Abstract
Few studies have directly compared the incidence of pneumonia in patients on common chronic obstructive pulmonary disease (COPD) treatments such as long-acting muscarinic antagonists (LAMA) with those on inhaled corticosteroids and long-acting β2-agonist (ICS/LABA). Moreover, risk factors for pneumonia in COPD are still unclear. We aimed to compare the incidence of pneumonia in COPD patients on LAMA and those on ICS/LABA and explored the risk factors associated with pneumonia. This nationwide cohort study used Korean National Health Insurance claim data from January 2002 to April 2016. Patients who received COPD medication, either LAMA or ICS/LABA, with the COPD diagnostic code, were selected. We enrolled patients with good compliance (medication possession ratio ≥ 80%). The primary outcome was pneumonia in COPD patients initiating LAMA or ICS/LABA. We investigated the risk factors associated with pneumonia, including the sub-types of ICS treatments. After propensity score matching, the incidence rate per 1000 person-years of pneumonia was 93.96 for LAMA (n = 1003) and 136.42 for ICS/LABA (n = 1003) patients (p < 0.001). The adjusted hazard ratio (HR) for pneumonia in patients on fluticasone/LABA was 1.496 (95% confidence interval [CI] 1.204–1.859) compared with LAMA (p < 0.001). In multivariable analysis, a history of pneumonia was a risk factor associated with pneumonia (HR 2.123; 95% CI 1.580–2.852; p < 0.001). The incidence of pneumonia was higher in COPD patients on ICS/LABA compared with those on LAMA. It is recommended that ICS use be avoided in COPD patients with high pneumonia risk.
Similar content being viewed by others
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive chronic disease characterized by persistent respiratory symptoms and airflow limitation1. According to the Global Burden of Disease, COPD was the third leading cause of death worldwide in 20102. Pharmacotherapy and hospitalization due to severe disease, exacerbations, and comorbidities contribute to the economic cost of COPD3. Therefore, appropriate medication selection can help control these factors and reduce both individual and societal costs.
Inhaled bronchodilators, such as long-acting muscarinic antagonists (LAMA) and long-acting β2-agonists (LABA) are regularly administered to reduce COPD symptoms while preventing progression and exacerbation1. Compared to monotherapy, fixed-dose combinations of LAMA and LABA within a single inhaler show improved lung function, symptom reduction, and overall improvement in quality of life4. The combination of LAMA/LABA with inhaled corticosteroids (ICS) is recommended for patients with severe COPD and frequent exacerbations1. An increased blood eosinophil count in COPD is associated with a higher exacerbation rate and favorable response to ICS5,6. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2019 initially recommended that, for patients in GOLD group D, an eosinophil count of ≥ 300 cells/μL was an indicator for ICS/LABA treatment, with this threshold identifying patients more likely to benefit from ICS treatment7.
ICS increase the risk of side effects such as oropharyngeal candidiasis, hoarse voice, skin bruising, tuberculosis, and pneumonia8,9; also, physicians should remain vigilant for pneumonia development in COPD patients on ICS-containing regimens10,11. However, in clinical practice, ICS is overused without due consideration of the risks and benefits12.
Randomized controlled trials do not always reflect the entire population affected by a disease. Compared to the general COPD population, COPD trial participants often have fewer comorbidities, lower age, and milder disease. There is already another study comparing the risk of pneumonia requiring hospitalization when patients with chronic airway disease used ICS/LABA in a real-world setting, using claims data from the National Health Insurance Service (NHIS) in South Korea13. However, there is a limitation in those patients with all chronic airway diseases such as COPD, asthma, bronchiectasis, and tuberculosis-destroyed lung were targeted, and the pneumonia risk was analyzed by dividing ICS/LABA by device—pressurized metered-dose inhalers (pMDIs) and dry powder inhalers (DPIs)—rather than a component. The objectives of our study were to compare the incidence and the risk of pneumonia between ICS/LABA therapy and LAMA monotherapy in COPD patients in the real-world.
Methods
Study design
This was a retrospective, observational, cohort study using claims data from the National Health Insurance Service (NHIS) to compare effectiveness and safety outcomes in COPD patients commencing LAMA or ICS/LABA treatment in South Korea. The study protocol followed the principles of the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of Konkuk University Medical Center (IRB No.: KUMC2020-06-013).
