications6,11,12,13. However, due to the actual worldwide regulation of lead, bismuth-germanate glasses have been proposed as an alternative glass matrix to be deployed in optical amplifiers for their appropriate properties. Thanks to their high refractive index (~ 2), low phonon energy (~ 700 cm−1), high REI solubility, high mid-infrared (MIR) transmission up to 5 µm, high thermal stability, good mechanical strength and good chemical durability, bismuth-germanate glasses are great candidates for optical devices and photonic applications14,15,16,17,18. Generally, in a glass doped with REIs, a high refractive index increases the spontaneous emission probability, while in the meantime, a low phonon energy enhances the radiative relaxation rate from an excited energy state to a lower energy state. Hence, the resulting combination of these two effects would help broaden the spectral distribution of fluorescence from REIs19,20,21.
Some researchers have studied the influence of the glass optical basicity on the spectral distribution of the Er3+ emission spectrum previously22,23,24. For each glass system under study, Tanabe et al. have evidenced that low glass optical basicity decreases the bond covalency between the Er3+ ions and glass matrix ions, allowing Er3+ ions to have a broader emission spectrum19. Additionally, it has been revealed that low optical basicity is achieved by incorporating cations with high electronegativity values into the glass matrix19. Bismuth-germanate glasses consist mainly of germanium and bismuth cations with electronegativities of 2.01 and 2.02, respectively, which are higher than that of silicon, 1.9, and other cations that silica glasses may include.
In this work, the electronegativity of erbium-doped bismuth-germanate glasses is increased by substituting germanium atoms with tungsten atoms with an electronegativity of 2.36, leading to a lower optical basicity of the glasses and a broader fluorescence spectrum. These glasses with varying tungsten contents are systematically studied in terms of thermal, structural, and spectroscopic properties, and lifetime measurements. Besides, gain bandwidth and gain properties in the samples are calculated.
Results and discussion
The thermal and physical properties of prepared glasses are given in Table 1.
The Tg of the glasses maintain similar values at around 496 ± 5 °C while there is a decrease in Tx, hence, the thermal stability of these samples weakens when the WO3 nominal content is greater than 10 mol%. The Tg and Tx values and the difference between them, ΔT, are shown in Table 1. The ΔT is described as the thermal stability of the glass and regarded as a measure of the crystallization tendency of the glass. Generally, the ΔT is desired to be greater than 100 °C for fiber fabrication. Therefore, the samples 5W and 10W seem to have appropriate thermal properties to be drawn into the fiber. The influence of the increase in WO3 content over the glass density and Er3+ ion concentration in the glass is also shown in Table 1. As the WO3 content increases from 5 to 25 mol%, the glass density rises from 6.04 to 6.63 g/cm3, while the Er3+ ion concentration decreases slightly from 1.78 \(\times\) 1020 to 1.72 \(\times\) 1020 ions/cm3. Additionally, the glass polarizabilities of the glasses at 1538 nm are calculated from the Clausius–Mossotti relation \(P=\frac{3\times ({n}^{2}-1)\times M}{4\times ({n}^{2}+2)\times \rho \times \pi \times N}\) where \(P\) is the glass polarizability, \(n\) is the glass refractive index at 1538 nm, \(M\) is the molecular weight of the glass, \(\rho\) is the glass density and \(N\) is Avogadro’s number25,26,27. Table 1 shows that the glass polarizability escalates with the WO3 content and, thus, with the glass density.
The refractive indices of the glass samples with respect to the WO3 content as a function of the wavelength are shown in Fig. 1. The refractive index increases from around 1.95 to 2.05 at 1538 nm as the WO3 content rises from 5 to 25 mol%. A decrease in the refractive indices at longer wavelengths is also exhibited.
The refractive index measurements for each sample in Fig. 1 are fitted with the Sellmeier equation28 \({n\left(\lambda \right)}^{2}=1+(\frac{{B}_{1}\times {\lambda }^{2}}{{\lambda }^{2}-{C}_{1}}+\frac{{B}_{2}\times {\lambda }^{2}}{{\lambda }^{2}-{C}_{2}})\), where \(n\) is the refractive index of the samples, \(\lambda\) is the wavelength, \({B}_{1}\), \({B}_{2}\), \({C}_{1}\) and \({C}_{2}\) are the dimensionless Sellmeier equation coefficients, which can be found in Supplementary Table S1. The fittings made by the Sellmeier constants agree with the refractive index values in Fig. 1. The \({B}_{1}\) parameter is associated with the network disorder in glasses and an increase in \({B}_{1}\) is measured, which might indicate that the introduction of a greater proportion of W–O bonds increases the network disorder in bismuth-germanate glasses. The negative values of Sellmeier’s coefficients have already been reported for a germanate matrix13,29. The regression curves for all samples are obtained with an R2 value higher than 0.99.
