Abstract
At least 65 million people suffer from long COVID. Treatment guidelines are unclear, especially pertaining to recommendations of increased activity. This longitudinal study evaluated safety, changes in functional level and sick leave following a concentrated rehabilitation program for patients with long COVID. Seventy-eight patients (19–67 years) participated in a 3-day micro-choice based rehabilitation program with 7-day and 3-month follow-up. Fatigue, functional levels, sick leave, dyspnea and exercise capacity were assessed. No adverse events were reported and 97.4% completed the rehabilitation. Fatigue measured with Chalder Fatigue Questionnaire decreased at 7-days [mean difference (MD = − 4.5, 95% CI − 5.5 to − 3.4) and 3-month (MD = − 5.5, 95% CI − 6.7 to − 4.3). Sick leave rates and dyspnea were reduced (p < 0.001) and exercise capacity and functional level increased (p < 0.001) at 3-month follow-up regardless of severity of fatigue at baseline. Micro-choice based concentrated rehabilitation for patients with long COVID was safe, highly acceptable and showed rapid improvements in fatigue and functional levels, sustaining over time. Even though this is a quasi-experimental study, the findings are of importance addressing the tremendous challenges of disability due to long COVID. Our results are also highly relevant for patients, as they provide the base for an optimistic outlook and evidence supported reason for hope.
Similar content being viewed by others
Introduction
Following the SARS-CoV-2 pandemic, we are now hit hard by a new wave, namely long COVID (post COVID-19 condition)1,2. Months or years after the infection up to 50% of patients face persistent symptoms, with fatigue as one of the most commonly reported, impacting their level of functioning, quality of life and ability to work1,3,4,5. Young adults as well as non-hospitalized patients are also affected1,5,6,7, resulting in huge societal costs8.
Worldwide, health care providers and researchers are scrambling for ways to help these patients in getting back their ordinary lives. Several studies on rehabilitation have been published9,10,11,12,13,14,15,16,17, however, no consensus or “gold standard” for treatment recommendations exists18. One cross-sectional study19 which was given substantial weight in a recently published review article18, even indicated that only 1% of patients with long COVID benefited from increased physical activity, and this study also reported that for 75% of the patients, physical activity worsened the symptoms19. Given this pessimistic, although, insufficient scientifically founded perspective, there is an urgent need to share research that might give nuances to this grim picture.
The group behind this study has developed a comprehensive concentrated interdisciplinary group rehabilitation program for long COVID, which is described in detail in the protocol paper20. One of its main features is a shift in focus from targeting symptoms to targeting and monitoring seemingly insignificant micro-choices that facilitate increased flexibility and levels of functioning. The approach has shown effectiveness across a number of different chronic health challenges21,22,23,24.
The aims of the study were to assess safety, acceptability, potential changes in fatigue, sick leave, functional level, dyspnea, and exercise capacity from pre-treatment to 3 months follow-up and to explore predictors for change in fatigue.
Methods
Study design and participants
This quasi-experimental study with 3-month follow-up is part of the “Project Development of Smarter Health Solutions” (PUSH project), Haukeland University Hospital (HUH: Bergen, Norway) and Helse i Hardanger (HiH: Øystese, Norway)20.
Patients were referred to the Department of Thoracic Medicine, HUH, by their general practitioner, or other physicians. Eligible patients were adults between 18 and 67 years, with long COVID, defined as confirmed SARS-CoV-2 infection and persisting symptoms at least 3 months from the onset of the infection leading to impaired everyday functioning which could not be explained by alternative diagnoses2. Patients who had improved from long COVID when waiting for treatment were excluded. Diseases where physical activity was not recommended was also reason for exclusion. Participants had to be fluent in oral and written Norwegian and have sufficient digital competence to handle online questionnaires.
Intervention
The clinical intervention consisted of three equally important phases (Fig. 1) and is outlined in a protocol paper for concentrated interdisciplinary group rehabilitation for patients with chronic illnesses20. Additionally, the specific questionnaires for fatigue and dyspnea, in addition to lung function and exercise measurements used in this study is not described in the generic protocol paper20.
