Abstract
Type 2 diabetes mellitus (T2DM) patients show brain tissue changes in mood and cognitive regulatory sites, but the nature and extent of tissue injury and their associations with symptoms are unclear. Our aim was to examine brain tissue damage in T2DM over controls using mean diffusivity (MD) computed from diffusion tensor imaging (DTI), and assess correlations with mood and cognitive symptoms in T2DM. We collected DTI series (MRI), mood, and cognitive data, from 169 subjects (68 T2DM and 101 controls). Whole-brain MD-maps were calculated, normalized, smoothed, and compared between groups, as well as correlated with mood and cognition scores in T2DM subjects. Type 2 diabetes patients showed altered cognitive and mood functions over control subjects. Multiple brain sites in T2DM patients showed elevated MD values, indicating chronic tissue changes, including the cerebellum, insula, and frontal and prefrontal cortices, cingulate, and lingual gyrus. Associations between MD values and mood and cognition scores appeared in brain sites mediating these functions. Type 2 diabetes patients show predominantly chronic brain tissue changes in areas mediating mood and cognition functions, and tissue changes from those regions correlate with mood and cognitive symptoms suggesting that the microstructural brain changes may account for the observed functional deficits.
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus is a chronic metabolic disorder, leading to multi-systemic impairments, including the brain with well-known vascular complications, cognitive, and mood dysfunction. Epidemiologic studies have shown that individuals with type 2 diabetes have an almost 1.5-fold higher incidence of cognitive deficits1 and a two-fold higher risk of mood disorders2,3 Type 2 diabetes mellitus has been recognized as an essential risk factor for early vascular dementia and Alzheimer’s disease4 Earlier studies suggest that the underlying mechanisms and risk factors for early dementia and Alzheimer’s disease are overlapping in type 2 diabetes patients5 and such risk factors are 2-fold higher in type 2 diabetes over healthy individuals6,7
The relationships between type 2 diabetes mellitus and mood, including depression and anxiety have been observed in several studies2,8 and these patients have an increased risk of mood dysfunction compared to people without diabetes. Depression in type 2 diabetes mellitus presents a major clinical challenge with worsening diabetes self-management skills, quality of life, incidence of complications, and life span9 In turn, these issues may increase type 2 diabetes treatment cost for patients with symptomatic or asymptomatic depression symptoms10 In addition, type 2 diabetes patient exhibit clinical and subclinical anxiety more frequently than the general population. Previous studies show link between anxiety and type 2 diabetes, with prevalence rates of clinically significant anxiety up to 55% in type 2 diabetes11,12 Also, anxiety symptoms are associated with poor metabolic outcomes and increased medical complications in this condition.
Cognitive and mood deficits in type 2 diabetes patients can be mediated through tissue changes in regulatory sites, and brain imaging studies may help identify markers of risk for psychological comorbidities including cognitive dysfunction, depression, and anxiety and clarify cerebral pathologies in type 2 diabetes condition. Subtle brain tissue abnormalities cannot be detected with regular (commonly used) structural magnetic resonance imaging (MRI) due to insufficient sensitivity to tissue changes, but can be studied using advanced techniques such as diffusion tensor imaging (DTI), a non-invasive technique that shows changes in the microstructural organization of the brain, including gray and white matter. Mean diffusivity (MD), which measures the average movement of water molecules and represents microstructural integrity, can be calculated from DTI. MD procedures distinguish acute from chronic stages of tissue change after hypoxia/ischemia, with decreased values in acute stages, and increased values in chronic stages, and can reveal the extent and nature of the tissue injury in type 2 diabetes condition.
Most previous studies of type 2 diabetes using DTI have used fractional anisotropy measurements, which are nonspecific for acute and chronic tissue changes, to assess microstructural tissue integrity of the whole brain or specific regions of white matter tracts13,14,15 In addition, previous MD studies have shown mixed brain abnormalities in patients with type 2 diabetes14,16 which may be due to limited sample sizes, differences in data acquisition parameters and analysis methods. Also, direct associations between brain tissue changes in sites that mediate cognition and mood based on MD and mood and cognitive symptom scores have not been documented in type 2 diabetes patients.
