Abstract
To review the current evidence on the risk of gestational diabetes mellitus (GDM) in women with endometriosis, taking into account relevant confounders such as the higher frequency of Assisted Reproductive Technologies (ART) conceptions. Database searches on PubMed, Medline, Embase and Scopus through June 2022, using combinations of relevant keywords. A total of 18 studies, involving N = 4,600,885 women, were included. The overall risk of GDM in endometriosis patients was significantly higher than in controls (OR, 1.23; 95% CI 1.07–1.51). This significant association persisted in natural pregnancies (OR, 1.08; 95% CI 1.04–1.12) but not in pregnancies conceived through ART (OR, 0.93;95% CI 0.70–1.24). Based on the limited number of studies that examined this association in relation to endometriosis phenotype, an increased risk was found in more severe stages (OR, 3.20; 95% CI 1.20–8.54) but independently from localization of the lesions. Endometriosis increases the risk of GDM, with a possible progressive effect in more advanced stages of the disease. Although the effect magnitude may be limited in some subgroups, this finding has a clinically relevant impact due to both the strong biological plausibility and to the relatively high incidence of both endometriosis and GDM.
Similar content being viewed by others
Introduction
Gestational diabetes mellitus (GDM) is one of the most common metabolic disorders in obstetrics and a growing public health concern, given its strong prediction of future type 2 diabetes mellitus (T2DM) in both mothers and infants1. The scientific literature defines GDM as a state of hyperglycemia developing in pregnancy and resolving following delivery, caused by insulin resistance or reduced insulin production. Risk factors for GDM encompass pre-pregnancy overweight and obesity, advanced maternal age, excessive weight gain during pregnancy, a family history of T2DM, previous pregnancies with GDM, having given birth to a baby weighing over 4000 g and having multiple pregnancies.
In GDM, the pregnant woman’s metabolism influences both maternal and fetal heath. Excessive carbohydrates/lipids intake raise glucose levels stimulating maternal pancreas to release additional insulin with production of excessive body fat. Immune and inflammatory responses are generated within white adipose tissue, resulting in a low-grade, systemic, chronic metabolic inflammation2. The inflammatory response reduces both insulin action and pancreatic β-cell compensatory reaction, promoting the development of GDM3. Uncontrolled maternal hyperglycemia contributes to obstetric complications, such as polyhydramnios, macrosomia, labor anomalies or premature birth, to adverse neonatal outcomes, such as hypoglycemia and delayed lung maturation, with long-term consequences for the offspring, like an increased risk of obesity, T2DM and cardiovascular diseases later in life4.
GDM is the most frequently reported pregnancy complication among women with polycystic ovary syndrome (PCOS)5,6,7, leading to a three-fold increase in risk8,9,10,11. PCOS determines pre-existing insulin resistance and compensatory hyperinsulinemia, which lead to hyperandrogenemia that exacerbates hyperglycemia12,13,14. The resulting inflammation impairs β-cell function, further contributing to hyperglycemia during pregnancy15.
The rationale for our research question is based upon two observations: (1) the growing interest in adverse pregnancy and neonatal outcomes among women with endometriosis16,17,18; within this line of research, the association between endometriosis and GDM remains unclear. Based on evidence that chronic inflammation and prolonged cytokine exposure increase the risk of GDM19, it is plausible that the subclinical inflammation underneath endometriosis might also exhibit this positive association. Notably, available findings are largely influenced by relevant confounders, such as the frequent need of affected women to undergo Assisted Reproductive Technologies (ART) procedures, which ‘per se’ increase the risk of GDM20; (2) a novel hypothesis by evolutionary biologists supporting the idea that endometriosis and PCOS represent extreme and diametric (opposite) outcomes of variations in the hypothalamic-pituitary–gonadal axis development and activity, with endometriosis mediated by low prenatal testosterone and PCOS mediated by high prenatal testosterone21,22. This diametric disorder hypothesis predicts that women with PCOS and those with endometriosis might display opposite hormonal and metabolic phenotypes also during pregnancy.
The purpose of this systematic review and meta-analysis was to synthesize the best available evidence regarding the association between GDM and endometriosis. The influence of medically assisted reproduction in assessing this association was also considered.
Results
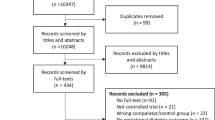
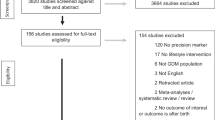
The PRISMA flow diagram of the review process is illustrated in Fig. 1. Out of the 330 full-text articles evaluated, 312 studies were excluded. In total, 18 studies23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40, involving N = 4,600,885 women, met the original inclusion criteria. Fifteen cohort studies23,24,25,26,27,28,29,31,32,34,35,36,38,39,40 (N = 4,600,016) and 3 case–control studies30,33,37 (N = 869) were included. Among the cohort studies, 10 were retrospective23,24,25,26,27,31,34,35,38,40 (with 2 of them27,31 employing a multicentric design), 2 were prospective32,36, 2 were based on a historical cohort28,29 and 1 was a nationwide study39. Out of the 3 case–control studies, 2 had a retrospective design30,33, while 1 was a prospective study37. A comprehensive summary of the characteristics of the included studies can be found in Table 1.
Risk of GDM in endometriosis patients versus controls
Studies overview
Out of the 1823,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40 studies included in the qualitative synthesis, two studies28,29 were based on the same historical cohort and study period, suggesting a high likelihood of data redundancy. Consequently, the quantitative synthesis was performed by omitting the study by Luke et al.28.
The population size of the included studies was highly variable: a total of N = 4,114,833 patients (n = 31,101 women with endometriosis and n = 4,083,732 controls) came from the largest study39 whereas only N = 88 women (n = 40 with endometriosis and n = 48 controls) were included in the smallest24.