Phase 1: Pre-treatment preparation
Standardized information about the intervention was given and physical examinations were done to exclude other medical conditions and to examine functional status (Fig. 1).
Phase 2: The concentrated micro-choice based rehabilitation
The core element in this out-patient rehabilitation, delivered in groups of 6–10 patients during three consecutive days (8.30–16.00), was a shift in focus, from targeting and monitoring symptoms, to focus on micro-choices in order to facilitate increased levels of physical activity and functioning (Fig. 1).
Phase 3: Integrating the changes into everyday living
The first 3 weeks after the concentrated rehabilitation, patients answered two questions digitally once a day (0–100) regarding strategies for handling symptoms: (1) To what extent did you allow the symptoms to decide today, and (2) to what extent did you make use of the principle of doing something else. An individual telephone consultation was conducted 10 days after the intervention (Fig. 1).
Measurements
An overview of the measurement tools and the respective assessment times are presented in Fig. 1.
Chalder Fatigue Scale (CFQ-11) was used to assess mental and physical fatigue25.
CFQ-11 was calculated with total score (0–33) and bimodally (0–11). The bimodal score provides a method for distinction between “cases” and “non-cases”26. A bimodal score of ≥ 4 was defined as a case of fatigue25,26. Severe fatigue was calculated as a bimodal score ≥ 4 and total score ≥ 236. Minimal clinically important difference (MCID) for total score is between 1.4 and 427.
Sick leave and % degree of sick leave were registered digitally by the participants.
The Work and Social Adjustment Scale (WSAS)28 was used to assess functional level. Total score ranges from 0 to 40, where higher scores indicate functional impairment. The MCID is a reduction of 3.6 points29. In addition a visual analogue scale (VAS) (0–100) was used30. Patients were asked “If 100 represents your functional level before COVID-19, what is your functional level today?”.
Modified Medical Research Council dyspnea scale (mMRC)31 and Dyspnea-12 were used to measure dyspnea32. A cut-off ≥ 1 for mMRC was used. MCID for Dyspnea-12 ranges between -3 and -6 points33.
Ongoing psychiatric symptoms and previous psychiatric illness were evaluated by a psychiatrist based on the clinical interview at baseline and information from medical records.
Spirometry was conducted on a Vyntus Body/APS Plethysmograph (Vyaire medical GmbH, Hochenberg, Germany) according to the American Thoracic Society/European Respiratory Society guidelines34.
Cardiopulmonary exercise test (CPET) was performed by uphill walking on a treadmill until exhaustion (Woodway, Würtzburg, Germany). Gas exchange and ventilatory variables were measured by breath-by-breath sampling averaged over 30 s intervals (Hans Rudolph two way breathing mask: V2 mask, Shawnee, USA). Heart rate was measured through a 12-lead electrocardiogram ECG (Custo Cardio 300, custo med, Ottobrun, Germany), and oxygen saturation (SpO2) with an ear probe using a stationary pulse oximeter (Xpod, Nonin, Minnesota, USA). Blood pressure was measured with Tango M2 (SunTech Medical, Morrisville, USA). The walking speed was set individually with an inclination at 0% at start with increased inclination every 60 s by 2% up to 20%. Thereafter the speed was increased by 0.5 km h−1 until exhaustion. Reasons for termination were pronounced pain or dizziness, ischaemic ECG changes or decreased systolic blood pressure below the resting pressure35. Dyspnea and leg fatigue were measured with Borg CR10 scale36. All assessments were measured at rest, throughout the test (Borg CR10 every second minute), at peak exercise and two minutes after termination of the test. Norwegian reference values for CPET variables were used37. Breathing reserve was calculated as maximal ventilatory limitation (MVV −peak ventilation \(\dot{V}\)Epeak/MVV × 100) using an estimate for MVV as forced expiratory volume in 1 s FEV1 × 4038. Ventilatory limitation was defined when breathing reserve < 15%38. Reduced exercise capacity was defined as peak oxygen uptake \(\dot{V}\)O2peak < 85% predicted38. CPET was considered maximal if respiratory exchange ratio RER ≥ 1.1.