In this study, we sought to examine microstructural tissue changes in patients with type 2 diabetes using whole-brain DTI based MD procedures compared to healthy controls, and assess associations between tissue integrity in mood and cognitive regulatory areas and depression, cognition, and anxiety scores in type 2 diabetes patients. We hypothesized that type 2 diabetes patients show altered MD values in mood and cognition regulatory areas compared to healthy control subjects, and tissue integrity of these areas will be associated with cognition and mood symptom scores in type 2 diabetes patients.
Results
Demographics, clinical, mood, and cognitive variables
Demographic, physical, and clinical data are outlined in Table 1. No significant differences in age (p=0.24), sex (p=0.62), and handedness (p=0.64) appeared between type 2 diabetes patients and healthy control subjects (Table 1). Body-mass-index (p<0.001) and systolic blood pressure (p=0.003) values were significantly higher in type 2 diabetes patients over control subjects. All type 2 diabetes patients were on oral diabetes medications with 17% also on insulin. Of 68 type 2 diabetes patients, 6 patients had retinopathy, 13 had neuropathy, and 7 had diabetes kidney disease. In addition, one patient had cerebrovascular disease, 3 patients had cardiovascular disease, and 2 had peripheral vascular disease. Type 2 diabetes patients had significantly higher depression [increased BDI-II] (p<0.001) and greater anxiety [increased BAI] (p<0.001) scores over healthy controls. In addition, global MoCA scores were significantly reduced in type 2 diabetes patients compared to controls (p=0.002), with significant differences observed in visuospatial (p=0.01), attention (p=0.005), and language cognitive subdomains (p=0.002).
Regional MD changes in type 2 diabetes patients
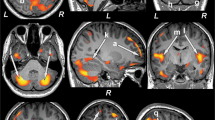
Multiple brain areas showed increased regional MD values, indicating chronic tissue changes, in type 2 diabetes patients compared to controls (Fig. 1), including the bilateral cerebellum, cerebellar vermis, left para-hippocampal gyrus, bilateral anterior and right posterior insula, and bilateral mid and right inferior frontal, and right prefrontal cortices. In addition, MD values were remarkably increased in the bilateral anterior, and posterior cingulate, left inferior, right superior, and bilateral mid temporal gyrus, left superior parietal cortices, bilateral lingual gyrus, and bilateral pre- and post-central gyri. Few brain regions showed decreased MD values, suggesting acute tissue changes, and included the bilateral thalamus, right putamen, pons, and right pallidum (Fig. 2). Regional brain MD values from sites showing significant differences between groups are summarized in Table 2.
Brain sites with increased regional mean diffusivity (MD) values in patients with type 2 diabetes compared to control subjects. Brain regions showed increased MD values in the bilateral insula (a, b), bilateral anterior (c, h), and posterior (d, i) cingulate, left superior parietal cortices (e), bilateral cerebellum (f, g), right inferior frontal cortices (j), right prefrontal cortices (k), left para-hippocampal gyrus (l), and bilateral lingual gyrus (m, n).
Correlations between MD and BDI-II, BAI, MoCA scores in type 2 diabetes patients
Depression (BDI-II) scores showed positive associations with MD values at the right prefrontal cortices, left inferior, bilateral mid, and right superior temporal gyrus, left mid and right inferior frontal cortices, left superior parietal cortices, right lingual gyrus, suggesting that the regions that showed chronic tissue changes are associated with higher depressive symptoms. Brain regions that showed decreased MD values in type 2 diabetes patients included the thalamus, putamen, and pons, and correlated with negative association with BDI-II scores, suggesting that the regions with acute tissue changes are related to depressive symptoms. Positive relationships were observed in the right mid and inferior frontal cortices, right anterior cingulate, bilateral mid temporal gyrus, and bilateral lingual gyrus; negative associations in pons between anxiety scores and MD values among the regions that showed altered MD values. MoCA scores were negatively correlated with MD values at the bilateral mid frontal, left superior parietal cortices, bilateral pre- and left post-central gyrus, and positively correlated with the thalamus. MoCA sub-scores, including the visuospatial function, language, attention, and abstraction were negatively associated with MD values from the cerebellar cortices, prefrontal cortices, parahippocampal gyrus, lingual gyrus, frontal, parietal, and temporal cortices, pre- and post-central gyrus, and positively correlated with the thalamus and putamen. The correlation coefficients for sites showing significant associations are summarized in Tables 3 and 4, which are significant based on the Benjamini-Hochberg procedures with false discovery rate of 0.05.
