Abstract
Heat stress (HS) is a long-standing hurdle that animals face in the living environment. Alpha-lipoic acid (ALA) is a strong antioxidant synthesized by plants and animals. The present study evaluated the mechanism of ALA action in HS-induced early porcine parthenotes development. Parthenogenetically activated porcine oocytes were divided into three groups: control, high temperature (HT) (42 °C for 10 h), and HT + ALA (with 10 µM ALA). The results show that HT treatment significantly reduced the blastocyst formation rate compared to the control. The addition of ALA partially restored the development and improved the quality of blastocysts. Moreover, supplementation with ALA not only induced lower levels of reactive oxygen species and higher glutathione levels but also markedly reduced the expression of glucose regulatory protein 78. The protein levels of heat shock factor 1 and heat shock protein 40 were higher in the HT + ALA group, which suggests activation of the heat shock response. The addition of ALA reduced the expression of caspase 3 and increased the expression of B-cell lymphoma-extra-large protein. Collectively, this study revealed that ALA supplementation ameliorated HS-induced apoptosis by suppressing oxidative and endoplasmic reticulum stresses via activating the heat shock response, which improved the quality of HS-exposed porcine parthenotes.
Similar content being viewed by others
Introduction
Heat stress (HS) is an environmental determinant that adversely affects the productivity of animals1, and greatly impairs reproductive performance by decreasing fertility during the summer season2. Elevation of ambient temperature adversely affects diverse aspects of pregnancy, including oocyte quality, fertilization efficiency, and successful embryonic development2,3. In recent years, the negative impacts of thermal stress in pigs have gradually increased, indicating that genetic selection for reproductive and lean tissue growth results in sensitivity to heat4. Although compromised embryo competence induced by HS has previously been reported3,5, further studies are required to fully understand the mechanisms in heat-stressed porcine embryos and to assess their therapeutic potential.
Mammalian cells respond differently to environmental stressors, including oxidative stress response, unfolded protein response (UPR), and heat shock response (HSR), to allow cell survival under unfavorable conditions. In response to HS, reactive oxygen species (ROS) production is increased and oxidative stress is induced, which can lead to apoptosis5. These environmental and cellular stresses trigger the accumulation of unfolded proteins in the endoplasmic reticulum (ER), which is termed ER stress. They also activate the UPR as a protective pathway to maintain protein homeostasis6. HSR is a fundamental protective mechanism against various stressors, and it normally upregulates heat shock factors (HSFs), which is followed by increased expression of heat shock proteins (HSPs) to suppress protein damage or aggregation7,8. The distinction between HSR and UPR allows independent responses to operate during protein restoration with their particular chaperones6.
In addition, HS compromised in vitro embryo production by increasing ROS in cows9, and induced oxidative and ER stresses that affect the activity of antioxidant enzymes and expression of ER stress-related genes in chicken testes10. Moreover, embryos produced in vitro had increased HSP70 mRNA expression after exposure to HS in mares11, and pig ovaries exposed to thermal stress showed upregulation of HSP70, HSP40, HSPH1, HSPA4, HSF1, and HSF2 genes12.
Alpha-lipoic acid (ALA), which is primarily involved in mitochondrial dehydrogenase reactions, has recently gained substantial attention as a potent antioxidant13. The therapeutic potential of ALA has been extensively studied in diverse oxidative stress-related diseases14. ALA scavenges ROS and provides reduced glutathione (GSH), inhibiting the formation of free radicals to maintain redox homeostasis in cells15,16. Moreover, ALA protects the testes against heat damage by suppressing oxidative and ER stresses in chickens10, improves embryo quality, and enhances cryotolerance by reducing ROS production in cows17,18. Despite numerous studies on the antioxidant effects of ALA, its impacts on heat-stressed porcine parthenotes remain to be elucidated. Thus, the purpose of this study was to examine the protective effects of ALA on HS-induced porcine parthenotes and further assess whether cellular stress and the HSR are associated with this protective process.
Results
HS disrupted parthenotes development in pigs
To confirm the harmful effects of high temperature (HT) on porcine parthenotes development, we first investigated the development of parthenotes exposed to HT at 42 °C for 10 h. After exposure to HT, the developmental rate from four-cell to morular stage was gradually decreased compared to that in the control group (four-cell stage: control: 87.56 ± 1.09% vs. HT: 71.99 ± 1.53%, p < 0.05; five to eight-cell stage: control: 78.85 ± 1.29% vs. HT: 54.73 ± 1.83%, p < 0.05; morular stage: control: 63.76 ± 1.07% vs. HT: 37.31 ± 1.1%, p < 0.001). Finally, the rate of blastocyst formation was significantly decreased (29.27 ± 2.99%, p < 0.01) compared to that in the control group (58.23 ± 4.61%, Fig. 1A,B), indicating that HT disrupts cleavage and consecutive progress during parthenotes development. These results show that HS reduced the potential for the development of porcine parthenotes.