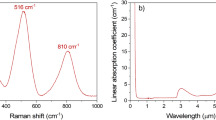
Figure 2 shows the normalized Raman spectra of the glass samples. For this measurement, a glass with 0 mol% WO3 is prepared to observe the effect of WO3 addition. Some changes take place in the glass structure as the WO3 content increases. The emergence of a band at 913 cm−1 is attributed to symmetric stretching vibrations of terminal W–O bonds (W–O− or W=O bonded species)30,31. The intensity increase in this band may be inferred as the addition of a higher amount of WO3 into the glass structure. Additionally, it can be seen that the intensity of the band at 413 cm−1 decreases while the one at 352 cm−1 improves as the WO3 content of the glasses increases. The band at 413 cm−1 is attributed to the symmetric vibrations of Ge–O bonds and the band at 352 cm−1 is associated with bending vibrations of W–O terminal bonds in WO6 octahedra32,33. The former band shrinks, whereas the latter grows as the WO3 content of the glass becomes greater. Due to GeO2 being the host element, the bridging oxygens (BO) are formed by O–Ge–O or Ge–O, but, when the WO3 content increases, the amount of BO decreases and non-bridging-oxygens (NBO) appears and increases with the WO3 content. The opposite change in the bands at 413 cm−1 and 352 cm−1 might be inferred as the breaking of Ge–O bonds and creation of W–O bonds. The increase in the NBOs quantity would increase the glass polarizability; thus, increase the refractive index as well34,35. This effect of the NBOs might confirm the glass density and polarizability increase in Tables 1 and 2. Moreover, the band at 800 cm−1 is due to the antisymmetric stretching vibrations of Ge–O–Ge bonds36. The appearance of a new band at 830 cm−1 is claimed to be due to W–O–W bonds31. Finally, the band at 145 cm−1 is attributed to the vibrations involving bismuth37.
Linear absorption coefficient spectra of the glasses are shown in Fig. 3. The absorption peaks at 451, 488, 521, 543, 653, 797, 977, and 1531 nm shown in Fig. 3 are due to the 4f–4f transitions of Er3+ ions, which are 4F3/2 + 4F5/2, 4F7/2, 2H11/2, 4S3/2, 4F9/2, 4I9/2, 4I11/2, and 4I13/2, respectively. From the inset of Fig. 3, it is shown that the UV absorption edge of the glasses exhibit a red shift of 30 nm because of the addition of tungsten since the tungsten atoms absorb the blue light38. Additionally, Fig. 3. reveals an absorption band at around 3000 nm, which is caused by the OH− present in the glasses. Furthermore, Fig. 3 shows that the glass samples reach their MIR transmission limit near 6000 nm. With the increase in the WO3 content, this MIR limit experience a blue shift which might be interpreted as that phonon energies of the glasses increase39.
The normalized Er3+ emission spectra of the prepared glasses excited at 200 and 250 mW laser power are presented in Fig. 4. These spectra in Fig. 4a,b express the emission broadening obtained by increasing the number of tungsten atoms in the glass matrix. By enhancing the number of excitation sites, the tungsten addition might have helped more electrons to be promoted on the different energy sublevels (due to the Stark effect) of the 4I13/2 of the Er3+ ions. The resulting emission spectra broadening is inhomogeneous and depends on the crystal field in different sites, hence, different transition probabilities can be produced with the increase in the tungsten content in the glass. Full width at half maximum (FWHM) values show an increase from 52 to 61 nm for 200 mW and from 70 to 80 nm for 250 mW pumping power. Additionally, the FWHM values for the same pumping power increase because of the higher amount of tungsten, which has a high electronegativity and low optical basicity, decreasing the bond covalency between the Er3+ and oxide ions which causes the Er3+ emission spectrum to broaden4. Figure 4a,b also reveal that the emission spectra broaden when the samples are excited by a higher laser power, which is not related to the sample reabsorption.
In contrast to the other oxide glass precursors, germanium oxide is not a conditional glass former. Nevertheless, no metallic germanium is present in our glasses, as it is only present in oxide form. Hence, no luminescence from such germanium form is observed. Therefore, the Er3+ emission band in the NIR is unaffected by germanium.
It is important to mention that the energy transfer from Bi3+ ions to Er3+ ions was discussed in the literature before40,41. However, this transfer takes place when the Bi3+ ions are excited in the UV range at around 340 nm. Here, the glasses were excited with a 980 nm and such excitation wavelength is outside of Bi3+ ions energy levels. Thus, an energy transfer process from Bi3+ ions to Er3+ ions is not expected. This is confirmed in the emission spectra, Fig. 4.
The Er3+ lifetime values were 4.06, 4.02, 3.79, 3.68 and 3.62 ms for the samples 5W, 10W, 15W, 20W, and 25W, respectively. In all samples, one exponential decay is observed. The decay curves are displayed in the inset of Supplementary Fig. S2 of Supplementary Material. Additionally, it is demonstrated that the radiative lifetime decreases with the increasing tungsten atoms in the glass network, which increases the refractive index of the glasses as well (Fig. 1)42. Also, the intensity of emission is related to the radiative transition probabilities and it is inversely related with the lifetime which are also true for these samples43.