Discussion
In the current study, we found that people living with type 2 diabetes mellitus showed widespread microstructural disruptions in various brain regions regulating cognition and mood functions compared to control subjects; these tissue changes were predominantly in chronic stages, with few sites in the acute stage. Congruent with study findings, overall cognitive deficits were observed in patients with type 2 diabetes, with impairment in visuospatial, attention, and language cognitive sub-domains. Depression and anxiety scores were significantly increased in type 2 diabetes patients over healthy controls, and significant associations were observed between microstructural tissue integrity of mood and cognitive regulatory sites and those symptoms, which is a first report in type 2 diabetes mellitus, based on our best knowledge. Considering that microstructural tissue impairment underlies common mechanisms of cognitive and mood dysfunction and disrupts the large-scale distributed brain cognitive and mood regulatory networks, our findings imply that extensive microstructural acute and chronic tissue changes in those areas play a distinct role in cognition and mood deficits in type 2 diabetes condition. Our investigation provides insight into the nature and extent of microstructural neuropathological changes on mood and cognition functions in type 2 diabetes mellitus.
Patients with type 2 diabetes showed extensive disruptions in the microstructural tissue integrity in areas predominantly located in the frontal, temporal, and parietal cortices, and subcortical regions reinforced by decreased nodal efficiency of these sites based on topological network analysis17 Several studies have shown microstructural tissue changes in the para-hippocampal gyrus, insular, prefrontal, and parietal cortices18 as was also demonstrated in our study with widespread changes in those areas with major sites showing chronic changes and a few regions with acute tissue injury. Brain fiber connectivity analyses from previous studies showed reduced connectivity in both cerebellar and cerebro-cerebellar circuits in patients with type 2 diabetes relative to control subjects15 These encompassed fibers connecting the cerebellum to vermis, and anterior crus to cortical areas, including the precentral, frontal, and superior parietal gyri, the regions that showed chronic tissue changes with increased MD values in type 2 diabetes patients in our study.
Previous studies have demonstrated that adults with type 2 diabetes have an increase in cognitive deficits compared to healthy controls in multiple domains, including attention, psychomotor efficiency, executive function, verbal/emotional/working memory, and information processing speed17,18 Our study showed similar findings with deficits in visuospatial processing, attention, and language domains. Prominent cognition and mild cognitive impairments are more likely to develop in individuals with type 2 diabetes, and the risks for Alzheimer's disease and vascular dementia are increased19,20 Reduced functional connectivity in patients with type 2 diabetes has been reported between regions of the default mode network, highly connected regions in the brain, including the frontal gyrus and medial temporal gyrus21 implicating an important role in global cognitive processing, memory, executive functioning, and processing speed. Such regions showed altered MD values and significant correlations with cognitive scores in type 2 diabetes patients in this study. Earlier studies have demonstrated that cognitive impairment in patients with type 2 diabetes is associated with more disruption in neural structures, including the fronto-temporal regions and thalamic radiations22 fewer white matter connections, and altered nodal network efficiency in temporal lobe13,23 these regions appeared to show abnormal MD values, which were significantly correlated with cognition scores in our study.
Depression and anxiety symptoms may result from difficulties in coping with chronic disease. However, the chronic metabolic consequences of type 2 diabetes may disturb cerebral neurotransmitters and impair brain tissue, thus predisposing people to depressive and anxiety symptoms24 In addition, mood changes in patients with type 2 diabetes might result from brain vascular damage25,26 On the other hand, the relationships between type 2 diabetes and mood deficits may be bidirectional, as these mood changes may even further contribute to enhanced brain tissue changes in patients with type 2 diabetes27 Neuroimaging studies have identified a consistently altered network of brain regions in anxious and depressed patients28,29 including the basal ganglia, prefrontal, and frontal cortices, anterior cingulate cortex, and thalamus, and these areas showed altered regional MD values and associations with BDI and BAI scores here. The cortico-striatal-pallidal-thalamic circuit, which comprises of neuroanatomical loops that connect various brain sites including anterior cingulate cortex, prefrontal cortex, basal ganglia and thalamus in a highly organized and integrated manner, supports diverse cognitive and emotional processes that are shown dysfunctional in depressed patients. In this study, we observed aberrant MD changes and correlations with BDI-II and BAI scores in these regions in patients with type 2 diabetes, which might be suggestive of bidirectional changes for patients with higher mood deficits.