ALA rescued HS-induced impairment of porcine parthenotes development
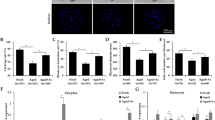
To explore the protective effects of ALA on HT-induced parthenotes, we first assessed the rate of parthenotes development following the addition of ALA in a dose-dependent manner. The results showed that the addition of 10 µM ALA partially restored the rate of blastocyst formation compared to the HT group (control: 40.38 ± 0.99% vs. HT: 25.35 ± 1.13%, p < 0.01; HT: 25.35 ± 1.13% vs. HT + 10 µM ALA: 35.54 ± 0.95%, p < 0.05; HT + 15 µM ALA: 30.33 ± 1.32%; HT + 20 µM ALA: 28.76 ± 1.15%, Fig. 2A). In addition, we evaluated the quality of blastocysts using two methods: assessment of blastocyst diameter and TUNEL assays, which detect the number of dead nuclei. As shown in Fig. 2B, although HT exposure markedly reduced the diameter of blastocysts, treatment with 10 µM ALA partially restored its full diameter. However, addition of 20 µM ALA was diminished the quality in blastocysts (control: 1.00 ± 0.05 vs. HT: 0.83 ± 0.05, p < 0.001; HT: 0.83 ± 0.05 vs. HT + 10 µM ALA: 0.94 ± 0.06, p < 0.05). Therefore, 10 µM of ALA was used in subsequent studies. Moreover, as shown in Fig. 2C, low blastocyst quality was observed in the HT group, indicating an increase in TUNEL-positive cells and a decrease in the total number of cells. In contrast, ALA addition reduced the number of TUNEL-positive cells and increased the total number of cells, suggesting that it improves the quality of blastocysts under HT (total number of cells: control: 46.38 ± 0.68 vs. HT: 32.43 ± 0.89, p < 0.01; HT: 32.43 ± 0.89 vs. HT + ALA: 43.22 ± 0.87, p < 0.05; Apoptosis index: control: 5.65 ± 0.39% vs. HT: 10.66 ± 0.73%, p < 0.01; HT: 10.66 ± 0.73% vs. HT + ALA: 6.89 ± 0.57%, p < 0.05, Fig. 2D,E). Collectively, these results indicate that ALA partially restored the rate of parthenotes development and quality in parthenotes subjected to HT.
Effect of alpha lipoic acid (ALA) on heat stressed-porcine parthenotes development. (A) Rate of blastocyst formation after HT exposure with the addition of 10, 15, and 20 µM ALA. (B) Relative diameter of blastocysts after HT exposure with ALA addition. (C) Representative images of the total cells and TUNEL-positive cells after HT exposure with ALA addition in porcine embryos. Blue, DNA; green, TUNEL-positive signal. (D) Total cell number in porcine blastocysts after HT exposure with ALA addition. (E) Apoptosis index calculated by number of TUNEL-positive cells by total cells after HT exposure with ALA addition. *p < 0.05; **p < 0.01; ****p < 0.0001.
ALA rescued HS-induced oxidative stress in porcine parthenotes
To evaluate the antioxidant effect of ALA on oxidative stress induced by HT, we first estimated ROS levels after ALA addition. As shown in Fig. 3A,B, HT exposure significantly increased ROS levels, resulting in increased oxidative stress. However, treatment with ALA partially reduced ROS levels compared to the HT group (control: 34.06 ± 0.73 vs. HT: 51.51 ± 1.48, p < 0.05; HT: 51.51 ± 1.48 vs. HT + ALA: 26.36 ± 0.93, p < 0.01). No difference was observed between the control and HT + ALA groups. We also investigated GSH levels as an indicator of antioxidant capacity under HS conditions. Although exposure to HT significantly reduced GSH levels compared to the control group, ALA addition markedly restored GSH levels compared to the HT group, showing it has a strong antioxidant defense capacity (control: 26.80 ± 0.57 vs. HT: 21.23 ± 0.43, p < 0.01; HT: 21.23 ± 0.43 vs. HT + ALA: 29.65 ± 0.79, p < 0.001, Fig. 3A,C). These results suggest that ALA addition partially ameliorated the oxidative stress induced by HT in porcine parthenotes.
Effect of ALA on oxidative stress in heat stressed-porcine parthenotes. (A) Representative images of reactive oxygen species (ROS) and glutathione (GSH) level after exposure in the presence of ALA in porcine parthenotes. Green, ROS; Blue, GSH. (B) Relative fluorescence intensity of ROS after HT exposure with ALA addition. (C) Relative fluorescence intensity of GSH after HT exposure with ALA addition. *p < 0.05; **p < 0.01; ***p < 0.001.
ALA rescued HS-induced ER stress in porcine parthenotes
Given the previous results on oxidative stress, we determined whether ALA had mitigating effects on ER stress after HT exposure. First, we measured the expression levels of glucose regulatory protein 78 (GRP78), an ER stress marker, among the groups. In the HT-treated group, the fluorescence intensity of GRP78 was significantly higher than that in the control group. However, treatment with ALA attenuated its expression, indicating a decrease in ER stress under HT (control: 24.62 ± 0.78 vs. HT: 44.60 ± 0.92, p < 0.01; HT: 44.60 ± 0.92 vs. HT + ALA: 27.40 ± 0.86, p < 0.0001). No differences were observed between the control and ALA groups (Fig. 4A,B). The relative band intensity of GRP78 was also higher in the HT-treated group than in the control group. ALA addition remarkably reduced its band intensity compared to the HT group (control: 1 vs. HT: 1.24 ± 0.04, p < 0.05; HT: 1.24 ± 0.04 vs. HT + ALA: 1.02 ± 0.08, p < 0.05, Fig. 4C,D). We also investigated the mRNA expression of other marker genes, such as activating transcription factor 4 (ATF4) and C/EBP homologous protein (CHOP). The results showed that expression of these marker genes was higher in the HT-treated group, whereas the ALA group exhibited lower expression levels compared to the HT group (GRP78: 1.00 vs. 1.99 vs. 1.01; p < 0.05 and p < 0.01; ATF4: 1.00 vs. 2.32 vs. 1.54; p < 0.05; CHOP: 1.00 vs. 2.02 vs. 1.08; p < 0.01and p < 0.001, Fig. 4E). Thus, these results suggest that ALA addition attenuates HT-induced ER stress in porcine parthenotes.