Stair Climbing Test (SCT) was used to assess submaximal exercise capacity and 30-s sit-to-stand-test (30STST) to assess lower extremity strength39. MCID is not reported for SCT and 30STST for patients with long COVID.
Statistical analyses
Data were analysed with IBM SPSS Statistics version 28 (SPSS Inc., Chicago, USA) and Stata version 17 (StataCorp). Descriptive statistics were used to characterize the study population (mean, standard deviation (SD), median, and percent). Logistic mixed effect models were used to estimate change from pre-treatment to 3-month follow-up in sick leave, and linear mixed effect models to estimate change in CFQ-11, Dyspnea-12, functional level, WSAS, 30STST, SCT and \(\dot{V}\)O2peak kg−1. The regression models were fitted with random intercepts and random slopes for time. Assumptions were checked with diagnostic plots. To explore predictors for change in CFQ-11, interaction terms between time and gender, age, time since infection, psychiatric illness, functional level and \(\dot{V}\)O2peak kg−1, respectively, were included in the models. A global test was performed to assess statistically significant interaction terms at 7-days and 3-month follow-up. Paired samples t-tests were used to analyse the change in CPET variables, lung function and Wilcoxon signed-rank test to analyse change in mMRC. Normality of change-data was checked by histograms, QQ-plots and Shapiro–Wilk’s test. Estimated changes from baseline to follow-up are presented with 95% confidence intervals (CI) and p values. Statistical significance was set at α = 0.05.
The data were collected electronically and by physical examinations and were stored on an encrypted server at Western Norway Regional Health Authority IKT.
Ethical approval
The PUSH project and the specific study protocol20 for patients with long COVID included in the current study were approved by the Western Norway Regional Committees for Medical and Health Research Ethics (REK 2020/101648), and is registered in Clinical Trials (NCT05234281, approval date: 10/02/2022). Informed consent was obtained from all participants included in the study. All methods were performed in accordance with the relevant guidelines and regulations.
Results
Safety of the intervention and baseline characteristics
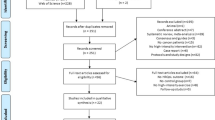
A total of 120 patients were assessed for eligibility to the intervention, of which 78 met the inclusion criteria (Fig. 2) and all accepted participation. The completion rate was 97.4% and no adverse events were reported (Fig. 2). Two patients (2.6%) reported slightly reduced levels of functioning at 7-days after the intervention, but at 3-month follow-up the functional level was improved. One patient (1.2%) reported reduced levels of functioning at 3-month. Table 1 summarises baseline characteristics. Mean duration of symptoms were 10.2 months, with fatigue (99%) and dyspnea (63%) as most frequently reported. Obesity (BMI > 30 kg m−2) was present in 16 (21%) participants, and 14 (18%) had been hospitalised during the acute infection, 3 (4%) needing intensive care treatment. For some of the measurements there were some missing data due to technical problems, pain or other symptoms that hampered completion of the physical tests or questionnaires in the mobile application. We report the exact number analysed for each measurement.
Changes in fatigue and predictors for change
Compared with baseline measures, mean CFQ-11 was reduced with 4.5 points (p < 0.001) at 7-days and 5.5 points at 3-month (p < 0.001) (Table 2). The reduction was larger than MCID. One patient reported an increased CFQ-11 larger than MCID at 7-days, however at 3-month symptoms of fatigue was clinically significant improved. Global tests of interactions showed that overall, the observed reduction in CFQ-11 was not modified by any of the investigated predictors (p ≥ 0.2). Accordingly, the decline in mean CFQ-11 was observed in participants irrespective of ongoing or previous psychiatric illness, or never experienced psychiatric illness (Fig. 3). Mean bimodal score was 8.8 (SD 2.2) at baseline, with reductions to 6.8 (SD 3.8) and 6.1 (SD 3.5) at 7-days and 3-month, respectively. At baseline, 61% of the patients had severe fatigue (Fig. 4). The proportion with severe fatigue was reduced to 26% at 7-days and 18% at 3-month follow-up, whereas 24% at 7-days and 23% at 3-month, did not meet the criteria for fatigue (Fig. 4).