Although the underlying mechanisms responsible for tissue changes in cognitive and mood regulatory areas contributing to functional deficits in type 2 diabetes mellitus are not fully elucidated, they may include hyperglycemia, insulin resistance, oxidative stress, and neuroinflammation-induced processes30,31 In addition, other common causes for depressive symptoms in type 2 diabetes may include lack of physical exercise, poor sleep, and diet changes, a common pathway that could activate and disturb the stress system, contributing to further brain tissue changes in cognition and mood regulatory areas. Chronic depressive and anxiety symptoms, components of stress leads to increased production of cortisol levels and inflammatory cytokines due to activated sympathetic nervous system (SNS), which is an integrative system that reacts to stress and a component of the autonomic nervous system and activated hypothalamus-pituitary-adrenal axis32 Glucocorticoid receptors are distributed in the hippocampus, hypothalamus, and other regions33 and increased cortisol levels can further contribute to tissue changes in those areas34,35 In addition, prolonged SNS activation and chronic hypercortisolemia elevate insulin resistance, visceral obesity, and lead to the metabolic syndrome; all factors leading to oxidative stress and inflammatory response contributing to type 2 diabetes mellitus. Also, chronic stress induced inflammatory responses interact with neurotransmitter metabolism, neuroendocrine function, synaptic plasticity, and behavior, and activate the fear system emerging anxiety and depression symptoms.
One of the limitations of our study is that some control subjects (50%) self-confirmed that they did not have type 2 diabetes, which may have contaminated data diluting findings. However, although their HbA1c levels were not available, those control subjects had normal cognitive and mood functions indicating very less influence, if any, in this study. Also, some of our T2DM adults had retinopathy, neuropathy, and hypertension, and the brain tissue changes observed in our study might be impacted with these comorbidities, and need to be explored in future with larger sample size.
Conclusions
In conclusion, people living with type 2 diabetes condition show widespread microstructural chronic brain tissue changes primarily in sites that mediate mood and cognition functions, and tissue integrity in those areas correlate with mood and cognitive symptoms. The data suggest that type 2 diabetes mellitus leads to an alteration in the microstructural chronic and acute cerebral tissue changes that may account for the observed impaired mood and cognition. The findings indicate that MRI-based biomarkers, such as MD, can be used for early detection of brain tissue changes in cognitive and mood regulatory regions in patients with type 2 diabetes.
Materials and methods
We recruited 68 people with type 2 diabetes mellitus from the University of California Los Angeles (UCLA) Gonda Diabetes Center, as well as from the surrounding community and all type 2 diabetes patients were on medications. 101 healthy, non-diabetic controls were recruited through flyer advertisement at the UCLA campus and the West Los Angeles area. Demographic, physiologic, mood, and cognitive data are summarized in Table 1. Inclusion criteria for type 2 diabetes patients included ages 40-65 years, on stable medications, and able to lay supine for MRI. Most of the control subjects were assessed for HbA1c levels using the point-of-care tests utilizing fingerstick blood, and some controls self-confirmed normoglycemia. Control subjects were 40-65 years of age, without hypertension, and diabetes mellitus. The exclusion criteria for type 2 diabetes patients and control subjects included psychiatric disease (clinical depression, schizophrenia, manic-depressive), diagnosed brain condition (seizure disorder, head trauma), history of stroke, heart failure, airway or chest deformities that would interfere with breathing, mechanical ventilator support, and renal failure (requiring dialysis). Subjects were also excluded if there was presence of sleep disordered breathing, dementia, cystic fibrosis, chronic obstructive pulmonary disease, brain mass lesions, or dependency on drugs (e.g., tobacco or cocaine use) that would introduce brain tissue changes, or the presence of claustrophobia, body weight more than 160 kg (limitations of scanner table), or metallic implants. The Diabetes Complications Severity Index (DCSI) was assessed from each patient’s clinical record to document complications including retinopathy, nephropathy, neuropathy, cerebrovascular, cardiovascular, and peripheral vascular disease. Written informed consent was obtained from all subjects prior to the study; the UCLA Institutional Review Board approved the entire protocol. All methods were performed in accordance with the relevant guidelines and regulations.