In most of the included studies23,24,25,26,27,30,31,33,34,35,36,37,38, the diagnosis of endometriosis was based on surgical and histological confirmation of the disease. However, only two studies27,33,35 provided a complete description of the anatomical localizations of endometriosis lesions, whereas five studies24,33,34,37,38 reported data on endometriosis severity according to the revised American Fertility Society (r-AFS) staging system41. Notably, two studies focused exclusively on women with deep endometriosis (DE)26,31: one study26 included women with nodules surgically treated by segmental bowel resection, while another study31 evaluated women still exhibiting a posterior DE lesion of at least 2 cm on ultrasound assessment after a previous incomplete surgical excision.
In most studies, controls were defined as women without endometriosis; however, only one study36 performed a diagnostic laparoscopy to rule out the disease in controls. Regarding diagnosis of gestational diabetes, 8 studies23,25,27,31,32,35,38,40 used a positive oral glucose tolerance test (OGTT) as the criterion. Specifically, one study25 followed the Canadian Guidelines42, one32 followed the Japanese Guidelines43, one38 adhered to the Polish Guidelines44, and another40 followed the American Guidelines45. Interestingly, one study diagnosed gestational diabetes in cases and controls through self-administered questionnaires36; the Authors reported that, according to their previous large prospective cohort study, self-reported adverse pregnancy outcomes are validly reported, with a 94% confirmation rate for GDM46,47.
Quality assessment
The risk of bias assessment revealed that 8 studies23,25,32,35,36,37,38,40 were at low risk of bias, 826,27,28,29,30,31,33,34,39 had a moderate risk of bias, and the remaining one24 was at high risk of bias (Supplemental Fig. S1). According to the GRADE approach48, the overall quality of the evidence ranged from low to moderate.
Data analysis
The meta-analysis comparing endometriosis cases to controls, regardless the mode of conception, revealed a significantly increased risk of GDM in endometriosis (OR, 1.23; 95% CI 1.07–1.51; 17 studies; N = 4,599,449) with moderate heterogeneity (I2 = 53.43%) and non-significant publication bias (Egger’s: z = -0.75, p = 0.4557; Begg’s: z = -0.87, p = 0.4338) (Fig. 2a,b). Sensitivity analyses by omitting one study at time confirmed the robustness of the pooled risk estimate (Supplemental Fig. S2).
Risk of GDM in endometriosis patients versus controls including only ART pregnancies
In total, 7 studies23,28,29,30,33,37,40 met the inclusion criteria: 2 were retrospective cohort studies23,40, 2 were retrospective case–control studies30,33, 1 was a prospective case–control study37 and 2 were historical cohort studies28,29. Population sizes varied significantly: the total sample ranged from N = 92 women (n = 49 with endometriosis and n = 43 controls) in the smallest study23 to N = 2,307 women (n = 406 with endometriosis and n = 1,901 controls) in the largest29.
Most included studies23,29,30,33 considered ART cycles with pregnancies achieved through both fresh and frozen embryo transfers. One study included only fresh cycles37, and another included only frozen ART cycles40. For the majority of the included studies, we were able to extract data on our primary outcome specifically for male factor infertility controls23,29,33,40. General characteristics of the included studies are summarized in Table 1.
Quality assessment
Three studies23,37,40 were judged at low risk of bias, and three29,30,33 had a moderate risk of bias (Supplemental Fig. S1). The overall quality of the evidence, according to the GRADE approach48, was deemed low.
Data analysis
The meta-analysis failed to reveal significant differences in GDM risk between women with endometriosis and controls in the ART population (OR, 0.93; 95% CI 0.70–1.24; 6 studies; N = 3,778; p = 0.63) (Fig. 3a), with no heterogeneity (I2 = 0%). Symmetry was observed upon visual inspection of the funnel plot (Fig. 3b), and both Egger’s test (z = 1.76, p = 0.0787) and Begg’s test (z = 1.13, p = 0.2597) showed no evidence of a small-study effect. Subgroup analyses according to predefined moderators did not find any group difference in the pooled risk estimates (Supplemental Fig. S3).
Risk of GDM in endometriosis patients versus controls including only spontaneous pregnancies
Studies overview
Overall, only 3 studies24,29,39 could be included in this comparison: one was a retrospective cohort study24, one was a historical cohort29 and one was a nationwide cohort study39. The total population size was however quite large (N = 4,413,498).
Notably, the study population of Mekaru et al.24 also included conceptions through first-level infertility treatments (ovulation induction and intrauterine insemination). However, infertility treatments were reported to be comparable between the groups. Since conceptions through in-vitro fertilization (IVF) were excluded a priori, we did not consider any of the pregnancies evaluated by Mekaru et al.24 as obtained by ART. General characteristics of the studies included in this comparison are summarized in Table 1.
Quality assessment
Two studies were at moderate risk of bias29,39 and one had a high risk of bias24 (Supplemental Fig. S1). The overall quality of the evidence, according to the GRADE approach48, was deemed low.
Data analysis
The meta-analysis revealed a significantly increased risk of GDM in endometriosis women compared to controls in the natural conception population (OR, 1.08; 95% CI 1.04–1.12; 3 studies; N = 4,413,498; p < 0.001) (Fig. 4a), with no heterogeneity (I2 = 0%) and a non-significant small-studies effect (Egger’s: z = − 0.08, p = 0.934; Begg’s: z = − 1.04, p = 1.00) (Fig. 4b).
Risk of GDM in endometriosis patients comparing ART and natural pregnancies
Studies overview
Overall, 4 studies met the inclusion criteria29,34,38,39: one was a historical cohort29, two were retrospective cohort studies34,38, and the remaining was a nationwide cohort study39. Study populations were smaller than those in other comparisons performed, yet sample size varied considerably between studies: the largest study39 assessed a total of N = 38,035 patients (n = 6,934 endometriosis women who conceived by ART and n = 31,101 who conceived naturally), while the smallest38 included only N = 64 women (n = 36 endometriosis women with ART conceptions and n = 28 endometriosis women with natural conception).