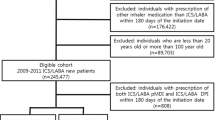
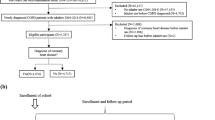
Patients receiving LAMA or ICS/LABA, with the COPD diagnostic code, between 1 January 2005 and 30 April 2015, were selected as the study group. The index date was the first prescription date of LAMA or ICS/LABA with the COPD diagnosis on record. A schematic diagram of the study design is shown in Fig. 1.
Inclusion criteria
To increase the likelihood of a COPD diagnosis, we only included patients aged ≥ 55 years on the index date; other inclusion criteria were two or more inpatient or outpatient claims for LAMA monotherapy or ICS/LABA fixed dose combination (FDC) with the International Classification of Disease-10 (ICD-10) code for COPD (J43.x–44.x [except J43.0]) recorded as any diagnosis in inpatient claims or primary to 4th secondary diagnosis in outpatient claims, and prescription of LAMA or ICS/LABA more than twice within 12 months of the index date (Fig. 2).
Exclusion criteria
Patients were excluded if prescribed LAMA and ICS/LABA; LABA or LAMA/LABA at the index date; ipratropium; leukotriene receptor antagonist; or ICS. Also excluded were patients diagnosed with lung cancer, interstitial lung disease, or lung transplantation during the baseline period prior to the index date (Fig. 2).
Outcomes
The primary outcome was pneumonia in COPD patients commencing LAMA or ICS/LABA. Pneumonia was defined as one or more inpatient or outpatient claims with (1) ICD-10 codes for pneumonia recorded as any diagnosis in inpatient claims or primary to 4th secondary diagnosis in outpatient claims; (2) diagnostic test codes for chest X-ray or computed tomography; and (3) antibiotic prescription after the index date.
Statistical analysis
The Chi-squared test was used for categorical variables and the t-test or Wilcoxon rank-sum test for continuous variables, to compare baseline characteristics between treatment groups. Hazard ratios (HRs) with 95% confidence intervals (CIs) were estimated using Cox-proportional hazards regression to compare the risk of primary outcomes between the study groups. All HRs were estimated using the LAMA group as a reference.
Propensity score (PS) matching was used to reduce confounding factors and to balance comparability between the study groups since there was a possibility that initial treatment could be based on patient demographics and baseline characteristics. 1:1 PS matching used age, sex, economic status, exacerbation history, pneumonia, comorbidities, and index year as co-variables. Significance was set at 0.05, two tailed. Software used for statistical analysis was SAS® 9.4 (SAS Institute, Cary, North Carolina, USA).
Ethics approval and consent to participate
The study protocol followed the principles of the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of Konkuk University Medical Center (IRB No.: KUMC2020-06-013). The requirement for informed consent from the participants was waived by the IRB of Konkuk University Medical Center (IRB No.: KUMC2020-06-013) due to the retrospective nature of this study.
Results
Baseline characteristics
The cohort included 4699 patients, 3479 and 1220 of whom received LAMA and ICS/LABA, respectively. After PS matching, the cohort included 2006 patients, with 1003 patients in each group. The LAMA group received either tiotropium, aclidinium, or glycopyrronium bromide. The ICS/LABA group received either fluticasone propionate/salmeterol, fluticasone propionate/formoterol, fluticasone furoate/vilanterol, beclometasone/formoterol or budesonide/formoterol.
After PS matching, the characteristics of the two groups (Table 1) showed good overall balance as each absolute standardized difference (ASD) was < 0.1. The observation period was significantly longer in the LAMA group (750.23 ± 670.21 days vs. 604.27 ± 476.13 days, p < 0.001).
Pneumonia incidence rate
Patients who received ICS/LABA had a higher pneumonia incidence rate than those who received LAMA (93.96/1000 PYs vs. 136.42/1000 PYs, p = 0.0004).
The pneumonia incidence rate was higher in ICS/LABA compared to LAMA in the 55–74 years age group (p = 0.0013) and in the group with no history of exacerbation (p = 0.0029) or one moderate exacerbation (p = 0.0116) (Table 2). The incidence rate of pneumonia-related hospitalization was also significantly higher with ICS/LABA compared with LAMA (p = 0.0001) (Additional file 1; Supplementary Table S1).