Effect of ALA on ER stress in heat stressed-porcine parthenotes. (A) Representative images of GRP78 intensity after HT exposure with ALA addition in porcine parthenotes. Red, GRP78; Blue, DNA. (B) Fluorescence intensity of GRP78 after HT exposure in the presence of ALA. (C) Western blotting of GRP78 protein expression after HT exposure with ALA treatment. Original blots are presented in Supplementary Fig. 1A,A′. (D) Relative band intensity analysis for GRP78/GAPDH after HT exposure with supplementation of ALA. (E) Relative mRNA expression of GRP78, ATF4, and CHOP among groups. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
ALA induced HSR in HS-treated parthenotes in pigs
HT exposure induces ER stress and results in an increase in damaged proteins. Based on the observed effect of ALA treatment on ER stress, we explored the effect of ALA on the HSR under HT conditions. As shown in Fig. 5A,B, the fluorescence intensity of heat shock factor 1 (HSF1) was significantly increased in the ALA treatment group compared to the other groups (control: 1 vs. HT + ALA: 1.26 ± 0.12, p < 0.05; HT: 1.05 ± 0.2 vs. HT + ALA: 1.26 ± 0.12, p < 0.05). The relative band intensity of HSP40 was also higher in the ALA group than in the other groups (control: 1 vs. HT + ALA: 1.13 ± 0.14, p < 0.05; HT: 1.01 ± 0.03 vs. HT + ALA: 1.13 ± 0.14, p < 0.05, Fig. 5C,D). Therefore, these results suggest that ALA addition induces an HSR to restore unfolded proteins under HT exposure in porcine parthenotes.
Effect of ALA on the heat shock response in heat stressed-porcine parthenotes. (A) Representative images of HSF1 after HT exposure in the presence of ALA in porcine parthenotes. Green, HSF1; Blue, DNA. (B) Relative fluorescence intensity of HSF1 after HT exposure with ALA addition. (C) Western blotting of HSP40 protein expression after HT exposure with ALA treatment. Original blots are presented in Supplementary Fig. 1B,B′. (D) Relative band intensity analysis for HSP40/GAPDH after HT exposure with supplementation of ALA. *p < 0.05.
ALA attenuated apoptosis induced by HS in porcine parthenotes
Considering the effects of oxidative and ER stresses, we evaluated the effect of ALA on apoptosis after HT exposure. We examined the expression of apoptotic genes such as caspase 3 and B-cell lymphoma-extra-large (Bcl-xL), which are involved in pro-apoptosis and anti-apoptosis, respectively. As shown in Fig. 6A,B, the fluorescence intensity of caspase 3 was significantly increased in the HT-treated group compared to that in the control group, whereas treatment with ALA restored its expression compared to the HT groups (control: 16.21 ± 0.48 vs. HT:19.65 ± 0.59, p < 0.05; HT: 19.65 ± 0.59 vs. HT + ALA: 15.30 ± 0.47, p < 0.01). In addition, the relative intensity of Bcl-xL was markedly increased in the ALA-treated group compared to that in the control group, suggesting that ALA suppressed apoptosis after HT exposure (control: 1 vs. HT + ALA: 1.18 ± 0.15, p < 0.05, Fig. 6C,D). Thus, these results indicate that ALA treatment attenuated apoptosis induced by HT exposure in porcine parthenotes.
Effect of ALA on apoptosis in heat stressed-porcine parthenotes. (A) Representative images of caspase3 after HT exposure in presence of ALA in porcine parthenotes. Red, caspase3; Blue, DNA. (B) Relative fluorescence intensity of caspase3 after HT exposure with ALA addition. (C) Western blotting of Bcl-xL protein expression after HT exposure with ALA treatment. Original blots are presented in Supplementary Fig. 1B′,C. (D) Relative band intensity analysis for Bcl-xL/GAPDH after HT exposure with ALA supplementation. *p < 0.05; **p < 0.01.
Discussion
The therapeutic properties of ALA against cellular stress have been previously described10,16. In the present study, we demonstrated that ALA significantly ameliorated the adverse effects of HS by decreasing cellular stress during porcine parthenotes development. Our results show that exposure to HT at 42 °C for 10 h reduced the rate of parthenotes development and its quality by decreasing the total cell number of blastocysts. In addition, HS conditions induced oxidative and ER stresses following an increase in unfolded proteins, which led to apoptosis caused by the increased expression of caspase3. In contrast, treatment with ALA partially ameliorated the oxidative and ER stresses induced by HS and also upregulated the HSR through higher expression levels of HSF1 and HSP40, which increased the refolding of unfolded proteins. Finally, it inhibited apoptosis under HT exposure by increasing Bcl-xL expression (Fig. 7).
Schematic diagram illustrating the ALA-mediated rescue of HS-induced impairment of parthenotes development. HT exposure induces oxidative and ER stresses, leading to an increase in unfolded proteins, which in turn leads to apoptosis. However, ALA treatment partially ameliorates oxidative and ER stresses and induces the HSR under HS conditions. Overall, ALA treatment attenuates apoptosis after HT exposure.