Depression and anxiety assessment
The Beck Anxiety Inventory (BAI)36 was administered in type 2 diabetes patients and control subjects to evaluate anxiety symptoms. Depression was measured by self-reported questionnaire using the Beck Depression Inventory (BDI-II)37 These self-administered questionnaires consisted of 21 questions per inventory, with scores ranging from 0-3 for each question and a total score for each inventory ranging from 0-63, depending on the severity of symptoms. Type 2 diabetic patients or healthy control subjects with BDI-II or BAI values > 9 were considered to have symptoms of depression or anxiety, respectively.
Cognition assessment
We administered Montreal Cognitive Assessment (MoCA)38 on patients with type 2 diabetes and control subjects. Multiple aspects of cognition, including visuospatial skills, attention and focus, executive function, concentration, delayed memory recall, naming, and language was assessed using the MoCA. The MoCA is widely used as a screening test for cognitive impairment, with scores ranging from 0 to 30 (≥ 26 normal).
Magnetic resonance imaging
Brain imaging studies were performed using a 3.0-Tesla MRI scanner (Siemens, Magnetom Prisma, Erlangen, Germany). High-resolution T1-weighted images were collected using the magnetization-prepared rapid acquisition gradient-echo pulse sequence [repetition-time (TR) = 2200 ms; echo-time (TE) = 2.4 ms; inversion time = 900 ms; flip angle (FA) = 9°; matrix size = 320 × 320; field-of-view (FOV) = 230 × 230 mm2; slice thickness = 0.9 mm)]. Proton-density (PD) and T2-weighted images (TR = 10,000 ms; TE1, 2 = 17, 134 ms; FA = 130°; matrix size = 256 × 256; FOV = 230 × 230 mm2; slice thickness = 3.5 mm) were collected using a dual-echo turbo spin-echo sequence in the axial plane. DTI data were collected using a single-shot echo planar imaging with twice-refocused spin-echo pulse sequence (TR = 12,200 ms; TE = 87 ms; flip angle = 90°; bandwidth = 1,345 Hz/pixel; matrix size = 128×128; FOV = 230×230 mm; slice thickness = 1.7 mm, diffusion values = 0 and 800 s/mm2, diffusion directions = 30, separate series = 2). The parallel imaging technique, generalized auto-calibrating partially parallel acquisition (GRAPPA), with an acceleration factor of two, was used for DTI data collection.
Data processing
This study used the SPM12 software package (Department of Cognitive Neurology, Wellcome, UK), Diffusion Toolkit (TrackVis.org, Massachusetts General Hospital, USA), MRIcroN, and MATLAB-based software for data processing, analysis, and visualization. We performed visual assessment of T1-, T2-, and PD-weighted images of all subjects for any major pathology (e.g., cystic lesion, infarct, or tumor) and subsequently excluded subjects if any abnormalities were found. Diffusion and non-diffusion weighted images of all type 2 diabetes patients and healthy control subjects were also assessed for any head-motion related or other imaging artifacts before quantifying MD maps.
MD calculation & processing
Diffusion tensor matrices were quantified with the Diffusion Toolkit software using diffusion-weighted (b=800 s/mm2) and non-diffusion weighted images (b=0 s/mm2)39,40 The diffusion tensor matrices were diagonalized, and principal eigenvalues (λ1, λ2, and λ3) were calculated. The principal eigenvalues were then used to calculate MD [MD = (λ1+λ2+λ3)/3] values at each voxel, with voxel intensities on the MD maps showing the corresponding diffusion values. A fixed threshold value was used to mask-out background noise and non-brain regions on MD maps in Diffusion Toolkit.