By using the linear absorption coefficients of Er3+ ions, absorption and emission cross-section spectra are calculated by the McCumber (MC) theory which are shown in Fig. 5. This theory uses the linear absorption coefficients of Er3+ ions for the emission cross-section approximation as in the formula: \({\sigma }_{ems}\left(v\right)={\sigma }_{abs}\left(v\right)\mathrm{exp }(\frac{hv-{E}_{0}}{{k}_{B}T})\) where \({\sigma }_{ems}\left(v\right)\) is the emission cross-section, \({\sigma }_{abs}\left(v\right)\) is the absorption cross-section, \(h\) is the Planck constant, \(v\) is the optical frequency, \({k}_{B}\) is the Boltzmann constant and \(T\) is temperature44. \({E}_{0}\) can be calculated from the energies of the single Stark levels. Figure 5 shows that the absorption and emission cross-sections of the Er3+ ions improved with increasing WO3 content from 5 to 25 mol%. In addition, the emission spectra of Er3+ ions broaden with the incorporation of more WO3.
The maximum values obtained from the emission cross-sections are 7.4 \(\times\) 10–21 cm2 and 7.7 \(\times\) 10–21 cm2 for 5W and 10W, respectively. These values and others from different glass systems are shown in Table 2. The emission cross-sections of 5W and 10W are similar to the other bismuth-germanate, lead-germanate and tellurite glasses, whereas they are higher than silicate glasses. A similar comparison can be made from Table 10 of Digonnet’s work45. To provide high gain to fabricate fiber amplifier, the emission cross-section is required to be as high as possible; therefore, bismuth-germanate glasses are a good candidate for such applications. Furthermore, Judd–Ofelt intensity parameters are calculated from the Judd–Ofelt theory46,47 and compared with other glass families in Table 2 as well. The Ω2 parameter describes the covalency between the REIs and the surrounding oxygen ions. The Ω2 values of 5W and 10W are greater than other germanate glasses, silicates and tellurites, which implies that these samples have much weaker covalency than other glasses which is obtained by tungsten addition as explained before. The Ω4 and Ω6 parameters exhibit the covalency of the glass matrix. Moreover, the Ω6 is shown to be the most important Judd–Ofelt parameter to increase the line strength of the electric dipole and the spontaneous emission probability19. It can be seen that the Ω6 is improved by increasing the WO3 content from 5 to 10 mol%. Therefore, samples 5W and 10W are capable of yielding a broader emission bandwidth than lead-germanate glasses.
After that, the gain spectra are calculated from the results of the MC theory, which the results for the samples 5W and 10W are shown in Fig. 6 since they are the candidates for fiber fabrication due to their ΔT being greater than 100 °C. However, the results of the other samples are quite similar. The gain spectra are calculated from \(Gain=[{p\times \sigma }_{ems}\left(v\right)-(1-p)\times {\sigma }_{abs}\left(v\right)]\) where \(p\) is the percentage of Er3+ ions at the excited level (the population inversion ratio between the 4I13/2 and 4I15/2 energy levels). From Fig. 6a,b, it is exhibited that at least 30% of the Er3+ ions should be at the excited state level to obtain gain in the L-band. For greater population inversion, gain becomes positive for the L-band spreading from 1565 to 1640 nm.
The emission cross-sections are also calculated by using the Füchtbauer–Ladenburg (FL) theory, which approximates the emission cross-sections from the Er3+ emission spectra by the formula \({\sigma }_{ems}\left(\lambda \right)=\frac{{\lambda }^{4}}{8\pi c{n}^{2}\tau }\frac{I(\lambda )}{\int I\left(\lambda \right) d\lambda }\) where \(\lambda\) is the wavelength, \(I\) is the emission intensity (see Fig. 4a,b), \(c\) is the vacuum velocity of light, \(n\) is the refractive index and \(\tau\) is the lifetime53. The emission cross-section spectra for the prepared samples by the FL theory are given in Fig. 7a. Namely, the \({\sigma }_{ems}\left(\lambda \right)\) can be determined from the line shape function and the intensity of the emission band “\(I(\lambda )\)”, as well as the radiative lifetime of the upper manifold and the refraction index. It is illustrated that no significant changes occur in the on the line shape of the Er3+ emission cross-sections as the WO3 content increases. It is important to mention that the maximum value of emission cross-section for the sample 5W obtained in Fig. 7a is 7.2 \(\times\) 10–21 cm2, which is slightly less than obtained from the MC theory (7.4 \(\times\) 10–21 cm2) in Fig. 5 under the same conditions. The gain spectra of the samples 5W and 10W at laser excitation power of 200 mW by the FL theory are shown in Fig. 7b,c. Similar to the emission spectra shape in Fig. 4, the gain spectra shape by the FL theory is significantly dependent on the laser power that might be attributed to the inhomogeneous broadening in which both laser power and amplifier efficiencies will be lower. Additionally, the line width of an amplifier is also affected due to the saturation only at a specific wavelength which will distort the gain spectrum45. Therefore, the dependence of the gain spectra shape by the FL theory on the laser power is an expected consequence of a gain system dominated by inhomogeneous broadening. The emission cross-section and the gain calculations for the samples 5W and 10W at 250 mW excitation power by the FL theory are demonstrated in Supplementary Fig. S3 of the Supplementary Material. However, the achieved values for the gain and gain bandwidth are similar to the ones reported in the literature54,55. Also, the FL theory shows that at least 30% of the Er3+ ions should be at the excited state level to obtain a gain signal in the L-band in Fig. 7b,c, which is similar to the result obtained by the MC theory when the samples are excited at 200 mW in both theories. Nevertheless, the obtained maximum gain values employing the MC theory are slightly larger, but the central peak does not show any change, i.e., a good agreement is observed between the two theories.