In the current study, HT exposure markedly reduced the rate of parthenotes development in pigs, thereby decreasing the cleavage rate throughout the developmental process. These results indicate that HS reduces the developmental potential of porcine parthenotes. In the 4-cell stage of porcine embryos, important conversions from maternal to zygote gradually occur19, called major zygotic genome activation. Epigenetic reprogramming can be influenced by increased ambient temperature, and maternal HS leads to global DNA methylation, which reduces antioxidant competence20. In addition, early embryos are extremely vulnerable to HS because they develop thermotolerance from the 2-cell stage to the morular stage21. HS in the 2-cell stage compromises the development of bovine embryos22. To assess the possible protective effect of ALA on heat-stressed porcine parthenotes, we compared three groups: control, HT 10 h, and HT + ALA. The results show that treatment with ALA partially restored the rate of parthenotes development and its quality compared to the HT group, resulting in an increase in the diameter of blastocysts, higher total cell numbers, and fewer TUNEL-positive cells. ALA addition increases the ratio of inner cell mass cells to total cells in blastocysts, suggesting that it may improve the quality of goat embryos23. In addition, to confirm whether ALA inhibited apoptosis in blastocysts, we detected the expression of the pro-apoptotic and anti-apoptotic genes caspase 3 and Bcl-xL, respectively. The results show that ALA treatment not only reduced the expression of caspase 3 compared to the HT group but also increased the expression of Bcl-xL compared to the other groups. ALA supplementation improves the quality of blastocysts exposed to ethanol, resulting in fewer TUNEL-positive cells, and decreases expression of apoptotic genes in ovine oocytes24. Given the previous results showing a decrease in oxidative and ER stresses, ALA treatment markedly inhibited apoptosis under HS conditions. Additionally, the present results showed a decrease in DNA damage followed by fewer TUNEL-positive cells in the HT group in the presence of ALA. In previous reports, relieving ER stress and promoting HSR have been regarded as potential therapeutic responses against several chronic diseases25,26. In addition, HSF1 induces the expression of HSP40 and HSP70 and stabilizes Bcl-xL as a protective response27. Therefore, these results reveal the potential benefits of ALA as a therapeutic agent in heat-stressed porcine parthenotes by inhibiting apoptosis.
Oxidative damage induced by HS leads to apoptosis with excess production of ROS5. To evaluate whether ALA can attenuate oxidative stress induced by HS in porcine parthenotes, we first detected ROS levels among the three groups. Although HT exposure induced ROS overproduction compared to the control, ALA treatment markedly reduced ROS levels compared to HT treatment. Moreover, higher GSH levels were observed in the ALA group than in the HT group, suggesting that it has strong antioxidant ability. GSH is mainly involved in scavenging ROS during the oocyte stage as a primary antioxidant component28. Several antioxidants have been shown to increase GSH levels and diminish ROS levels in oocytes exposed to HS. This suggests that the modulation of antioxidants under HS conditions may relieve thermal-oxidative stress in oocytes and enhance fertility5. In addition, ALA dissolves in both water and lipids, which enables it to freely pass through biological membranes, and generally serves as an antioxidant in the cytosol, extracellular spaces, and plasma, thereby effectively protecting cells against ROS injury29. Thus, these results demonstrate that ALA ameliorates ROS production by regenerating GSH, resulting in a decrease in oxidative stress under HS conditions.
A few pathological signal transduction pathways, as well as ER stress and apoptosis, are promoted by oxidative stress30. Accumulation of unfolded or aggregated proteins activates ER stress, which induces many chaperones to restore these proteins, regarded as the UPR pathway31. Although the UPR protects cells from several stresses and exerts its effects on protein homeostasis, prolonged ER stress can induce cell death30. Based on our results regarding oxidative stress after HT exposure, we further examined ER stress and evaluated the curative effect of ALA under HT exposure. Our results revealed that HT exposure resulted in higher protein expression of GRP78 and mRNA levels of GRP78, ATF4, and CHOP, which are ER stress markers, indicating the occurrence of ER stress. ALA supplementation markedly reduced the expression of all marker genes and GRP78 protein levels in porcine parthenotes, suggesting that it inhibited ER stress under HS conditions. In previous studies, thermal stress stimulated the formation of protein aggregates in the ER, resulting in oxidative stress-related damage31. ALA has the potential to regenerate endogenous antioxidants such as vitamin C, vitamin E, and GSH and is attracting attention as a therapeutic support to ameliorate ER stress32. ALA protects against cadmium-induced ER stress in rats and attenuates heat-damaged injury by inhibiting ER stress in chicken testes10,33. Taken together, these results suggest that ALA attenuates ER stress in HS-induced porcine parthenotes.
Interestingly, we found that HT exposure in the presence of ALA induced the HSR in porcine parthenotes, indicating an increase in the expression of HSF1 and HSP40. The HSR is induced by HS or oxidative damage, and HSPs serve as molecular chaperones and protect against misfolded or aggregated proteins34. Therefore, heat damage immediately activates HSFs, which leads to the synthesis of HSP70 and HSP40 as cell survival signaling molecules35. However, in the present study, there were no differences in the expression of HSF1 and HSP40 between the control and HT groups. ER stress induced by thermal stress suppresses HSR via translational blockade in rats35. ALA has a beneficial effect on modulating diverse components of the HSR including HSP25, HSP72, and HSF1, which results in the attenuation of ER stress and improvement in insulin sensitivity36. In addition, ALA prevents heat stroke-induced myocardial damage by acting as an antioxidative and anti-inflammatory agent with the induction of HSP7037. Thus, based on the previous results on ER stress recovery by ALA treatment, these results suggest that ALA supplementation induces HSR in parallel with suppression of ER stress in heat-stressed porcine parthenotes.