Changes in fatigue, functional status, exercise capacity and dyspnea from baseline to 3-month follow up. (a) The unadjusted mean values are presented for patients with no, previous or ongoing psychiatric illness, respectively, and in (b–f) the unadjusted mean values are presented for patients with fatigue (a bimodal CFQ-11 score ≥ 4), and severe (bimodal CFQ-11 score ≥ 4 and total score ≥ 23). The four patients with no fatigue at baseline are included in the fatigue group. CFQ Chalder Fatigue Questionnaire, \(\dot{V}\)O2peak peak oxygen uptake, WSAS Work and Social Adjustment Scale.
Changes in sick leave and functional level
The proportion of employed participants on sick leave was reduced from 63% at baseline to 43% at 3-month (OR = 0.2, p = 0.02) (Table 2). Further, the mean degree of sick leave was reduced from 51 to 30%. The sick leave rate was reduced in those with fatigue from 42 to 22% (p = 0.019) and severe fatigue from 59 to 36% (p = 0.002) (not shown in table).
Self- reported functional level increased from 53% at baseline to 63% and 69% at 7-days and 3-month follow-up, respectively (p < 0.001) (Table 2). At baseline the mean WSAS score was 21.9 with a reduction of 6.9 at 3-month, which was larger than MCID (Table 2). The findings for functional level and WSAS for those with fatigue and severe fatigue were similar (Fig. 3).
Changes in dyspnea, lung function and exercise capacity
At baseline 69.7% of the participants scored ≥ 1 on mMRC while at 3-month follow-up this was reduced to 57.7% (p = 0.03) (Table 3). Dyspnea-12 total score was reduced significantly with 3.3 points which is within the range of MCID between 3 and 6 points at 3-month (Table 2). For the first patient group there were technical problems in the mobile application which resulted in missing data storage for 10 patients at baseline.
Lung function was within normal values at baseline and 3-month (Table 3).
At baseline the participants had a mean \(\dot{V}\)O2peak kg−1 of 92 (17) % of predicted value (Table 3), 41% had \(\dot{V}\)O2peak < 80% predicted. At 3-month this was reduced to 32%. \(\dot{V}\)O2peak kg−1 increased significantly (p = 0.002), and the group with very severe fatigue at baseline had a significantly larger improvement compared to the others (Fig. 3).
SCT and 30STST also showed significant improvement at 3-month follow-up (Table 2).
Discussion
A highly pessimistic picture has been painted for patients suffering from long COVID. The current paper present results that are in clear contrast to this: Following a 3-day, micro-choice based group intervention, the patients’ level of functioning increased significantly and there was a rapid, significant, and clinically important reduction in fatigue at 3-month follow-up, in addition to significantly reduced dyspnea and improved exercise capacity. Mean pre-treatment symptom duration was 10.2 months. There were no indications of post-exertional malaise or other adverse events.
These results are important, both for health care workers and for patients, given that the current treatment guidelines are unclear, especially with regards to recommendations of increased activity. Strikingly, one cross-sectional study indicated that less than 1% of long COVID victims benefited from physical activity, with detrimental effects seen in 75%19. In clear contrast, the results of our study showed rapid, consistent and highly relevant improvements in fatigue, physical functioning and work participation, with no harmful effects. The approach may thus represent one answer to the call for evidence supported treatments for long COVID18. At 3-month follow-up the improvements in our study were sustained, in addition to significantly reduced dyspnea and improved exercise capacity.