The MC theory is often used to predict the emission cross-section of the 1.53 μm electronic transition of Er3+-doped bulk glasses from the transitions of measured absorption spectrum. Furthermore, with any modification or not, it is generally employed to determine the emission cross-section in EDFAs. In this frame, FL theory is occasionally employed to predict the emission cross-section in Er3+-doped bulk glasses. A possible explanation for such fact is that MC theory is easy to determine since it just depends experimentally on the absorption spectrum. However, FL theory depends on the measurements of glass refractive index, emission intensity and Er3+ lifetime, which could result in a poor approximation of the emission cross-section unless carefully done. Besides, such measurements are uncommon in optical fibers; therefore, it is easy to apply the MC theory to determine the emission cross-section.
The comparison of the Er3+ emission cross-sections calculated from the MC theory and FL theory at 200 and 250 mW for the sample 5W is shown in Fig. 8a. The FL theory yields a broader spectrum than the MC theory. Such a difference can be related to the fact that MC theory employs the absorption spectra, which are measured with the beam of the transmission spectrometer lamp, i.e., without pumping power control, which can produce different absorption probabilities. Hence, these effects are not considered in the calculations of the MC theory. However, it should be noted that the peak intensities do not change at higher laser power according to the FL theory. Moreover, the MC theory results in larger emission cross-section values at longer wavelengths due to the presence of the factor that multiplies the absorption cross-section in its formula, also called reciprocity relation, which might overestimate the emission cross-section after the highest intensity peak56. Additionally, the areas under the emission cross-sections of all samples by both theories are compared in Fig. 8b. The emission cross-section areas increase with the WO3 incorporation according to the MC theory, but the FL theory suggests the emission cross-section areas experience a very little increase. Similar results are observed also in the emission spectrum in Fig. 4. Furthermore, FL theory exhibits that the emission cross-section areas increase with the laser power. Finally, Fig. 8c shows that the gain spectra calculated by both theories for 5W when there is 50% population inversion differ significantly. There is a higher gain at higher laser power by the FL theory and the MC theory yields lower gain at shorter wavelengths but higher gain at longer wavelengths.
Conclusion
Five GeO2–Bi2O3–WO3 glasses doped with Er2O3 are prepared with varying WO3 content. No examples of glasses with this composition are found in the literature review. The thermal characterizations reveal that the samples with 5 and 10 mol% WO3 can have good thermal stabilities for fiber fabrication to be used in the L-band fiber amplifier applications. Additionally, it is shown that the Er3+ emission bandwidth broadens, absorption and emission cross-sections improve with the increase of tungsten atoms in the glass matrix whereas the Er3+ ion lifetime decreases. The emission cross-section values of the bismuth-germanate glasses in this study are similar to the tellurite glasses but larger than silica glasses reported in the literature. Moreover, the gain spectra by the MC and FL theories are calculated and 30% population inversion is found to be enough to get gain signal in the L-band. Furthermore, a comparison of both theories, which are widely used in the calculation of Er3+ emission cross-section, are made. The FL theory claims higher emission cross-section intensity and gain signal at shorter wavelengths, whereas the MC theory provides greater values for longer wavelengths. Also, the emission cross-section areas have similar values for all samples by the FL theory; however, they increase with the tungsten content by the MC theory. Therefore, bismuth-germanate glasses with 5 and 10 mol% of WO3 open new opportunities for glass photonics, which could be used in integrated optics with well-developed silicon technologies and in EDFA applications.
Experimental methods
The used precursors for glass preparation are Germanium oxide (Adv. Materials Tech. Co., 99.999% 5N), Bismuth (III) oxide (Strem Chemicals, 99.9%-Bi), Tungsten (VI) oxide (Alfa Aesar, 99.8% (metals basis)), Antimony (III) oxide (Sigma-Aldrich, 99%), and Erbium (III) oxide (Strem Chemicals, 99.995%-Er). The precursors were melted in alumina crucibles at 1200 °C in ambient atmosphere for 45 min and cast at 1100 °C into stainless steel molds which were preheated at 460 °C. After the casting, the glasses were annealed at 460 °C for 2 h. Finally, the glasses were optically polished on both parallel sides. The fabricated samples have the composition of 97.5[(75 − x)GeO2–25Bi2O3–(x)WO3]–2Sb2O3–0.5Er2O3 where x = 5, 10, 15, 20, and 25 mol%. The labels of the samples are “5W”, “10W”, “15W”, “20W”, and “25W” concerning their WO3 content.