In conclusion, ALA ameliorated HT-induced apoptosis by suppressing oxidative and ER stresses by inducing the HSR in porcine parthenotes. Moreover, these results revealed that ALA had a strong protective function against HS in pigs and demonstrate its therapeutic role in protein stabilization via activation of the HSR.
Materials and methods
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise indicated, and all animal studies were conducted following the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Chungbuk National University, South Korea. In present study, parthenogenetic diploids were used due to the relatively high occurrence of polyspermy with in vitro fertilization of porcine embryos. However, the development of porcine parthenogenetic diploids to the blastocyst stage is comparable to the normal development of embryos38,39,40.
Oocyte collection and in vitro maturation
Porcine ovaries were acquired from a local slaughterhouse (Farm Story Dodram B&F, Umsung, Chungbuk, South Korea) and transported to the laboratory at 38.5 °C in saline. Cumulus–oocyte complexes (COCs) were aspirated from ovarian follicles (3–6 mm in diameter), and oocytes enclosed by at least three layers of cumulus cells were collected for further experiments. After washing thrice with Tyrode lactate HEPES (TL-HEPES) buffer, and the COCs were transferred to an in vitro maturation medium containing TCM-199 (Invitrogen, Carlsbad, CA, USA), supplemented with 10% (v/v) porcine follicular fluid, 0.91 mM sodium pyruvate, 0.6 mM l-cysteine, 10 ng/mL epidermal growth factor, 10 μg/mL luteinizing hormone, and 0.5 μg/mL follicle-stimulating hormone, and were cultured for 44 h at 38.5 °C in a humidified 5% CO2 incubator.
Parthenogenetic activation and in vitro culture
Parthenogenetic activation and in vitro culture were conducted as previously reported41. After removing the cumulus cells by repeated pipetting in 1 mg/mL hyaluronidase, denuded oocytes were parthenogenetically activated by two direct-current pulses of 120 V for 60 µs in 297 mM mannitol (pH 7.2) containing 0.1 mM CaCl2, 0.05 mM MgSO4, 0.01% polyvinyl alcohol (PVA, w/v), and 0.5 mM HEPES. Then, these oocytes were incubated in bicarbonate-buffered porcine zygote medium 5 (PZM‐5) containing 4 mg/mL bovine serum albumin (BSA) and 7.5 µg/mL cytochalasin B for 3 h to inhibit extrusion of the pseudo-second polar body. Next, the oocytes were thoroughly washed and incubated in bicarbonate‐buffered PZM‐5 supplemented with 4 mg/mL BSA in 4-well plates for 7 days at 38.5 °C in humidified atmosphere containing 5% CO2. Four-cell cleavage rate and morula and blastocyst formation rates were recorded at 48, 96, and 144 h after activation, respectively. Blastocyst diameter was examined in parthenotes at the blastocyst stage on day 7 using ImageJ v.l.44 g software (National Institutes of Health, Bethesda, MD, USA).
TUNEL assay
The intracellular apoptosis standard of blastocysts was determined with TUNEL assay using an In Situ Cell Death Detection Kit (11684795910; Roche, Basel, Switzerland), as described earlier42. Blastocysts were fixed in 3.7% formaldehyde for 30 min at room temperature (RT) and permeabilized by incubation in 0.5% Triton X-100 for 30 min at RT. Next, the blastocysts were cultured with fluorescein-conjugated dUTP and terminal deoxynucleotidyl transferase enzyme for 1 h at 38.5 °C. After washing thrice with phosphate-buffered saline/polyvinyl alcohol (PBS/PVA), the blastocysts were treated with 10 µg/mL Hoechst 33,342 for 10 min and mounted onto glass slides. Images were obtained using a confocal microscope (LSM 710 Meta; Zeiss, Oberkochen, Germany). The apoptosis index was calculated by dividing the TUNEL-positive cell number by the total cell number.
GSH and ROS measurements
To determine GSH levels, blastocysts were cultured for 30 min at 38.5 °C in PBS/PVA containing 10 μM 4-chloromethyl-6,8-difluoro-7-hydroxycoumarin dye (CellTracker™ Blue CMF2HC, Thermo Fisher Scientific, Waltham, USA) as previously described43. To determine total ROS levels, blastocysts were incubated for 30 min at 38.5 °C in PBS/PVA containing 10 μM 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA, Cat # D399, Molecular Probes, Eugene, OR, USA). After incubation, the blastocysts were washed thrice with PBS/PVA. Fluorescence signals were detected as TIFF files using a digital camera (DP72; Olympus, Tokyo, Japan) connected to a fluorescence microscope (IX70; Olympus). GSH and ROS levels were quantified by analyzing the fluorescence intensity in blastocysts using ImageJ v.1.44g software (National Institutes of Health, Bethesda, MD, USA).