A rapid return to work, as shown in our study, can reduce societal costs substantially and increase the quality of life for the individuals. The results are in line with findings from concentrated treatment for other conditions21,22,23,24, and this further development of the concentrated treatment format may thus have implications for the way we provide rehabilitation for patients with long COVID.
Most participants in our study had not been hospitalized during the acute phase, making these results relevant for the majority of patients suffering from long COVID6,7. Furthermore, the study population is comparable to a cohort of home-isolated COVID-19 patients recruited from the same geographical area in the same period6,40. In this cohort, fatigue was significantly higher compared to healthy controls and was not reduced 12 months after the acute infection. In comparison, our participants reported a much higher fatigue at baseline yielding a higher proportion of fatigue (96% vs 30%) and severe fatigue (61% vs 7%)6, indicating that our patients were severely affected by long COVID and that spontaneous recovery before participation was unlikely. In addition, the long duration of symptoms before the intervention makes it less likely that the rapid improvement was due to natural recovery and rather a result of the intervention. Our results are relevant for patients struggling with long COVID, as they provide the base for an optimistic outlook and evidence-based reason for hope.
The greatest proportion of fatigue reduction was seen rapidly, already 7 days after the intervention. The decrease was clinically significant and maintained after 3 months27. The large reduction in severe fatigue after 1 week, suggests that the intervention is particularly useful for the most severely affected. Reduction in fatigue has also been reported after other rehabilitation interventions10,15,16,17. Jimeno-Almazan et al.15 showed a large reduction in fatigue following an 8-week supervised exercise intervention compared to a control group.
The fact that we did not reveal any baseline predictors of change in fatigue indicates that the intervention may be beneficial to patients with long COVID, regardless of duration, age or gender. Furthermore, the findings imply that previous or ongoing psychiatric illnesses do not constitute barriers to improvement through concentrated rehabilitation and that these patient groups should therefore not be excluded. This might also be noted as interesting given the potential effects on mental health related to the lock-down41,42,43. In line with this, our group has previously found increased functional levels and decreased symptoms following concentrated rehabilitation for patients with longstanding anxiety and/or depression24.
Despite baseline \(\dot{V}\)O2peak values within the normal range of predicted values, the participants significantly improved \(\dot{V}\)O2peak at 3-month. We also observed an increase in SCT and 30STST. The increases were consistent in both maximal and submaximal exercise capacity as well as for lower limb strength. These findings are in line with other studies on rehabilitation for patients with long COVID10,11,12,15,17. While no significant differences in exercise capacity were found between those with fatigue and severe fatigue at baseline, patients with severe fatigue had a significantly larger increase in \(\dot{V}\)O2peak. This underlines that the intervention seems particularly useful in the most seriously affected. Adverse reactions to physical activity were closely monitored in our study. We found no indication of this occurring in our patient cohort, quite the contrary, as both self-reported activity levels as well as objective measures improved. Hence, our results support the notion of targeting the seemingly insignificant micro-choices in order to achieve a substantial increase in functional levels.
Strengths and limitations
This study represents a novel way of providing rehabilitation for patients with long COVID, based on experiences from the concentrated treatment format21,22,23,24, and shows that a concentrated intervention may result in large changes in both symptoms and functional levels. Compliance with the intervention was high, and the results were highly significant and consistent across a number of subjective assessments of symptoms and function and objective tests examining exercise capacity. Due to the lack of a control group, it is not possible to rule out that the treatment effect can be non-specific and due to attention. However, due to long waiting time, we had the possibility to exclude those with spontaneous improvement, allowing only participants with persistent symptoms and no improvement to participate. The long duration of symptoms and reduced functional level before participation contrasts with the rapid improvement after the intervention. Hence, it seems unlikely that the changes observed were due to spontaneous recovery. Moreover, follow-up data at 3 months showed a sustained improvement over time. It might be noted that the complex intervention has elements from a number other approaches, for example the cognitive behaviour therapy for chronic fatigue syndrome44. However, the design in the current study does not allow for identifying the relative importance of the component(s).