We used the MATLAB-based SPM12 software for pre-processing of the MD maps. The MD maps, derived from each DTI series, were realigned to remove any potential variation from head motion and averaged. Similarly, non-diffusion weighted images were also realigned and averaged. The averaged MD maps were normalized to Montreal Neurological Institute (MNI) space. Non-diffusion weighted (b0) images were normalized to MNI space using a unified segmentation approach41 and the resulting normalization parameters were applied to corresponding MD maps. The normalized MD maps were smoothed with a Gaussian filter (8 mm).
Statistical analyses
We used the statistical package for social sciences (SPSS V28) for assessment of demographic, physiological, mood, and cognitive data. The numerical demographic and clinical variables were compared between groups with independent samples t-tests, and categorical characteristics were compared using the Chi-square test. Mood and cognition data were compared between type 2 diabetes patients and control subjects using ANCOVA (covariates, age and sex). Statistical threshold values of p<0.05 were considered as significant differences.
For regional brain MD differences between type 2 diabetes patients and control subjects, the smoothed MD maps were compared voxel-by-voxel using ANCOVA (SPM12; covariates, age and sex; false discovery rate, p<0.05). The statistical parametric maps showing brain sites with significant MD differences between groups were superimposed onto a mean anatomical image for structural identification using MRIcroN software. Regions of interest (ROI) values were obtained from brain regions with significant differences between T2DM and controls (ANCOVA, SPSS, covariates, age and sex, Bonferroni corrected).
Whole-brain MD maps were correlated voxel-by-voxel with mood and cognition scores including BAI, BDI-II, MoCA, and MoCA sub-scores in type 2 diabetes patients using partial correlations (SPM12; covariates, age and sex; p<0.005). The ROI values were obtained from brain sites showing significant associations with mood and cognition values. The Benjamini-Hochberg method was used to determine if the associations survived for multiple corrections, controlling for the false discovery rate with p<0.05.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Abbreviations
- BAI:
-
Beck anxiety inventory
- BDI-II:
-
Beck depression inventory
- DTI:
-
Diffusion tensor imaging
- FA:
-
Flip angle
- FOV:
-
Field-of-view
- MD:
-
Mean diffusivity
- MNI:
-
Montreal neurological Institute
- MoCA:
-
Montreal cognitive assessment
- PD:
-
Proton-density
- ROI:
-
Region of interest
- SNS:
-
Sympathetic nervous system
- SPM12:
-
Statistical parametric mapping package
- SPSS V28:
-
Statistical package for social sciences
- TR:
-
Repetition-time
- TE:
-
Echo-time
References
Cukierman, T., Gerstein, H. C. & Williamson, J. D. Cognitive decline and dementia in diabetes–systematic overview of prospective observational studies. Diabetologia 48, 2460–2469. https://doi.org/10.1007/s00125-005-0023-4 (2005).
Anderson, R. J., Freedland, K. E., Clouse, R. E. & Lustman, P. J. The prevalence of comorbid depression in adults with diabetes: A meta-analysis. Diabetes care 24, 1069–1078. https://doi.org/10.2337/diacare.24.6.1069 (2001).
Nichols, G. A. & Brown, J. B. Unadjusted and adjusted prevalence of diagnosed depression in type 2 diabetes. Diabetes Care 26, 744–749. https://doi.org/10.2337/diacare.26.3.744 (2003).
Cheng, G., Huang, C., Deng, H. & Wang, H. Diabetes as a risk factor for dementia and mild cognitive impairment: A meta-analysis of longitudinal studies. Int. Med. J. 42, 484–491. https://doi.org/10.1111/j.1445-5994.2012.02758.x (2012).
Exalto, L. G., Whitmer, R. A., Kappele, L. J. & Biessels, G. J. An update on type 2 diabetes, vascular dementia and Alzheimer’s disease. Exp. Gerontol. 47, 858–864. https://doi.org/10.1016/j.exger.2012.07.014 (2012).
Peila, R., Rodriguez, B. L., Launer, L. J. & Honolulu-Asia Aging, S. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The honolulu-asia aging study. Diabetes 51, 1256–1262. https://doi.org/10.2337/diabetes.51.4.1256 (2002).
Crane, P. K. et al. Glucose levels and risk of dementia. N. Engl. J. Med. 369, 540–548. https://doi.org/10.1056/NEJMoa1215740 (2013).
Egede, L. E., Zheng, D. & Simpson, K. Comorbid depression is associated with increased health care use and expenditures in individuals with diabetes. Diabetes Care 25, 464–470 (2002).