Immunofluorescence staining
Immunostaining was conducted as previously described41. After washing thrice with PBS/PVA, blastocysts were fixed with 3.7% formaldehyde at RT for 30 min. Next, blastocysts were permeabilized in 0.5% Triton X-100 for 30 min at RT, and incubated in PBS/PVA containing 3.0% BSA at RT for 1 h. Subsequently, these blastocysts were incubated overnight at 4 °C with rabbit anti-GRP78 (1:100; ab21685, Abcam, Cambridge, United Kingdom), rabbit anti-HSF1 antibody (1:100; 12972S, Cell Signaling, Danvers, MA, United States), and rabbit anti-caspase3 antibodies (1:20; 9664S, Cell Signaling). After washing thrice with PBS/PVA, the blastocysts were incubated with Alexa Fluor 488™ goat anti-rabbit IgG (1:200; A32731, Invitrogen) or Alexa Flour 546™ donkey anti-rabbit IgG (1:200; A10040, Invitrogen, Carlsbad, CA, United States) at 37 °C for 1 h. After three washes, the blastocysts were incubated for 10 min with 5 μg/mL Hoechst 33342. Finally, the blastocysts were mounted on slides and examined under a confocal microscope (LSM 710 META; Zeiss, Oberkochen, Germany). Images were processed using Zen software (v.8.0; Zeiss, Jena, Germany) and then analyzed using ImageJ v.l.44 g software (National Institutes of Health, Bethesda, MD, USA).
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
As previously described42, mRNA was extracted from 30 embryos in each group using a Dynabeads mRNA Direct Kit (61012; Thermo Fisher Scientific, Waltham, MA, USA), and cDNA was obtained using a First Strand Synthesis Kit (cat# 6210; LeGene, San Diego, CA, USA) following the manufacturer’s instructions. qRT-PCR was performed using a WizPure qPCR Master mix (W1731-8; Wizbio Solutions, Seongnam, South Korea) according to the manufacturer’s instructions, using a QuantStudio™ 6 Flex Real-Time PCR System (Applied Biosystems, Waltham, MA, USA). Amplification was performed as follows: initial denaturation at 95 °C for 10 min, followed by 40 cycles of amplification at 95 °C for 15 s, 60 °C for 20 s, and 72 °C for 15 s, and a final extension at 95 °C for 15 s. Relative gene expression was calculated using the ∆∆CT method. The primers used to amplify each gene are listed in Table 1.
Western blotting analysis
As previously reported43, 80 porcine embryos from each group were collected in sodium dodecyl sulfate sample buffer and heated at 95 °C for 5 min. Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and electrotransferred to polyvinylidene fluoride membranes. Next, the membranes were blocked in Tris-buffered saline containing Tween 20 (TBST) with 5% skim milk for 1 h and incubated overnight at 4 °C with rabbit anti-GRP78 antibody (1:1000; ab21685, Abcam), rabbit anti-HSP40 antibody (1:1000; ab69402, Abcam), rabbit anti-Bcl-xL antibody (1:1000; #2762, Cell Signaling), and rabbit anti-GAPDH antibody (1:1000; 5174S, Cell Signaling). After washing thrice with TBST, the membranes were incubated with horseradish peroxidase-conjugated goat anti-mouse IgG or goat anti-rabbit IgG (1:2000) at RT for 1 h. The blots were cut prior to hybridization with antibodies and have the absence of images of full length (Supplementary Fig. 1). Signals from blots were captured using a charge-coupled device camera and UviSoft software (Uvitec, Cambridge, United Kingdom).
Experimental design
To ensure parthenotes of steady quality, the 2-cell cleavage rate was confirmed after 24 h of parthenogenetic activation. Parthenotes of the one-cell and two-cell stages and the same quality were separated evenly into three groups: control, HT, and HT + ALA (10 µM). ALA was diluted in ethanol 0.1% (the toxicity of which has been previously evaluated44 and added at 10, 15, or 20 µM to porcine parthenotes cultured in vitro. The concentration of ALA was selected based on the results. The control group was cultured at 38.5 °C. HT and HT + ALA groups were cultured at 42 °C for 10 h and then returned to 38.5 °C45 and continuously cultured for 6 days.
Statistical analysis
Each experiment was performed three times, each sample in triplicates. Data were analyzed using one-way analysis of variance (ANOVA) or the Student’s t-test. All percentage data were subjected to arcsine transformation before statistical analysis and are presented as mean ± SEM. Differences were considered statistically significant at p < 0.05. All calculations were performed using the GraphPad Prism 6 software (GraphPad, San Diego, CA, USA).
Data availability
The datasets used or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Belhadj Slimen, I., Najar, T., Ghram, A. & Abdrrabba, M. Heat stress effects on livestock: Molecular, cellular and metabolic aspects, a review. J. Anim. Physiol. Anim. Nutr. 100, 401–412. https://doi.org/10.1111/jpn.12379 (2016).
Rensis, F. D. & Scaramuzzi, R. J. Heat stress and seasonal effects on reproduction in the dairy cow—a review. Theriogenology 60, 1139–1151. https://doi.org/10.1016/S0093-691X(03)00126-2 (2003).
Sartori, R. et al. Fertilization and early embryonic development in heifers and lactating cows in summer and lactating and dry cows in winter. J. Dairy Sci. 85, 2803–2812. https://doi.org/10.3168/jds.S0022-0302(02)74367-1 (2002).
Renaudeau, D., Gourdine, J. L. & St-Pierre, N. R. A meta-analysis of the effects of high ambient temperature on growth performance of growing-finishing pigs. J. Anim. Sci. 89, 2220–2230. https://doi.org/10.2527/jas.2010-3329 (2011).