Of all, 2 studies29,39 included ART cycles with pregnancies obtained by both fresh and frozen embryo transfers; the remaining two studies34,38 did not mention if ART cycles included only fresh or only frozen embryo transfers or both. Notably, data from Warzecha et al.38 for natural conception also included first-level infertility treatments (intrauterine insemination). General characteristics of the included studies are summarized in Table 1.
Quality assessment
Overall, one study was at low risk of bias38, while the remaining three were at moderate risk of bias29,34,39 (Supplemental Fig. S1). The overall quality, as judged by the GRADE approach48, was considered low or very low.
Data analysis
The meta-analysis failed to find any significant difference in GDM risk in pregnancies of patients affected by endometriosis with different modes of conception (ART versus natural) (OR, 0.97; 95% CI 0.89–1.06; 4 studies; N = 39,193; p = 0.51), with no heterogeneity in pooling data (I2 = 0%) (Fig. 5a). Symmetry of funnel plot and non-significant tests for small-studies effect (Egger’s: z = 1.06, p = 0.287; Begg’s: z = 1.02, p = 0.308) showed absence of significant publication biases (Fig. 5b).
Risk of GDM in endometriosis pregnancies by ART versus endometriosis spontaneous pregnancies. Legend: Forest plot summarizing the results of the meta-analysis (a). Funnel plot for publication bias (b). Abbreviations: ART = assisted reproductive techniques; CI = confidence interval; GDM = gestational diabetes mellitus.
Risk of GDM in patients with deep endometriosis versus all other localization of endometriosis
Studies overview
Overall, only 2 studies33,35 provided data on the prevalence of GDM in DE compared to all other localizations of endometriosis (ovarian and/or superficial and/or DE with concomitant ovarian and/or superficial lesions). Both had a retrospective design; one was a cohort study35 and one was a case–control study33. The overall study population was relatively small (N = 350). Interestingly, the prevalence of DE in the original study populations was 15.3% in the study by Mannini et al.35 and 43.4% in that by Jacques et al.33. General characteristics of the included studies are summarized in Table 1.
Quality assessment
Of the two included studies, one was at low risk of bias35 and the other was at moderate risk of bias33 (Supplemental Fig. S1). The overall quality of the evidence, according to the GRADE48 approach, was judged as very low.
Data analysis
The meta-analysis failed to show a significant difference in the risk of GDM in pregnant patients with different localizations of endometriosis: DE versus other disease localizations (OR, 0.67; 95% CI 0.32–1.40; 2 studies; N = 350; p = 0.29), with no heterogeneity (I2 = 0%) (Fig. 6). Relative symmetry was observed on visual inspection of the funnel plot (data not shown); however, due to the very low number of publications, publication bias could not be entirely ruled out.
Risk of GDM in stage III-IV versus stage I-II endometriosis
Studies overview
Overall, only two studies provided complete data on the prevalence of GDM according to endometriosis severity33,38. One was a retrospective cohort study38 and the other was a retrospective case–control study33. The total study population was very small (N = 175). The prevalence of stage III–IV endometriosis patients according to the r-AFS classification41 was similar in the two study populations: 57.81% in Warzecha et al.’s cohort38 and 46.85% in Jacques et al.’s sample33. General characteristics of the two included studies are summarized in Table 1.
Quality assessment
One study was at low risk of bias38, and the other study was at moderate risk of bias33 (Supplemental Fig. S1). Due to the very low number of publications available, the overall quality of the evidence, as judged by the GRADE approach48, was considered very low.
Data analysis
The meta-analysis found a significantly increased risk of GDM in patients with stage III-IV disease severity compared to stage I-II (OR, 3.20; 95% CI 1.20–8.54; 2 studies; N = 175; p = 0.02). Patients with advanced stages of the disease showed more than threefold increase in the risk of the outcome, with no study heterogeneity (I2 = 0%) (Fig. 7). Relative symmetry was observed upon visual inspection of the funnel plot (data not shown), demonstrating non-significant evidence of publication bias despite the very low number of publications available.
Discussion
This meta-analysis demonstrates that the GDM risk is increased in pregnancies with endometriosis compared to unaffected controls. We could observe a noteworthy sequence of progression with significantly greater risk of GDM in more severe stages of endometriosis. The association of endometriosis and GDM remained stable in most subgroups analyses, including those related to study design, method of diagnosis of endometriosis and/or of GDM. Overall, the association of endometriosis and GDM appeared unrelated to the method of conception, given the absence of significance difference in the risk when comparing endometriosis patients conceiving spontaneously with those achieving pregnancy by medically assisted reproduction. The overall quality of the evidence for the main comparison according to GRADE approach48 is low to moderate, heterogeneity is low-moderate, and publication bias or small-study effects were not demonstrated.
Endometriosis has been consistently associated with several adverse pregnancy outcomes, and mounting evidence suggests an increased risk especially of preterm birth, pregnancy hypertension and small-for-gestational age16,17. This comes in line with our results, given the known association of some of these unfavorable pregnancy outcomes with GDM.
However, to date, the real association between endometriosis and many pregnancy complications remains rather controversial. The fact that the most women with endometriosis suffer from infertility and are thus ART-users is probably the main driver of such discordant findings. Indeed, women conceiving by ART are known to be at high risk for several obstetric complications, including GDM20, independently of the cause of infertility.
To account for ART influence on pooled risk estimates, in this meta-analysis we stratified comparisons of endometriosis to unaffected controls according to the method of conception; interestingly, results remained stable only when accounting for spontaneous conceptions. Infertile PCOS women, who are known to carry a higher risk of GDM7,8,9,10,11,12, are often ART users. The possibility of PCOS as indication for ART in our control population is unlikely, as we have excluded controls affected by this disease (unless they were undetectable from other causes of infertility in original studies). On the other hand, ovulatory disorders, in general, have been shown to be associated with GDM49 and could have been represented consistently within the control groups of the ART studies. In any case, our results confirm the suggested role of ART as a major confounder in interpreting current research data and the imperative need to weigh comparisons according to this eventual parallel risk factor for GDM in controls.