Holt, R. I. & Katon, W. J. Dialogue on diabetes and depression: dealing with the double burden of co-morbidity. J. Affect. Disord. 142(Suppl), S1-3. https://doi.org/10.1016/S0165-0327(12)00632-5 (2012).
Egede, L. E., Walker, R. J., Bishu, K. & Dismuke, C. E. Trends in costs of depression in adults with diabetes in the United States: Medical expenditure panel survey, 2004–2011. J. Gen. Intern. Med. 31, 615–622. https://doi.org/10.1007/s11606-016-3650-1 (2016).
Grigsby, A. B., Anderson, R. J., Freedland, K. E., Clouse, R. E. & Lustman, P. J. Prevalence of anxiety in adults with diabetes: A systematic review. J. Psychosom. Res. 53, 1053–1060. https://doi.org/10.1016/s0022-3999(02)00417-8 (2002).
Tovilla-Zarate, C. et al. Prevalence of anxiety and depression among outpatients with type 2 diabetes in the Mexican population. PLoS One 7, e36887. https://doi.org/10.1371/journal.pone.0036887 (2012).
van Bussel, F. C. et al. Altered hippocampal white matter connectivity in type 2 diabetes mellitus and memory decrements. J. Neuroendocrinol. 28, 12366. https://doi.org/10.1111/jne.12366 (2016).
Zhang, A. et al. White matter tract integrity of anterior limb of internal capsule in major depression and type 2 diabetes. Neuropsychopharmacology 38, 1451–1459. https://doi.org/10.1038/npp.2013.41 (2013).
Fang, P. et al. Changes in the cerebellar and cerebro-cerebellar circuit in type 2 diabetes. Brain Res. Bull. 130, 95–100. https://doi.org/10.1016/j.brainresbull.2017.01.009 (2017).
Nouwen, A. et al. Microstructural abnormalities in white and gray matter in obese adolescents with and without type 2 diabetes. Neuroimage. Clin. 16, 43–51. https://doi.org/10.1016/j.nicl.2017.07.004 (2017).
Zhang, J. et al. Disrupted white matter network and cognitive decline in type 2 diabetes patients. J. Alzheimers Dis. 53, 185–195. https://doi.org/10.3233/JAD-160111 (2016).
Yau, P. L., Kluger, A., Borod, J. C. & Convit, A. Neural substrates of verbal memory impairments in adults with type 2 diabetes mellitus. J. Clin. Exp. Neuropsychol. 36, 74–87. https://doi.org/10.1080/13803395.2013.869310 (2014).
Luchsinger, J. A. et al. Relation of diabetes to mild cognitive impairment. Arch. Neurol. 64, 570–575. https://doi.org/10.1001/archneur.64.4.570 (2007).
Yaffe, K. et al. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology 63, 658–663. https://doi.org/10.1212/01.wnl.0000134666.64593.ba (2004).
Musen, G. et al. Resting-state brain functional connectivity is altered in type 2 diabetes. Diabetes 61, 2375–2379. https://doi.org/10.2337/db11-1669 (2012).
Xiong, Y. et al. A diffusion tensor imaging study on white matter abnormalities in patients with type 2 diabetes using tract-based spatial statistics. AJNR Am. J. Neuroradiol. 37, 1462–1469. https://doi.org/10.3174/ajnr.A4740 (2016).
Zhang, Y. et al. Altered brain structural topological properties in type 2 diabetes mellitus patients without complications. J. Diabetes 11, 129–138. https://doi.org/10.1111/1753-0407.12826 (2019).
Krabbe, K. S. et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia 50, 431–438. https://doi.org/10.1007/s00125-006-0537-4 (2007).
Baldwin, R. C. & O’Brien, J. Vascular basis of late-onset depressive disorder. Br. J. Psychiatry: J. Mental Sci. 180, 157–160. https://doi.org/10.1192/bjp.180.2.157 (2002).
Bruce, D. G. et al. Vascular depression in older people with diabetes. Diabetologia 49, 2828–2836. https://doi.org/10.1007/s00125-006-0478-y (2006).