Sakatani, M. Effects of heat stress on bovine preimplantation embryos produced in vitro. J. Reprod. Dev. 63, 347–352. https://doi.org/10.1262/jrd.2017-045 (2017).
Singh, M. B., Lohani, N. & Bhalla, P. L. The role of endoplasmic reticulum stress response in pollen development and heat stress tolerance. Front. Plant Sci. https://doi.org/10.3389/fpls.2021.661062 (2021).
Buchberger, A., Bukau, B. & Sommer, T. Protein quality control in the cytosol and the endoplasmic reticulum: Brothers in arms. Mol. Cell 40, 238–252. https://doi.org/10.1016/j.molcel.2010.10.001 (2010).
Hartl, F. U., Bracher, A. & Hayer-Hartl, M. Molecular chaperones in protein folding and proteostasis. Nature 475, 324–332. https://doi.org/10.1038/nature10317 (2011).
Amaral, C. S. et al. Heat stress on oocyte or zygote compromises embryo development, impairs interferon tau production and increases reactive oxygen species and oxidative stress in bovine embryos produced in vitro. Mol. Reprod. Dev. 87, 899–909. https://doi.org/10.1002/mrd.23407 (2020).
Xiong, Y., Yin, Q., Li, J. & He, S. Oxidative stress and endoplasmic reticulum stress are involved in the protective effect of alpha lipoic acid against heat damage in chicken testes. Animals 10, 384 (2020).
Mortensen, C. J. et al. Heat shock protein 70 gene expression in equine blastocysts after exposure of oocytes to high temperatures in vitro or in vivo after exercise of donor mares. Theriogenology 74, 374–383. https://doi.org/10.1016/j.theriogenology.2010.02.020 (2010).
Pennarossa, G. et al. Characterization of the constitutive pig ovary heat shock chaperone machinery and its response to acute thermal stress or to seasonal variations1. Biol. Reprod. https://doi.org/10.1095/biolreprod.112.104018 (2012).
Packer, L., Witt, E. H. & Tritschler, H. J. Alpha-lipoic acid as a biological antioxidant. Free Radic. Biol. Med. 19, 227–250. https://doi.org/10.1016/0891-5849(95)00017-R (1995).
Wollin, S. D. & Jones, P. J. Α-lipoic acid and cardiovascular disease. J. Nutr. 133, 3327–3330 (2003).
Shay, K. P., Moreau, R. F., Smith, E. J., Smith, A. R. & Hagen, T. M. Alpha-lipoic acid as a dietary supplement: Molecular mechanisms and therapeutic potential. Biochim. Biophys. Acta-Gener. Subjects 1790, 1149–1160 (2009).
Skibska, B. & Goraca, A. The protective effect of lipoic acid on selected cardiovascular diseases caused by age-related oxidative stress. Oxid. Med. Cell. Longev. 2015, 313021. https://doi.org/10.1155/2015/313021 (2015).
Fabra, M. C. et al. Alpha-lipoic acid improves bovine preimplantation blastocyst quality and cryotolerance. Theriogenology 198, 61–68. https://doi.org/10.1016/j.theriogenology.2022.12.025 (2023).
Fabra, M. C. et al. Effect of alpha-lipoic acid during preimplantation development of cattle embryos when there were different in vitro culture conditions. Anim. Reprod. Sci. 221, 106550. https://doi.org/10.1016/j.anireprosci.2020.106550 (2020).
Du, Z. Q. et al. Single cell rna-seq reveals genes vital to in vitro fertilized embryos and parthenotes in pigs. Sci. Rep. 11, 14393. https://doi.org/10.1038/s41598-021-93904-3 (2021).
Zhu, Y. et al. Maternal dietary manganese protects chick embryos against maternal heat stress via epigenetic-activated antioxidant and anti-apoptotic abilities. Oncotarget 8, 89665 (2017).
Edwards, J. L. & Hansen, P. J. Differential responses of bovine oocytes and preimplantation embryos to heat shock. Mol. Reprod. Dev. 46, 138–145. https://doi.org/10.1002/(SICI)1098-2795(199702)46:2%3c138::AID-MRD4%3e3.0.CO;2-R (1997).
Al-Katanani, Y. M. & Hansen, P. J. Induced thermotolerance in bovine two-cell embryos and the role of heat shock protein 70 in embryonic development. Mol. Reprod. Dev. 62, 174–180. https://doi.org/10.1002/mrd.10122 (2002).
He, Y., Wang, Y., Zhang, H., Zhang, Y. & Quan, F. Alpha-lipoic acid improves the maturation and the developmental potential of goat oocytes in vitro. Reprod. Dom. Anim. 56, 545–554. https://doi.org/10.1111/rda.13892 (2021).
Moghimi Khorasgani, A. et al. Alpha-lipoic acid can overcome the reduced developmental competency induced by alcohol toxicity during ovine oocyte maturation. Cell J. 23, 164–173. https://doi.org/10.22074/cellj.2021.7071 (2021).
Literáti-Nagy, B. et al. Improvement of insulin sensitivity by a novel drug candidate, bgp-15, in different animal studies. Metab. Syndr. Relat. Disord. 12, 125–131 (2014).
Chung, J. et al. Hsp72 protects against obesity-induced insulin resistance. Proc. Natl. Acad. Sci. USA 105, 1739–1744 (2008).