Interestingly, unlike pregnancy outcomes related to placental dysfunctions in which endometriosis and ART conceptions somehow present additive risks50, this does not seem to happen for GDM. Our findings, supporting no differences in risk estimates between endometriosis pregnancies conceived naturally or by ART, strengthen the idea that endometriosis itself and not ART treatment determines an increase in GDM risk in women affected by the disease. This is in line with recent findings suggesting that endometriosis is associated with adverse pregnancy outcomes independently from infertility diagnosis or fertility treatment51.
An effect gradient between r-AFS stage41 of endometriosis and GDM was observed in this meta-analysis. This is quite interesting considering that endometriosis progression has been related to increased levels of circulating and peritoneal fluid interleukins, systemic inflammation, and immune activation, with an overall higher prevalence of autoimmune diseases52.
The possible etiology of GDM in endometriosis patients is likely linked to the systemic inflammation associated with the disease53,54. GDM itself is not only related to increased insulin resistance and glucose intolerance, but also to low-grade systemic inflammation55. While adipose tissue is increasingly recognized as a legitimate immune organ in PCOS patients, in endometriosis patients, the disease itself contributes to the production of inflammatory effectors such as leptin, tumor necrosis factor-alpha and interleukin-6 with reduced production of adiponectin, potentially leading to insulin resistance. Leptin levels are increased and those of adiponectin decreased in women with endometriosis56, particularly during pregnancy when the mother frequently increases carbohydrates intake.
Our results do not seem to support the diametric model proposed by the Crespi’s group21,22, which suggests that PCOS and endometriosis would arise as maladaptive extremes due to variation in hypothalamic–pituitary–gonadal axis development and intrauterine androgens levels. According to this hypothesis, the metabolic and endocrine alterations observed in PCOS, such as GDM, would not be present in women with endometriosis21,22. On the other hand, we cannot exclude that GDM in endometriosis arises as a phenomenon secondary to sustained inflammation and immune dysregulation and therefore would be totally unrelated to the mechanisms underlying the disease etiology. Indeed, endometriosis is a lifelong disease in which chronic inflammation acts as one of the main drivers possibly involved not only in the genesis and maintenance of endometrial ectopic lesions, but also in the establishment of a susceptibility status for several comorbidities in the life course of women affected.
The major strength of our work is the high biological plausibility justifying the association found, and the fact that the incidence of both endometriosis and GDM is such that the emerged risk increase has a significant impact on clinical practice. The finding that endometriosis increases GDM risk further supports the idea that women with endometriosis may represent a unique population at greater risk for adverse outcomes across pregnancy. If currently GDM diagnosis is based on evaluation of blood glucose levels at late stages of pregnancy, the presence of endometriosis should potentially modify this criterion. Therefore, endometriosis may be considered as a red flag and should be included among the routinely early assessed risk factors for GDM. In this sense, the earlier and more specific detection of GDM in women with endometriosis could improve pregnancy management and final maternal–fetal outcomes. Indeed, elucidating pathways for prevention, screening and intervention in pregnancies of women with endometriosis will be critical to improve the health of these women and their children.
This study presents other important strengths. First, only one meta-analysis57 to date has specifically investigated the association between endometriosis and GDM, concluding that endometriosis had no significant effect on GDM risk. Our analysis has several added values, including the fact that we have incorporated more recent data. Even more importantly, the novelty of our meta-analysis is that results were provided weighting and stratifying the estimates accounting for clinically relevant confounders, managing the possible over-estimate of the effect at a population level. Third, the estimation of the certainty of the evidence following GRADE guidelines58 allowed us to identify missing gaps in current knowledge. Forth, the estimation method59 adopted allowed us to produce a robust, unbiased, nonnegative estimate of between-study variability. In this meta-analysis there are also some limitations that must be acknowledged. First, the evidence was mainly generated by observational studies, so the quality of the evidence is moderate to low according to GRADE guidelines58. Secondly, sample sizes of original studies were quite heterogeneous, with very large study populations that could have strongly influenced our results. To limit the larger weight in risk estimate from larger studies, sensitivity analysis was obtained by omitting one study at time, giving consistent results. Thirdly, substantial heterogeneity across studies was observed particularly in the population under study and the definition of endometriosis and/or GDM. These limitations were managed with subgroup analysis, even if the number of studies available was limited and the resulting quality of the evidence was graded as low, hindering generalization of some results. This suggests that further research is needed, possibly standardizing the reporting of disease prevalence by endorsing major international guidelines to reach more robust conclusions.
In conclusion, this systematic review and meta-analysis showed that endometriosis is associated with an increased rate of GDM. Therefore, a positive anamnesis for endometriosis must be considered in the prevention, early diagnosis and management of GDM, both in clinical practice and in research settings. It is important to consider the risk of other coexisting conditions frequently encountered in patients with endometriosis, such as autoimmune diseases, as clearly these risks contribute to the risk of adverse pregnancy outcomes60. We can also speculate on the possibility of GDM prevention based upon adequate treatment of endometriosis with pharmacological or surgical methods. More research is required to examine this topic in more detail, including investigations of the underlying mechanisms explaining the association of endometriosis with GDM.
Methods
This is a systematic review and meta-analysis performed according to the Preferred Reporting Item for Systematic Reviews and Meta-analysis (PRISMA)61 and the Meta-analysis Of Observational Studies in Epidemiology (MOOSE)62 guidelines. The study protocol was prospectively registered (date registered: March 13, 2022) on the publicly accessible database PROSPERO with the registration ID CRD42022309113.