The sample size was moderate, although larger than several comparable studies10,15,17. Further research should therefore be conducted to investigate transferability to larger group of patients with long COVID. However, further strengthening our conclusions, a similar format of concentrated rehabilitation has also been shown effective in reducing fatigue for patients with chronic fatigue syndrome21.
Conclusion
This study of a micro-choice based 3-day concentrated group rehabilitation for long COVID yielded strong results. Rapid, sustained, and consistent improvements were observed for fatigue, dyspnea, sick leave, functional level, and exercise capacity. No safety issues were detected. The findings are in agreement with results of the concentrated treatment format for other chronic conditions and may be of importance for the large numbers of individuals worldwide experiencing persistent symptoms and disability due to long COVID.
Data availability
In accordance with the approvals granted for this study by the Regional Committee on Medical Research Ethics and the Norwegian Data Inspectorate, the data files will be stored securely and in accordance with the Norwegian Law of Privacy Protection. A subset of the data file with anonymized data will be made available to interested researchers upon reasonable request to Bente Frisk: bente.frisk@hvl.no, providing that Norwegian privacy legislation and the General Data Protection Regulation are respected, and that permission is granted from the Norwegian Data Inspectorate and the data protection officer at Haukeland University Hospital.
References
Nalbandian, A. et al. Post-acute COVID-19 syndrome. Nat. Med. 27, 601–615. https://doi.org/10.1038/s41591-021-01283-z (2021).
World Health Organization. A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (2021).
Fernandez-de-Las-Penas, C. et al. Prevalence of post-COVID-19 symptoms in hospitalized and non-hospitalized COVID-19 survivors: A systematic review and meta-analysis. Eur. J. Intern Med. 92, 55–70. https://doi.org/10.1016/j.ejim.2021.06.009 (2021).
Huang, L. et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: A longitudinal cohort study. Lancet Respir. Med. https://doi.org/10.1016/S2213-2600(22)00126-6 (2022).
Alkodaymi, M. S. et al. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: A systematic review and meta-analysis. Clin. Microbiol. Infect. 28, 657–666. https://doi.org/10.1016/j.cmi.2022.01.014 (2022).
Blomberg, B. et al. Long COVID in a prospective cohort of home-isolated patients. Nat. Med. 27, 1607–1613. https://doi.org/10.1038/s41591-021-01433-3 (2021).
Caspersen, I. H., Magnus, P. & Trogstad, L. Excess risk and clusters of symptoms after COVID-19 in a large Norwegian cohort. Eur. J. Epidemiol. 37, 539–548. https://doi.org/10.1007/s10654-022-00847-8 (2022).
Westerlind, E., Palstam, A., Sunnerhagen, K. S. & Persson, H. C. Patterns and predictors of sick leave after Covid-19 and long Covid in a national Swedish cohort. BMC Public Health 21, 1–9 (2021).
Al-Mhanna, S. B. et al. in Healthcare. 2130 (MDPI).
Compagno, S. et al. Physical and psychological reconditioning in long COVID syndrome: Results of an out-of-hospital exercise and psychological-based rehabilitation program. IJC Heart Vasc. 41, 101080 (2022).
Daynes, E., Gerlis, C., Chaplin, E., Gardiner, N. & Singh, S. J. Early experiences of rehabilitation for individuals post-COVID to improve fatigue, breathlessness exercise capacity and cognition—a cohort study. Chron. Respir. Dis. 18, 14799731211015692 (2021).
Fugazzaro, S. et al. Rehabilitation interventions for post-acute COVID-19 syndrome: A systematic review. Int. J. Environ. Res. Public Health https://doi.org/10.3390/ijerph19095185 (2022).
Gloeckl, R. et al. Benefits of pulmonary rehabilitation in COVID-19: A prospective observational cohort study. ERJ Open Res. 7, 25 (2021).