The glass with 5 mol% WO3 was fabricated with higher amount of erbium content up to 2 mol% Er2O3. However, the glass composition with 0.5 mol% Er2O3 concentration presents the best trade-off between glass stability and high concentration of Er2O3. Above 25 mol% of WO3, some crystallization spots were observed during the casting. As these glasses would be used for fiber drawing, the glass must be stable enough to be casted into a preform. Hence, we do not consider adding glasses with higher amount of WO3 considering the focus of this work. Sb2O3 was used to limit the reduction of bismuth and tungsten ions during the glass synthesis57. The glass with 5 mol% WO3 was fabricated with up to 8 mol% Sb2O3 in our preliminary study and the spectroscopic properties of Er3+ ions was not affected by higher concentration of Sb2O3. Therefore, this concentration of antimony oxide was demonstrated to be sufficient for this purpose.
The characteristic temperatures of the glasses, glass transition (Tg) and crystallization onset (Tx) temperatures were determined by differential scanning calorimetry (DSC) measurements, which were performed in a Pt/Rh crucible using a Netzsch DSC 404 F3 Pegasus apparatus at a heating rate of 10 °C min−1, from 25 to 800 °C. The glass thermal stability is determined as, \(\Delta T={T}_{x}-{T}_{g}\).
Glass densities were measured by an Alfa Mirage MD-300S electronic densimeter using Archimedes’ principle. The Er3+ ion concentrations were calculated from: \({Er}^{3+}=M\times \rho \times x/N\) where \(M\) is the molecular weight of the glass, \(\rho\) is the glass density, \(x\) is the Er2O3 mol% and \(N\) is Avogadro’s number58.
The refractive indices of the glasses were measured by a prism coupling technique (Metricon 2010) at five different wavelengths 532, 633, 972, 1308 and 1538 nm with TE polarization.
The transmission spectra in the UV–Vis-NIR region were measured by Cary 60 and Cary 5000 spectrometers and in the MIR region by a Frontier FTIR spectrometer. The Raman spectra of the samples were recorded with a Renishaw in Via™ confocal Raman microscope under laser excitation at 785 nm.
The luminescence measurements were carried out with a diode laser at 980 nm with 200 and 250 mW power pumping. The luminescence light is detected with a Jobin–Yvon Nanolog spectrometer coupled with a nitrogen-cooled InGaAs detector. The Er3+ lifetime of the 4I13/2 → 4I15/2 radiative decay was measured by exciting the samples with an OPO at 980 nm with a frequency of 10 Hz and an average power of 30 mW per pulse. The signal was collected with an Edinburgh FLS1000 spectrometer connected to a Tektronix MDO 3022/200 MHz oscilloscope.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Barnes, W. L., Laming, R. I., Tarbox, E. J. & Morkel, P. R. Absorption and emission cross section of Er3+ doped silica fibers. IEEE J. Quantum Electron. 27(4), 1004–1010. https://doi.org/10.1109/3.83335 (1991).
Quimby, R. S., Miniscalco, W. J. & Thompson, B. Clustering in erbium-doped silica glass fibers analyzed using 980 nm excited-state absorption. J. Appl. Phys. 76(8), 4472–4478. https://doi.org/10.1063/1.357278 (1994).
Carvalho, D. O., Kassab, L. R. P., Del Cacho, V. D., da Silva, D. M. & Alayo, M. I. A review on pedestal waveguides for low loss optical guiding, optical amplifiers and nonlinear optics applications. J. Lumin. 203, 135–144. https://doi.org/10.1016/j.jlumin.2018.06.037 (2018).
Tanabe, S., Sugimoto, N., Ito, S. & Hanada, T. Broad-band 1.5 μm emission of Er3+ ions in Bismuth-based oxide glasses for potential WDM amplifier. J. Lumin. 87–89, 670–672. https://doi.org/10.1016/S0022-2313(99)00352-X (2000).
Zhang, Y. et al. Optical properties of Er3+/Yb3+ co-doped phosphate glass system for NIR lasers and fiber amplifiers. Ceram. Int. 44(18), 22467–22472. https://doi.org/10.1016/j.ceramint.2018.09.015 (2018).
Yuan, J. et al. Enhanced 1.5 µm emission from Yb3+/Er3+ codoped tungsten tellurite glasses for broadband near-infrared optical fiber amplifiers and tunable fiber lasers. RSC Adv. 11(45), 27992–27999. https://doi.org/10.1039/D1RA05269A (2021).