Eligibility criteria, information sources, search strategy
A systematic literature search from April 2022 through June 2022 was performed using the following databases: PubMed, Medline, Embase and Scopus. MeSH terms for PubMed and comparable terms for other databases were used. Literature search was based on the following search terms’ combination: ((gestational diabetes) OR (pregnancy diabetes) OR (pregnancy complication) OR (maternal outcomes) OR (pregnancy out-comes)) AND ((endometriosis) OR (endometrioma)) AND ((IVF) OR (ICSI) OR (ART) OR (natural conception) OR (spontaneous conception)) and limited to studies on humans. No restrictions for year of publication and geographic location were applied. Only full-length manuscripts written in English language and published in peer-reviewed journals were screened. Bibliography of relevant papers was also examined to identify any relevant article not captured by the electronic searches. Duplicates were removed by Endnote Software (available online: https://endnote.com, accessed on 02 July 2022). The literature search and the article eligibility were independently assessed by two Authors (N.S. and L.L.P). Disagreements were resolved by discussion with a third reviewer (P.V.).
Inclusion and exclusion criteria
Case–control and cohort studies reporting the incidence of GDM in pregnant women with diagnosis of endometriosis compared with a control group were included. We did not include descriptive studies (case-reports and case-series) and studies that did not reported original results (reviews, abstracts, editorials, comments).
We included only original studies reporting a confirmed diagnosis of endometriosis in cases; women with endometriosis were included regardless of their medical/surgical treatment history or symptoms before pregnancy. The controls were women without a diagnosis of endometriosis, including both fertile and infertile women referred to a specialized Fertility Centre. When original studies reported data in controls stratified by specific indications for ART, we used the reference group whose infertility was due to male factor as the first option or, as a second option, to other indications for ART with no diagnosis of endometriosis. Male factor infertility was used as a reference group in several other studies of ART outcomes63,64,65,66, suggesting absence of infertility issues for the female partner (misdiagnosis of endometriosis is expected to be below 5%: similar to the general population). Controls with PCOS diagnosis were always excluded, in light of our research question. Additionally, studies where the entire population of controls was reported to have PCOS and/or altered glucose tolerance or insulin-resistance were a priori excluded. Studies in which for controls it was impossible to ascertain that infertility was unrelated to endometriosis were excluded.
Medically assisted reproduction was defined as a pregnancy achieved by second-line ART treatments, including IVF or intracytoplasmic sperm injections (ICSI) procedures. Studies not mentioning the mode of conception (either natural or medically assisted or both) for endometriosis cases and/or controls were excluded.
Data extraction
Data from original studies were extracted by two independent reviewers (N.S. and L.L.P). The following data were collected and tabulated: author; publication year; study country; study design; sample size; frequency of GDM in cases and controls; diagnostic modality for endometriosis; criteria followed to define gestational diabetes; endometriosis localization (ovarian, superficial, deep); endometriosis severity according to r-AFS41; type of controls (fertile or infertile, infertility etiology); mode of conception (natural or medically assisted); type of ART (IVF/ICSI, fresh or frozen embryo transfer); demographic data (maternal age, body mass index (BMI) and parity); other possible confounding variables at multivariate analysis.
Assessment of risk of bias
The risk of bias within and across studies was assessed referring to the Risk of Bias In Non-randomized Studies of Exposures (ROBINS-E) tool from the updated Cochrane collaboration guidelines67. Based on answers to the signaling questions of the seven bias domains for ROBINS-E tool68, an overall judgment was reached so that each study was classified as follows: low risk of bias, when the study was considered comparable to a well-performed randomized trial; moderate risk of bias, for studies providing sound evidence for a non-randomized study but still not comparable to those coming from a well-performed randomized trial; serious risk of bias, when the study had one or more important problems (serious risk of bias in at least one domain, but not at critical risk of bias in any domain); critical risk of bias, when the study was judged as too problematic to provide any useful evidence.
To guide interpretation of the confidence in the effect estimates, the certainty of the evidence was graded into four levels according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) guidelines58: high, moderate, low or very low. Risk of bias was evaluated by two independent reviewers (N.S. and L.L.P); where disagreement occurred, consensus was reached with input from a third team member (P.V.).
Study outcomes and outcomes measures
The a priori planned primary outcome of the current meta-analysis was the incidence of GDM in pregnancies from endometriosis patients compared to unaffected controls. Original studies enrolling women with pre-gestational diabetes or conditions characterized by altered glucose metabolism not satisfying the diagnosis of GDM (i.e., impaired fasting glucose or impaired glucose tolerance) were excluded. Also, according with American Diabetes Association69, studies where the diagnosis of diabetes occurred during the 1st trimester of pregnancy were not included. In light of the absence of a worldwide adopted diagnostic criteria for GDM, we planned a subgroup analysis for the primary outcome according with the diagnostic modality for GDM reported in original studies.
Meta-analysis methods
Data synthesis
To provide a theoretical underpinning of the qualitative synthesis, a quantitative synthesis of included studies was also performed by an independent reviewer (N.S.). Original data on binary outcome measures were extrapolated so that Log Odds Ratios (ORs) with 95% Confidence Intervals (CIs) and corresponding standard errors (SEs) were computed from original data and pooled together. A random-effects (RE) meta-analysis model was performed to estimate pooled effect sizes and a restricted maximum likelihood (REML) estimation method was used to compute between-study variabilities (\(\uptau _{2}\))59. When the assumption of study homogeneity was reasonable, a fixed-effects (FE) model using Mantel–Haenszel method was also performed70.
The overall effect size was estimated as the weighted average of study-specific effect sizes, with larger studies having greater weights and smaller studies having lesser weights. Forest plots were used to present study-specific and overall effect sizes with their respective CIs. Sensitivity analyses were conducted by omitting one study at time to present relative influence of each study on pooled risk estimate.
STATA version 17 software (Stata Corp LLC, 2021, College Station, TX, USA) was used for all statistical analyses. A p-value < 0.05 was considered to be statistically significant.