Harenwall, S. et al. Post-covid-19 syndrome: Improvements in health-related quality of life following psychology-led interdisciplinary virtual rehabilitation. J. Prim. Care Community Health 12, 21501319211067670 (2021).
Jimeno-Almazán, A. et al. Rehabilitation for post-COVID-19 condition through a supervised exercise intervention: A randomized controlled trial. Scand. J. Med. Sci. Sports 20, 25 (2022).
Mammi, P. et al. Post-COVID-19 ongoing symptoms and health-related quality of life: Does rehabilitation matter?: Preliminary evidence. Am. J. Phys. Med. Rehabil. 102, 241–244 (2023).
Nopp, S. et al. Outpatient pulmonary rehabilitation in patients with long COVID improves exercise capacity, functional status, dyspnea, fatigue, and quality of life. Respiration 101, 593–601 (2022).
Davis, H. E., McCorkell, L., Vogel, J. M. & Topol, E. J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 20, 1–14 (2023).
Wright, J., Astill, S. L. & Sivan, M. The relationship between physical activity and long COVID: A cross-sectional study. Int. J. Environ. Res. Public Health 19, 5093 (2022).
Kvale, G. et al. Evaluation of novel concentrated interdisciplinary group rehabilitation for patients with chronic illnesses: Protocol for a nonrandomized clinical intervention study. JMIR Res. Protoc. 10, e32216. https://doi.org/10.2196/32216 (2021).
Stubhaug, B., Lier, H. O., Aßmus, J., Rongve, A. & Kvale, G. A 4-day mindfulness-based cognitive behavioral intervention program for CFS/ME: An open study, with 1-year follow-up. Front. Psychiatry 9, 720 (2018).
Hansen, B. et al. The Bergen 4-day treatment for panic disorder: A pilot study. Front. Psychol. 3(9), 639 (2018).
Launes, G. et al. A randomized controlled trial of concentrated ERP, self-help and waiting list for obsessive-compulsive disorder: The Bergen 4-day treatment. Front. Psychol. 15(10), 2500 (2019).
Kvale, G. et al. Concentrated transdiagnostic and cross-disciplinary group treatment for patients with depression and with anxiety: A pilot study. BMC Psychiatry 22, 1–11 (2022).
Chalder, T. et al. Development of a fatigue scale. J. Psychosom. Res. 37, 147–153. https://doi.org/10.1016/0022-3999(93)90081-p (1993).
Loge, J. H., Ekeberg, Ø. & Kaasa, S. Fatigue in the general Norwegian population: Normative data and associations. J. Psychosom. Res. 45, 53–65 (1998).
Tack, M., Tuller, D. M. & Struthers, C. Bias caused by reliance on patient-reported outcome measures in non-blinded randomized trials: An in-depth look at exercise therapy for chronic fatigue syndrome. Fatigue Biomed. Health Behav. 8, 181–192 (2020).
Mundt, J. C., Marks, I. M., Shear, M. K. & Greist, J. M. The Work and Social Adjustment Scale: A simple measure of impairment in functioning. Br. J. Psychiatry 180, 461–464 (2002).
Tack, M. & Tuller, D. M. PRINCE Secondary: Transdiagnostic CBT is not effective for persistent physical symptoms. Psychol. Med. 20, 1–2 (2021).
Guyatt, G. H., Townsend, M., Berman, L. B. & Keller, J. L. A comparison of Likert and visual analogue scales for measuring change in function. J. Chronic Dis. 40, 1129–1133 (1987).
Bestall, J. C. et al. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 54, 581–586 (1999).
Yorke, J., Moosavi, S. H., Shuldham, C. & Jones, P. W. Quantification of dyspnoea using descriptors: Development and initial testing of the Dyspnoea-12. Thorax 65, 21–26. https://doi.org/10.1136/thx.2009.118521 (2010).