Jacobs, A. T. & Marnett, L. J. Heat shock factor 1 attenuates 4-hydroxynonenal-mediated apoptosis: Critical role for heat shock protein 70 induction and stabilization of bcl-xl*. J. Biol. Chem. 282, 33412–33420. https://doi.org/10.1074/jbc.M706799200 (2007).
Edwards, J. L., King, W. A., Kawarsky, S. J. & Ealy, A. D. Responsiveness of early embryos to environmental insults: Potential protective roles of hsp70 and glutathione. Theriogenology 55, 209–223. https://doi.org/10.1016/S0093-691X(00)00455-6 (2001).
Rochette, L. et al. Direct and indirect antioxidant properties of α-lipoic acid and therapeutic potential. Mol. Nutr. Food Res. 57, 114–125 (2013).
Dandekar, A., Mendez, R. & Zhang, K. Cross talk between er stress, oxidative stress, and inflammation in health and disease. Stress Resp. 20, 205–214 (2015).
Homma, T. & Fujii, J. Heat stress promotes the down-regulation of ire1α in cells: An atypical modulation of the UPR pathway. Exp. Cell Res. 349, 128–138 (2016).
Jiang, Q. et al. Apoptotic events induced by high glucose in human hepatoma hepg2 cells involve endoplasmic reticulum stress and Mapk’s activation. Mol. Cell. Biochem. 399, 113–122. https://doi.org/10.1007/s11010-014-2238-5 (2015).
Yuan, Y. et al. Alpha-lipoic acid protects against cadmium-induced neuronal injury by inhibiting the endoplasmic reticulum stress eif2α-atf4 pathway in rat cortical neurons in vitro and in vivo. Toxicology 414, 1–13. https://doi.org/10.1016/j.tox.2018.12.005 (2019).
Benjamin, I. J. & McMillan, D. R. Stress (heat shock) proteins: Molecular chaperones in cardiovascular biology and disease. Circ. Res. 83, 117–132 (1998).
Liu, Y. et al. Heat stress activates ER stress signals which suppress the heat shock response, an effect occurring preferentially in the cortex in rats. Mol. Biol. Rep. 39, 3987–3993. https://doi.org/10.1007/s11033-011-1179-2 (2012).
Diane, A. et al. Alpha lipoic acid attenuates ER stress and improves glucose uptake through dnajb3 cochaperone. Sci. Rep. 10, 20482. https://doi.org/10.1038/s41598-020-77621-x (2020).
Shen, H.-H. et al. Alpha-lipoic acid protects cardiomyocytes against heat stroke-induced apoptosis and inflammatory responses associated with the induction of hsp70 and activation of autophagy. Mediators Inflamm. 2019, 8187529. https://doi.org/10.1155/2019/8187529 (2019).
Shen, X.-H. et al. High mobility group box 1 (hmgb1) enhances porcine parthenotes developing in vitro in the absence of BSA. Theriogenology 66, 2077–2083. https://doi.org/10.1016/j.theriogenology.2006.05.019 (2006).
Thuan, N. V., Harayama, H. & Miyake, M. The development of porcine parthenogenetic diploid oocytes with homogeneous genomic components in vitro. J. Reprod. Dev. 48, 157–166. https://doi.org/10.1262/jrd.48.157 (2002).
Lee, J. W., Tian, X. C. & Yang, X. Optimization of parthenogenetic activation protocol in porcine. Mol. Reprod. Dev. Incorp. Gamete Res. 68, 51–57 (2004).
Zhou, D., Niu, Y. & Cui, X.-S. M-ras regulate cdh1 function in blastomere compaction during porcine embryonic development. J. Anim. Reprod. Biotechnol. 35, 12–20. https://doi.org/10.12750/JARB.35.1.12 (2020).
Zhou, D., Sun, M. H., Lee, S. H. & Cui, X. S. Romo1 is required for mitochondrial metabolism during preimplantation embryo development in pigs. Cell. Div. 16, 7. https://doi.org/10.1186/s13008-021-00076-7 (2021).
Zhou, D. et al. Epigallocatechin-3-gallate protects porcine oocytes against post-ovulatory aging through inhibition of oxidative stress. Aging 14, 8633–8644. https://doi.org/10.18632/aging.204368 (2022).
Nikoloff, N. et al. Effects of epa on bovine oocytes matured in vitro with antioxidants: Impact on the lipid content of oocytes and early embryo development. Theriogenology 146, 152–161. https://doi.org/10.1016/j.theriogenology.2019.11.028 (2020).
Jin, Y.-X. et al. Heat shock induces apoptosis related gene expression and apoptosis in porcine parthenotes developing in vitro. Anim. Reprod. Sci. 100, 118–127. https://doi.org/10.1016/j.anireprosci.2006.06.017 (2007).
Acknowledgements
This work was supported by the National Research Foundation (NRF) of Korea funded by the Korean Government (MSIT) (Nos. 2020R1A4A1017552 and 2022R1A2C300769), Republic of Korea.
Author information
Authors and Affiliations
Contributions
S.H.L. and X.S.C. designed the study. S.H.L. and M.H.S. performed the experiments. W.J.J., X.H.L., G.H. analyzed the data. S.H.L., D.Z., and Z.C. prepared the figures. S.H.L. and X.S.C. wrote the manuscript. X.S.C. revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, SH., Sun, MH., Jiang, WJ. et al. Alpha-lipoic acid attenuates heat stress-induced apoptosis via upregulating the heat shock response in porcine parthenotes. Sci Rep 13, 8427 (2023). https://doi.org/10.1038/s41598-023-35587-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-35587-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.