Groups’ comparisons
The primary analysis to answer the research question was obtained comparing pooled data on the risk of GDM in endometriosis patients versus controls, independently from mode of conception (both medically assisted and spontaneous). Pooled risk estimates for the primary outcome were also provided separately according to the following groups comparisons: (1) pregnancies from endometriosis patients conceived by ART versus pregnancies from controls conceived by ART; (2) spontaneous pregnancies in endometriosis women versus spontaneous pregnancies in controls; (3) pregnancies in endometriosis patients conceived by ART versus pregnancies in endometriosis cases conceived by natural conception; (4) DE versus all other localizations of endometriosis; (5) cases affected by stage III-IV endometriosis versus stage I-II endometriosis.
Assessment of publication bias and small-study effects
To investigate the impact of publication bias and small-study effects on final results, funnel plots were implemented scattering the logarithm of the study-specific effect sizes (log ORs) against their SEs. Funnel plot asymmetry was tested using both the linear regression-based method according to Egger et al.71 and the adjusted rank correlation test proposed by Begg et al.72.
Assessment of heterogeneity
Between-study heterogeneity was explored throughout the I2 statistics which estimates the percentage of total variation across studies that is due to between-studies heterogeneity rather than to sampling variation73. I2 index values were interpreted as follows: 0–25%, insignificant heterogeneity; 25–50%, low heterogeneity; 50–75%, moderate heterogeneity; > 75%, high heterogeneity1. The Chi-squared statistic was also interpreted as a result of heterogeneity, so that a low p-value (< 0.10) questioned the validity of the pooled risk estimates74.
Subgroup analysis and investigation of heterogeneity
Subgroup-analyses were performed according to Wang et al.75 to explore the level of heterogeneity explained by study-level covariates. We planned to carry out subgroup-analyses accounting for the following study moderators: study design, study country, endometriosis diagnosis (self-reported/questionnaire or surgical/histological), GDM diagnosis (medical record review/database search/questionnaire or positive glucose tolerance test or unknown), age of population (≤ 35 years or > 35 years or unknown), BMI categories (normal or unknown) and parity (both multiparous and nulliparous or only nulliparous). For subgroup-analyses performed selectively on ART population, the following covariates were also included: type of control for endometriosis cases (all causes of infertility or only male factor) and type of ART cycle (fresh and frozen or only fresh or only frozen).
Data availability
The data for this meta-analysis are freely available. The PROSPERO protocol can be found at https://www.crd.york.ac.uk/prospero CRD ID: CRD42022309113.
References
Salmeri, N. et al. Maternal arsenic exposure and gestational diabetes: A systematic review and meta-analysis. Nutrients 12, 3094 (2020).
McElwain, C. J., McCarthy, F. P. & McCarthy, C. M. Gestational diabetes mellitus and maternal immune dysregulation: What we know so far. Int. J. Mol. Sci. 22, 4261 (2021).
Lorenzo, P. I., Martín-Montalvo, A., Cobo Vuilleumier, N. & Gauthier, B. R. Molecular modelling of islet β-cell adaptation to inflammation in pregnancy and gestational diabetes mellitus. Int. J. Mol. Sci. 20, 6171 (2019).
Mistry, S. K. et al. Gestational diabetes mellitus (GDM) and adverse pregnancy outcome in South Asia: A systematic review. Endocrinol Diabetes Metab. 4, e00285 (2021).
Shek, N. W., Ngai, C. S., Lee, C. P., Chan, J. Y. & Lao, T. T. Lifestyle modifications in the development of diabetes mellitus and metabolic syndrome in Chinese women who had gestational diabetes mellitus: A randomized interventional trial. Arch. Gynecol. Obstet. 289, 319–327 (2014).
Poolsup, N., Suksomboon, N. & Amin, M. Effect of treatment of gestational diabetes mellitus: A systematic review and meta-analysis. PLoS ONE 9, e92485 (2014).
Yu, H. F., Chen, H. S., Rao, D. P. & Gong, J. Association between polycystic ovary syndrome and the risk of pregnancy complications: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 95, e4863 (2016).
Boomsma, C. M. et al. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum. Reprod. Update 12, 673–683 (2016).
Kjerulff, L. E., Sanchez-Ramos, L. & Duffy, D. Pregnancy outcomes in women with polycystic ovary syndrome: A metaanalysis. Am. J. Obstet. Gynecol. 204, 558 (2011).
Qin, J. Z. et al. Obstetric complications in women with polycystic ovary syndrome: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 11, 56 (2013).
Wei, Y. M. et al. Risk of adverse pregnancy outcomes stratified for pre-pregnancy body mass index. J. Matern. Fetal. Neonatal Med. 29, 2205–2209 (2016).
Toulis, K. A. et al. Risk of gestational diabetes mellitus in women with polycystic ovary syndrome: A systematic review and a meta-analysis. Fertil. Steril. 92, 667–677 (2009).
Baptiste, C. G., Battista, M. C., Trottier, A. & Baillargeon, J. P. Insulin and hyperandrogenism in women with polycystic ovary syndrome. J. Steroid Biochem. Mol. Biol. 122, 42–52 (2010).
Ding, H. et al. Resistance to the insulin and elevated level of androgen: A major cause of polycystic ovary syndrome. Front. Endocrinol. (Lausanne) 12, 741764 (2021).
Moyce, B. L. & Dolinsky, V. W. Maternal β-cell adaptations in pregnancy and placental signalling: Implications for gestational diabetes. Int. J. Mol. Sci. 19, 3467 (2018).
Zullo, F. et al. Endometriosis and obstetrics complications: A systematic review and meta-analysis. Fertil. Steril. 108, 667-672.e5 (2017).
Salmeri, N. et al. Endometriosis and impaired placentation: A prospective cohort study comparing uterine arteries doppler pulsatility index in pregnancies of patients with and without moderate-severe disease. Diagnostics (Basel) 12, 1024 (2022).
Vigano, P., Corti, L. & Berlanda, N. Beyond infertility: Obstetrical and postpartum complications associated with endometriosis and adenomyosis. Fertil. Steril. 104, 802–812 (2015).