Williams, M. T. et al. Dyspnoea-12 and multidimensional dyspnea profile: Systematic review of use and properties. J. Pain Symptom Manage. 63, e75–e87 (2022).
Miller, M. R. et al. Standardisation of spirometry. Eur. Respir. J. 26, 319–338. https://doi.org/10.1183/09031936.05.00034805 (2005).
Albouaini, K., Egred, M., Alahmar, A. & Wright, D. J. Cardiopulmonary exercise testing and its application. Postgrad. Med. J. 83, 675–682 (2007).
Borg, G. Borg’s Perceived Exertion and Pain Scales (Human Kinetics, 1998).
Edvardsen, E. et al. Reference values for cardiorespiratory response and fitness on the treadmill in a 20- to 85-year-old population. Chest 144, 241–248. https://doi.org/10.1378/chest.12-1458 (2013).
Radtke, T. et al. ERS statement on standardisation of cardiopulmonary exercise testing in chronic lung diseases. Eur. Respir. Rev. 28, 25 (2019).
Tveter, A. T., Dagfinrud, H., Moseng, T. & Holm, I. Measuring health-related physical fitness in physiotherapy practice: Reliability, validity, and feasibility of clinical field tests and a patient-reported measure. J. Orthop. Sports Phys. Ther. 44, 206–216. https://doi.org/10.2519/jospt.2014.5042 (2014).
Fjelltveit, E. B. et al. Symptom burden and immune dynamics 6 to 18 months following mild SARS-CoV-2 infection—a case–control study. Clin. Infect. Dis. https://doi.org/10.1093/cid/ciac655 (2022).
Hagen, K. et al. Changes in mental health symptoms from April (COVID-19 outbreak) to December 2020 in Norway: A two-wave study. Cogent Psychol. 10, 2173998 (2023).
Kampf, G. & Kulldorff, M. Calling for benefit–risk evaluations of COVID-19 control measures. Lancet 397, 576–577 (2021).
Paltrinieri, S. et al. Beyond lockdown: The potential side effects of the SARS-CoV-2 pandemic on public health. Nutrients 13, 1600 (2021).
Sharpe, M. et al. Cognitive behaviour therapy for the chronic fatigue syndrome: A randomised controlled trial. BMJ 312, 22–26 (1996).
Acknowledgements
First and foremost, we want to thank the patients for participating in the study. We also thank Helse in Hardanger and Bergen Hospital Trust for funding this study.
Funding
Open access funding provided by University of Bergen. This study was part of the PUSH project which was funded by Helse in Hardanger and Bergen Hospital Trust.
Author information
Authors and Affiliations
Contributions
B.F. and M.J. wrote the main manuscript text and prepared the figures. B.F., M.J. and B.E. did the statistical analyses and interpretation of data for the work and BE revised the figures. K.L.N. was responsible for the mobile application the patients used to answer questionnaire. B.F., M.J., K.L.N., B.B.A., E.S. and G.K. had substantial contributions to the conception and design of the work. K.L.N., B.E., B.B.A., E.S. and G.K. were revising the article critically for important intellectual content. B.F., M.J., B.E., K.L.N., E.S., B.B.A. and G.K. did final approval of the version to be published. B.F., M.J., B.E., K.L.N., E.S., B.B.A. and G.K. agreed to be accountable for all aspects of the work, in ensuring that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
Bernt Bøgvald Aarli has received Grants, consulting fees or honoraria from Boehringer Ingelheim, GlaxoSmithKline, Astra Zeneca, Novartis and Sanofi-Aventis. Bente Frisk, Marte Jürgensen, Birgitte Espehaug, Kiri Lovise Njøten, Eirik Søfteland and Gerd Kvale have none competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Frisk, B., Jürgensen, M., Espehaug, B. et al. A safe and effective micro-choice based rehabilitation for patients with long COVID: results from a quasi-experimental study. Sci Rep 13, 9423 (2023). https://doi.org/10.1038/s41598-023-35991-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-35991-y
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.