Pinto, I. C. et al. Novel insights on energy transfer processes in [Ce4+/Ce3+]-Er3+-doped tellurite glass. Ceram. Int. https://doi.org/10.1016/j.ceramint.2022.10.156 (2022).
Kassab, L. R. P. et al. Fabrication and characterization of Er3+-doped GeO2–PbO and GeO2–PbO–Bi2O3 glass fibers. J. Non-Cryst. Solids 352(32–35), 3530–3534. https://doi.org/10.1016/j.jnoncrysol.2006.03.086 (2006).
Kassab, L. R. P., Hora, W. G., Lozano, B. W., de Oliveira, M. A. S. & Maciel, G. S. Optical properties of Er3+ doped GeO2–PbO glass: Effect of doping with Bi2O3. Opt. Commun. 269(2), 356–361. https://doi.org/10.1016/j.optcom.2006.08.014 (2007).
Yang, G. F., Zhang, Q. Y., Li, T., Shi, D. M. & Jiang, Z. H. Laser-diode-excited intense luminescence and green-upconversion in erbium-doped bismuth–germanate–lead glasses. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 69(1), 41–48. https://doi.org/10.1016/j.saa.2007.03.006 (2008).
Wells, A. Structural Inorganic Chemistry (Oxford Classic Texts in the Physical Sciences; Oxford University Press, 2012).
Boivin, M. et al. Germanate-tellurite composite fibers with a high-contrast step-index design for nonlinear applications. Opt. Mater. Express 4(8), 1740–1746. https://doi.org/10.1364/OME.4.001740 (2014).
Silva, O. B., Rivera, V. A. G., Ledemi, Y., Messaddeq, Y. & Marega, E. Germanium concentration effects on the visible emission properties of Er3+ in tellurite glasses. J. Lumin. 232, 117808. https://doi.org/10.1016/j.jlumin.2020.117808 (2021).
Wetter, N. U., da Silva, D. S., Kassab, L. R. P. & Jimenez-Villar, E. Improving performance in ytterbium-erbium doped waveguide amplifiers through scattering by large silicon nanostructures. J. Alloys Compd. 794, 120–126. https://doi.org/10.1016/j.jallcom.2019.04.141 (2019).
Zhou, Y., Gai, N., Wang, J., Chen, F. & Yang, G. Effect of Ce3+(Eu3+) codoping on the spectroscopic properties of Er3+ in bismuth-germanate glass. Opt. Mater. 31(11), 1595–1599. https://doi.org/10.1016/j.optmat.2009.03.007 (2009).
Zhou, Y., Wang, S., Lin, J., Ye, M. & Yang, G. Effect of Ce3+ codoping on Er3+-doped bismuth-germanate glass and fiber under 980 nm excitation. Opt. Commun. 284(9), 2312–2316. https://doi.org/10.1016/j.optcom.2011.01.012 (2011).
Prakash, M. R. et al. Optical and radiative properties of Sm3+ ions activated alkali-bismuth-germanate glasses. J. Lumin. 214, 116566. https://doi.org/10.1016/j.jlumin.2019.116566 (2019).
Gökçe, M. Development of Eu3+ doped bismuth germanate glasses for red laser applications. J. Non-Cryst. Solids 505, 272–278. https://doi.org/10.1016/j.jnoncrysol.2018.11.011 (2019).
Sugimoto, N. & Tanabe, S. Correlation between emission bandwidth of Er3+: 1.5 µm band and optical basicity of host oxide glasses. J. Ceram. Soc. Jpn. 113(1313), 120–122. https://doi.org/10.2109/jcersj.113.120 (2005).
Yong, G. C. & Heo, J. 1.3 µm emission and multiphonon relaxation phenomena in PbO–Bi2O3–Ga2O3 glasses doped with rare-earths. J. Non-Cryst. Solids 217(2), 199–207. https://doi.org/10.1016/S0022-3093(97)00189-0 (1997).
Peacock, R. D. The intensities of lanthanide f ↔ f transitions. In Rare Earths Structure and Bonding, vol. 22, 83–122 (eds. Nieboer, E., Jørgensen, C. K., Peacock, R. D., Reisfeld, R.) (Springer, 1975). https://doi.org/10.1007/BFb0116556.
Tanabe, S. Optical transitions of rare earth ions for amplifiers: How the local structure works in glass. J. Non-Cryst. Solids 259(1), 1–9. https://doi.org/10.1016/S0022-3093(99)00490-1 (1999).
Tanabe, S., Ohyagi, T., Soga, N. & Hanada, T. Compositional dependence of Judd–Ofelt parameters of Er3+ ions in alkali-metal borate glasses. Phys. Rev. B 46(6), 3305–3310. https://doi.org/10.1103/PhysRevB.46.3305 (1992).
Tanabe, S. Rare-earth-doped glasses for fiber amplifiers in broadband telecommunication. Comptes Rendus Chim. 5(12), 815–824. https://doi.org/10.1016/S1631-0748(02)01449-2 (2002).