Gomes, C. P. et al. Cytokine levels in gestational diabetes mellitus: A systematic review of the literature. Am. J. Reprod. Immunol. 69, 545–557 (2013).
Bosdou, J. K. Risk of gestational diabetes mellitus in women achieving singleton pregnancy spontaneously or after ART: A systematic review and meta-analysis. Hum. Reprod. Update 26, 514–544 (2020).
Dinsdale, N. L. & Crespi, B. J. Endometriosis and polycystic ovary syndrome are diametric disorders. Evol. Appl. 14, 1693–1715 (2021).
Dinsdale, N., Nepomnaschy, P. & Crespi, B. The evolutionary biology of endometriosis. Evol. Med. Public Health 9, 174–191 (2021).
Kuivasaari-Pirinen, P., Raatikainen, K., Hippeläinen, M. & Heinonen, S. Adverse outcomes of IVF/ICSI pregnancies vary depending on aetiology of infertility. ISRN Obstet. Gynecol. 2012, 451915 (2012).
Mekaru, K. et al. Endometriosis and pregnancy outcome: Are pregnancies complicated by endometriosis a high-risk group?. Eur. J. Obstet. Gynecol. Reprod. Biol. 172, 36–39 (2014).
Aris, A. A 12-year cohort study on adverse pregnancy outcomes in Eastern Townships of Canada: Impact of endometriosis. Gynecol. Endocrinol. 30, 34–37 (2014).
Baggio, S. et al. Delivery and pregnancy outcome in women with bowel resection for deep endometriosis: A retrospective cohort study. Gynecol. Surg. 112, 279–285 (2015).
Conti, N. et al. Women with endometriosis at first pregnancy have an increased risk of adverse obstetric outcome. J. Matern. Fetal. Neonatal Med. 28, 1795–1798 (2015).
Luke, B. et al. Birth outcomes by infertility diagnosis analyses of the Massachusetts outcomes study of assisted reproductive technologies (MOSART). J. Reprod. Med. 60, 480–490 (2015).
Stern, J. E. et al. Adverse pregnancy and birth outcomes associated with underlying diagnosis with and without assisted reproductive technology treatment. Fertil. Steril. 103, 1438–1445 (2015).
Benaglia, L. et al. Pregnancy outcome in women with endometriosis achieving pregnancy with IVF. Hum. Reprod. 31, 2730–2736 (2016).
Exacoustos, C., Lauriola, I., Lazzeri, L., De Felice, G. & Zupi, E. Complications during pregnancy and delivery in women with untreated rectovaginal deep infiltrating endometriosis. Fertil. Steril. 106, 1129-1135.e1 (2016).
Harada, T. et al. Obstetrical complications in women with endometriosis: A cohort study in Japan. PLoS ONE 11, e0168476 (2016).
Jacques, M., Freour, T., Barriere, P. & Ploteau, S. Adverse pregnancy and neo-natal outcomes after assisted reproductive treatment in patients with pelvic endometriosis: A case-control study. Reprod. Biomed. Online 32, 626–634 (2016).
Li, H. et al. Effects of previous laparoscopic surgical diagnosis of endometriosis on pregnancy outcomes. Chin. Med. J. (Engl.) 130, 428–433 (2017).
Mannini, L. et al. New adverse obstetrics outcomes associated with endometriosis: A retrospective cohort study. Arch. Gynecol. Obstet. 295, 141–151 (2017).
Farland, L. V. et al. Endometriosis and risk of adverse pregnancy outcomes. Obstet. Gynecol. 134, 527–536 (2019).
Sharma, S. et al. Pregnancy and live birth rates are comparable in young infertile women presenting with severe endometriosis and tubal infertility. Reprod. Sci. 27, 1340–1349 (2020).
Warzecha, D., Pietrzak, B., Szymusik, I., Smiech, Z. & Wielgos, M. Should the patients with endometriosis be treated as a risk group of pregnancy complications? Single center experience and literature review and literature review. Ginekol. Pol. 91, 383–388 (2020).
Epelboin, S. et al. Endometriosis and assisted reproductive techniques independently related to mother–child morbidities: A French longitudinal national study. Reprod. Biomed. Online 42, 627–633 (2021).
Wang, J. et al. Pregnancy outcomes of Chinese women undergoing IVF with embryonic cryopreservation as compared to natural conception. BMC Pregnancy Childbirth. 21, 39 (2021).
Canis, M. et al. Revised American society for reproductive medicine classification of endometriosis: 1996. Fertil. Steril. 67, 817–821 (1997).
Bhattacharyya, O. K., Estey, E. A., Cheng, A. Y., Canadian Diabetes Association 2008. Update on the Canadian Diabetes Association 2008 clinical practice guidelines. Can. Fam. Phys. Med. Fam. Can. 55, 39–43 (2009).
Minakami, H. et al.; Japan Society of Obstetrics and Gynecology; Japan Association of Obstetricians and Gynecologists. Guidelines for obstetrical practice in Japan: Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists (JAOG) 2011 edition. J. Obstet. Gynaecol. Res. 37, 1174–1197 (2011).
Wender-Ożegowska, E. et al. Standards of Polish society of gynecologists and obstetricians in management of women with diabetes. Ginekol. Pol. 89, 341–350 (2018).
ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet Gynecol. 131, e49–e64 (2018).
Stuart, J. J. et al. Hypertensive disorders of pregnancy and maternal cardiovascular disease risk factor development: An observational cohort study. Ann. Intern. Med. 169, 224–232 (2018).
Tanz, L. J. et al. Preterm delivery and maternal cardiovascular disease in young and middle-aged adult women. Circulation 135, 578–589 (2017).
Schünemann, H., Brożek, J., Guyatt, G., Oxman, A. GRADE Handbook. https://gdt.gradepro.org/app/handbook/handbook.html (The GRADE Working Group, 2013).
Stern, J. E. et al. Assisted reproductive technology treatment increases obstetric and neonatal risks over that of the underlying infertility diagnosis. Fertil. Steril. 117, 1223–1234 (2022).