Knol, M. J. et al. Depression as a risk factor for the onset of type 2 diabetes mellitus A meta-analysis. Diabetologia 49, 837–845. https://doi.org/10.1007/s00125-006-0159-x (2006).
Holzschneider, K. & Mulert, C. Neuroimaging in anxiety disorders. Dialogues Clin. Neurosci. 13, 453–461 (2011).
Kanner, A. M. Structural MRI changes of the brain in depression. Clin. EEG Neurosci. 35, 46–52. https://doi.org/10.1177/155005940403500111 (2004).
Feinkohl, I., Price, J. F., Strachan, M. W. & Frier, B. M. The impact of diabetes on cognitive decline: Potential vascular, metabolic, and psychosocial risk factors. Alzheimers Res. Ther. 7, 46. https://doi.org/10.1186/s13195-015-0130-5 (2015).
Geijselaers, S. L. C., Sep, S. J. S., Stehouwer, C. D. A. & Biessels, G. J. Glucose regulation, cognition, and brain MRI in type 2 diabetes: A systematic review. Lancet. Diabetes Endocrinol. 3, 75–89. https://doi.org/10.1016/S2213-8587(14)70148-2 (2015).
Kyrou, I. & Tsigos, C. Stress hormones: Physiological stress and regulation of metabolism. Curr. Opin. Pharmacol. 9, 787–793. https://doi.org/10.1016/j.coph.2009.08.007 (2009).
Alexis, M. N., Stylianopoulou, F., Kitraki, E. & Sekeris, C. E. The distribution and properties of the glucocorticoid receptor from rat brain and pituitary. J. Biol. Chem. 258, 4710–4714 (1983).
Sapolsky, R. M., Uno, H., Rebert, C. S. & Finch, C. E. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J. Neurosci: Off. J. Soc. Neurosci. 10, 2897–2902 (1990).
Sheline, Y. I. Neuroimaging studies of mood disorder effects on the brain. Biol.Psychiatry 54, 338–352. https://doi.org/10.1016/s0006-3223(03)00347-0 (2003).
Beck, A. T., Epstein, N., Brown, G. & Steer, R. A. An inventory for measuring clinical anxiety: Psychometric properties. J. Consult. Clin. Psychology 56, 893–897. https://doi.org/10.1037//0022-006x.56.6.893 (1988).
Beck, A. T., Steer, R. A., Ball, R. & Ranieri, W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J. Pers. Asses. 67, 588–597. https://doi.org/10.1207/s15327752jpa6703_13 (1996).
Nasreddine, Z. S. et al. The montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53, 695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x (2005).
Basser, P. J. & Pierpaoli, C. A simplified method to measure the diffusion tensor from seven MR images. Magn. Reson. Med. 39, 928–934. https://doi.org/10.1002/mrm.1910390610 (1998).
Pierpaoli, C., Jezzard, P., Basser, P. J., Barnett, A. & Di Chiro, G. Diffusion tensor MR imaging of the human brain. Radiology 201, 637–648. https://doi.org/10.1148/radiology.201.3.8939209 (1996).
Ashburner, J. & Friston, K. J. Unified segmentation. Neuroimage 26, 839–851. https://doi.org/10.1016/j.neuroimage.2005.02.018 (2005).
Acknowledgements
Authors would like to thank Mrs. Karen A. Harada, Ms. Cristina Cabrera-Mino, Ms. Samantha Liebmann, and Ms. Daisy Mercado for assistance with data collection, and Ms. Megan Carrier for editorial assistance.
Funding
This research work was supported by National Institutes of Health R01 NR017190 and 3R01NR017190-03S1.
Author information
Authors and Affiliations
Contributions
R.K., and S.E.C. were involved in the conception, and design of the study. B.R., S.E.C., M.J.F., and R.K. were involved in the conduct of the study. B.R., and R.K. performed the analysis and interpretation of the results. B.R. wrote the first draft of the manuscript, and all authors edited, reviewed, and approved the final version of the manuscript. R.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Roy, B., Choi, S.E., Freeby, M.J. et al. Microstructural brain tissue changes contribute to cognitive and mood deficits in adults with type 2 diabetes mellitus. Sci Rep 13, 9636 (2023). https://doi.org/10.1038/s41598-023-35522-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-35522-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.