Rivera, V. A. G. & Barbosa, L. C. Spectroscopic properties of Er3+-doped sodium-modified tellurite glasses for use as optical amplifiers at 1540 nm. J. Lumin. 156, 116–123. https://doi.org/10.1016/j.jlumin.2014.07.023 (2014).
Ryazanov, M. I. & Tishchenko, A. A. Clausius–Mossotti–type relation for planar monolayers. J. Exp. Theor. Phys. 103(4), 539–545. https://doi.org/10.1134/S1063776106100049 (2006).
Drexhage, M. G. et al. Preparation and properties of heavy-metal fluoride glasses containing ytterbium or lutetium. J. Am. Ceram. Soc. 65(10), c168–c171. https://doi.org/10.1111/j.1151-2916.1982.tb10353.x (1982).
Sellmeier. Zur Erklärung Der Abnormen Farbenfolge Im Spectrum Einiger Substanzen. Ann. Phys. 219(6), 272–282 (1871). https://doi.org/10.1002/andp.18712190612.
Tatian, B. Fitting refractive-index data with the sellmeier dispersion formula. Appl. Opt. 23(24), 4477–4485. https://doi.org/10.1364/AO.23.004477 (1984).
Poirier, G., Messaddeq, Y., Ribeiro, S. J. L. & Poulain, M. Structural study of tungstate fluorophosphate glasses by raman and X-ray absorption spectroscopy. J. Solid State Chem. 178(5), 1533–1538. https://doi.org/10.1016/j.jssc.2004.10.032 (2005).
de Araujo, C. C. et al. Structural studies of NaPO3–WO3 glasses by solid state NMR and Raman spectroscopy. J. Mater. Chem. 16(32), 3277–3284. https://doi.org/10.1039/B605971F (2006).
Cozar, O., Magdas, D. A., Nasdala, L., Ardelean, I. & Damian, G. Raman spectroscopic study of some lead phosphate glasses with tungsten ions. J. Non-Cryst. Solids 352(28), 3121–3125. https://doi.org/10.1016/j.jnoncrysol.2006.03.066 (2006).
Sharma, S. K., Matson, D. W., Philpotts, J. A. & Roush, T. L. Raman study of the structure of glasses along the join SiO2-GeO2. J. Non-Cryst. Solids 68(1), 99–114. https://doi.org/10.1016/0022-3093(84)90037-1 (1984).
Duffy, J. A. The electronic polarisability of oxygen in glass and the effect of composition. J. Non-Cryst. Solids 297(2), 275–284. https://doi.org/10.1016/S0022-3093(01)00940-1 (2002).
Lanagan, M. T. et al. Dielectric polarizability of alkali and alkaline-earth modified silicate glasses at microwave frequency. Appl. Phys. Lett. 116(22), 222902. https://doi.org/10.1063/5.0008646 (2020).
Alvarado-Rivera, J. et al. Effect of CeO2 on the glass structure of sodium germanate glasses. J. Am. Ceram. Soc. 97(11), 3494–3500. https://doi.org/10.1111/jace.13202 (2014).
Beneventi, P. et al. Raman study of Bi2O3–GeO2–SiO2 glasses. J. Non-Cryst. Solids 192–193, 258–262. https://doi.org/10.1016/0022-3093(95)00360-6 (1995).
Schieder, M. et al. Hierarchically porous tungsten oxide nanotubes with crystalline walls made of the metastable orthorhombic polymorph. J. Mater. Chem. A 1(2), 381–387. https://doi.org/10.1039/C2TA00169A (2012).
Harrison, A. J. Water content and infrared transmission of simple glasses. J. Am. Ceram. Soc. 30(12), 362–366. https://doi.org/10.1111/j.1151-2916.1947.tb18868.x (1947).
Xu, Q., Lin, B. & Mao, Y. Photoluminescence characteristics of energy transfer between Er3+ and Bi3+ in Gd2O3: Er3+, Bi3+. J. Lumin. 128(12), 1965–1968. https://doi.org/10.1016/j.jlumin.2008.06.007 (2008).
Talewar, R. A., Mahamuda, S. K., Rao, A. S. & Moharil, S. V. Intense infrared emission of Er3+ in ZnB2O4 phosphors from energy transfer of Bi3+ by broadband UV excitation. J. Lumin. 244, 118706. https://doi.org/10.1016/j.jlumin.2021.118706 (2022).
Herrmann, A., Tewelde, M., Kuhn, S., Tiegel, M. & Rüssel, C. The effect of glass composition on the luminescence properties of Sm3+ doped alumino silicate glasses. J. Non-Cryst. Solids 502, 190–197. https://doi.org/10.1016/j.jnoncrysol.2018.09.008 (2018).
Reisfeld, R. & Eckstein, Y. Radiative and non-radiative transition probabilities and quantum yields for excited states of Er3+ in germanate and tellurite glasses. J. Non-Cryst. Solids 15(1), 125–140. https://doi.org/10.1016/0022-3093(74)90117-3 (1974).