Ibiebele, I. et al. Pregnancy outcomes in women with endometriosis and/or ART use: A population-based cohort study. Hum. Reprod. 37, 2350–2358 (2022).
Velez, M. P. et al. Mode of conception in patients with endometriosis and adverse pregnancy outcomes: A population-based cohort study. Fertil. Steril. 118, 1090–1099 (2022).
Vanni, V. S. et al. Concomitant autoimmunity may be a predictor of more severe stages of endometriosis. Sci. Rep. 11, 15372 (2021).
Gentilini, D. et al. Gene expression profiling of peripheral blood mononuclear cells in endometriosis identifies genes altered in non-gynaecologic chronic inflammatory diseases. Hum. Reprod. 26, 3109–3117 (2011).
Giacomini, E. et al. Genetics and inflammation in endometriosis: Improving knowledge for development of new pharmacological strategies. Int. J. Mol. Sci. 22, 9033 (2021).
Sharma, S., Banerjee, S., Krueger, P. M. & Blois, S. M. Immunobiology of gestational diabetes mellitus in post-medawar era. Front. Immunol. 12, 758267 (2022).
Zhao, Z. et al. Association of leptin and adiponectin levels with endometriosis: A systematic review and meta-analysis. Gynecol. Endocrinol. 37, 591–599 (2021).
Pérez-López, F. R. et al. Endometriosis and gestational diabetes mellitus risk: A systematic review and meta-analysis. Gynecol. Endocrinol. 34, 363–369 (2018).
Guyatt, G. H. et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336, 924–926 (2008).
Raudenbush, S. W. Analyzing effect sizes: Random effects models. In The Handbook of Research Synthesis and Meta-Analysis 2nd edn (eds Cooper, H. et al.) 295–316 (Russell Sage Foundation, New York, 2009).
Chatzakis, C., Cavoretto, P. & Sotiriadis, A. Gestational diabetes mellitus pharmacological prevention and treatment. Curr. Pharm. Des. 27, 3833–3840 (2021).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 151, 264–269 (2019).
Stroup, D. F. et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283, 2008–2012 (2000).
Kawwass, J. F. et al. Tubal factor infertility and perinatal risk after assisted reproductive technology. Obstet. Gynecol. 121, 1263–1271 (2013).
Chen, Z., Zhang, D., Zhen, J., Sun, Z. & Yu, Q. Predicting cumulative live birth rate for patients undergoing in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) for tubal and male infertility: A machine learning approach using XGBoost. Chin. Med. J. (Engl.) 135, 997–999 (2022).
Alasmari, W. et al. Comparable reproductive outcomes of ICSI for couples with unexplained infertility and couples with male factor infertility. Middle East Fertil. Soc. J. 23, 393–398 (2018).
Rees, C. O. et al. Women with combined adenomyosis and endometriosis on MRI have worse IVF/ICSI outcomes compared to adenomyosis and endometriosis alone: A matched retrospective cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 271, 223–234 (2022).
Deeks, J. J., Higgins, J. P. T., Altman, D. G. on behalf of the Cochrane Statistical Methods Group. Assessing risk of bias in a non-randomized study in Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (ed. Higgins, J.P.T. et al.) https://training.cochrane.org/handbook/current/chapter-25 (The Cochrane Collaboration, 2022).
Higgins, J. P. T. et al. on behalf the ROBINS-E Development Group. Risk of Bias In Non-randomized Studies - of Exposure (ROBINS-E). https://www.riskofbias.info/welcome/robins-e-tool (2022).
American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 43, S14-S31 (2020).
Mantel, N. & Haenszel, W. Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 22, 719–748 (1959).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997).
Begg, C. B. & Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 (1994).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558 (2002).
Deeks, J. J., Higgins, J. P. T., Altman, D. G. on behalf of the Cochrane Statistical Methods Group. Analysing data and undertaking meta-analyses in Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (ed. Higgins, J.P.T. et al.) https://training.cochrane.org/handbook/current/chapter-10 (The Cochrane Collaboration, 2022).
Wang, R., Lagakos, S. W., Ware, J. H., Hunter, D. J. & Drazen, J. M. Statistics in medicine–reporting of subgroup analyses in clinical trials. N. Engl. J. Med. 357, 2189–2194 (2007).
Funding
This study was partially funded by Ministry of Health-Current research IRCCS.
Author information
Authors and Affiliations
Contributions
Conceptualization, P.V. and N.S.; methodology, N.S.; software, N.S.; validation, P.V., E.S., P.I.C. and M.C.; formal analysis, N.S.; investigation, N.S and L.L..; resources, N.S and P.V.; data curation, N.S.; writing—original draft preparation, N.S. and P.V.; writing—review and editing, E.S. and P.I.C.; visualization, N.S., L.L., P.I.C., E.S., P.V. and M.C.; supervision, P.V.; project administration, N.S. and P.V. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Salmeri, N., Li Piani, L., Cavoretto, P.I. et al. Endometriosis increases the risk of gestational diabetes: a meta-analysis stratified by mode of conception, disease localization and severity. Sci Rep 13, 8099 (2023). https://doi.org/10.1038/s41598-023-35236-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-35236-y
This article is cited by
-
Effect of arsenic on the risk of gestational diabetes mellitus: a systematic review and meta-analysis
BMC Public Health (2024)
-
Causal effects of glycemic traits and endometriosis: a bidirectional and multivariate mendelian randomization study
Diabetology & Metabolic Syndrome (2024)
-
Determinants of Conception and Adverse Pregnancy Outcomes in Women with Endometriosis: A Longitudinal Study
Reproductive Sciences (2024)
-
Alteration in Effects of Endometriosis on Fecundity According to Pregnancy Experience in Mouse Model
Reproductive Sciences (2024)
-
Effects of dysregulated glucose metabolism on the occurrence and ART outcome of endometriosis
European Journal of Medical Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.