McCumber, D. E. Einstein relations connecting broadband emission and absorption spectra. Phys. Rev. 136(4A), A954–A957. https://doi.org/10.1103/PhysRev.136.A954 (1964).
Rare-Earth-Doped Fiber Lasers and Amplifiers, Revised and Expanded, 2nd ed. (ed. Digonnet, M. J. F.) (CRC Press, 2001).
Judd, B. R. Optical absorption intensities of rare-earth ions. Phys. Rev. 127(3), 750–761. https://doi.org/10.1103/PhysRev.127.750 (1962).
Ofelt, G. S. Intensities of crystal spectra of rare-earth ions. J. Chem. Phys. 37(3), 511–520. https://doi.org/10.1063/1.1701366 (1962).
Kassab, L. R. P., de Oliveira Preto, A., Lozano, W., de Sá, F. X. & Maciel, G. S. Optical properties and infrared-to-visible upconversion in Er3+-doped GeO2–Bi2O3 and GeO2–PbO–Bi2O3 Glasses. J. Non-Cryst. Solids 351(43–45), 3468–3475. https://doi.org/10.1016/j.jnoncrysol.2005.09.013 (2005).
Ono, S. & Tanabe, S. Evidence of enhanced hypersensitive transition in erbium-doped fibers with different Al2O3 content. IEEE J. Quantum Electron. 40(12), 1704–1708. https://doi.org/10.1109/JQE.2004.837665 (2004).
Jlassi, I., Elhouichet, H., Ferid, M. & Barthou, C. Judd–Ofelt analysis and improvement of thermal and optical properties of tellurite glasses by adding P2O5. J. Lumin. 130(12), 2394–2401. https://doi.org/10.1016/j.jlumin.2010.07.026 (2010).
Zhang, L., Hu, H. & Lin, F. Emission properties of highly doped Er3+ fluoroaluminate glass. Mater. Lett. 47(4–5), 189–193. https://doi.org/10.1016/S0167-577X(00)00233-0 (2001).
Wetenkamp, L., West, G. F. & Többen, H. Optical properties of rare earth-doped ZBLAN glasses. J. Non-Cryst. Solids 140, 35–40. https://doi.org/10.1016/S0022-3093(05)80737-9 (1992).
Moulton, P. F. Spectroscopic and laser characteristics of Ti:Al2O3. JOSA B 3(1), 125–133. https://doi.org/10.1364/JOSAB.3.000125 (1986).
Song, X., Jin, D., Zhou, D., Xu, P. & Han, K. Er3+/Yb3+ co-doped bismuthate glass and its large-mode-area double-cladding fiber for 1.53 µm laser. J. Alloys Compd. 853, 157305. https://doi.org/10.1016/j.jallcom.2020.157305 (2021).
Ragin, T., Czajkowski, K., Żmojda, J., Kochanowicz, M., Dorosz, D. Investigation of thermal and spectroscopic properties of Bi2O3–GeO2–Ga2O3–Na2O glasses doped with Er3+ ions (eds. Romaniuk, R. S.) 92900Q (Warsaw, 2014). https://doi.org/10.1117/12.2075861.
Digonnet, M. J. F., Murphy-Chutorian, E. & Falquier, D. G. Fundamental limitations of the McCumber relation applied to Er-doped silica and other amorphous-host lasers. IEEE J. Quantum Electron. 38(12), 1629–1637. https://doi.org/10.1109/JQE.2002.805111 (2002).
Winterstein, A., Manning, S., Ebendorff-Heidepriem, H. & Wondraczek, L. Luminescence from Bismuth–Germanate Glasses and Its Manipulation through Oxidants. Opt. Mater. Express 2(10), 1320. https://doi.org/10.1364/OME.2.001320 (2012).
Rivera, V. A. G., El-Amraoui, M., Ledemi, Y., Messaddeq, Y. & Marega, E. Expanding broadband emission in the near-IR via energy transfer between Er3+–Tm3+ Co-doped tellurite-glasses. J. Lumin. 145, 787–792. https://doi.org/10.1016/j.jlumin.2013.08.071 (2014).
Acknowledgements
We thank Iago Carvalho Pinto, Gabriel Toshiaki Tayama, Wagner Correr and Nathaly Colmenares for their experimental help and their discussions. This research was funded by the Natural Sciences and Engineering Research Council (NSERC) of Canada through a partnership grant CRDPJ 538379-18.
Author information
Authors and Affiliations
Contributions
H.C.Ç. carried out the experiments and calculations, wrote the manuscript and prepared the figures. T.G. and V.A.G.R. assisted experiments and helped with the research concept and the manuscript. R.F.F. helped with the research concept and the calculations. S.L. and Y.M. led the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Çamiçi, H.C., Guérineau, T., Rivera, V.A.G. et al. The role of tungsten oxide in Er3+-doped bismuth-germanate glasses for optical amplification in L-band. Sci Rep 13, 8835 (2023). https://doi.org/10.1038/s41598-023-35995-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-35995-8
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.