There were no significant differences in the incidence rate of outpatient pneumonia and pneumonia-related death between the patients on LAMA and ICS/LABA according to age groups, sex, and higher exacerbation history (Additional file 1; Supplementary Tables S2 and S3).
Frequency of pneumonia
After PS matching, the frequency of pneumonia events showed no significant difference between LAMA and ICS/LABA (p = 0.172) (Additional file 1; Supplementary Table S4). The frequency of pneumonia-related hospitalization events was higher in the ICS/LABA group than in the LAMA group (Additional file 1; Supplementary Table S5). The frequency of outpatient pneumonia events was not significantly different between LAMA and ICS/LABA (Additional file 1; Supplementary Table S6).
Risk of pneumonia
Treatment with ICS/LABA compared with LAMA was associated with a higher risk of pneumonia (HR 1.374; 95% CI 1.116–1.692; p = 0.0028; Additional file 1, Supplementary Table S7). This also applied in the 55 to < 75 years age group (p = 0.0126), males (p = 0.0187), patients treated in general hospitals (hospitals with > 100 beds with internal medicine specialists, p = 0.0122), patients with no history of COPD exacerbation (p = 0.0150) or a history of one moderate exacerbation (p = 0.0050), and patients with no prior history of pneumonia (p = 0.0010).
A higher proportion of patients on ICS/LABA were hospitalized with pneumonia events compared with LAMA (HR 1.610; 95% CI 1.234–2.101; p = 0.0005; Additional file 1, Supplementary Table S8), and this applied irrespective of age group. The risk was significantly higher in ICS/LABA patients in the lower quartile of income (p = 0.0081), and those who had never experienced COPD exacerbation (p = 0.0021) or had experienced one moderate exacerbation (p = 0.0168).
There were no significant differences in outpatient pneumonia events and pneumonia-related deaths between ICS/LABA and LAMA (Additional file 1; Supplementary Tables S9 and S10).
Pneumonia probability
Kaplan–Meier probability estimates revealed that the risk of pneumonia, based on the time to first pneumonia events, was significantly higher in ICS/LABA (p = 0.0026; Fig. 3A). The risk of pneumonia-related hospitalization was also significantly higher in ICS/LABA (p = 0.00034; Fig. 3B). However, Kaplan–Meier probability estimates showed no significant differences in the risks of outpatient pneumonia events or pneumonia-related deaths between the two groups (Fig. 3C,D).
(A) Time to first pneumonia event in patients on ICS/LABA vs. LAMA. (B) Time to first pneumonia-related hospitalization event in patients on ICS/LABA vs. LAMA. (C) Time to first outpatient pneumonia event in patients on ICS/LABA vs. LAMA. (D) Time to pneumonia-related death in patients on ICS/LABA vs. LAMA. ICS inhaled corticosteroids, LABA long acting β2-agonists, LAMA long-acting muscarinic antagonists.
Risk factors associated with pneumonia
In the PS-matched population, ICS/LABA treatment was a risk factor associated with pneumonia (HR 1.389; 95% CI 1.127–1.713; p = 0.0021; Table 3). A secondary analysis was performed on patients on ICS/LABA per ICS treatment. In patients using fluticasone, ICS/LABA was a risk factor associated with pneumonia (HR 1.496; 95% CI 1.204–1.859; p = 0.0003). However, in patients on non-fluticasone ICS/LABA, ICS/LABA was not a risk factor associated with pneumonia (HR 1.002; 95% CI 0.682–1.473; p = 0.9914). In COPD patients, another significant pneumonia-associated risk factor was a history of pneumonia (HR 2.123; 95% CI 1.580–2.852; p < 0.0001).
Discussion
In this study, we compared the risk of pneumonia associated with long-term ICS/LABA or LAMA treatment for COPD and found that the overall risk of pneumonia was significantly higher in ICS/LABA treatment. The incidence rates of pneumonia and pneumonia-related hospitalization were higher in patients on ICS/LABA, especially in the youngest included age group (55 to < 75 years). This trend was even observed in COPD patients with COPD exacerbation history (no or one moderate exacerbation).
Additionally, the subgroups with higher pneumonia risk on ICS/LABA—compared to LAMA—were those with no history of pneumonia; treatment at a hospital-level medical institution with inpatient beds rather than a primary medical institution such as primary care, or a lower income class in the 4th quartile. Regarding comorbidities, pneumonia risk was higher when ICS/LABA was used in patients with chronic pulmonary diseases such as bronchiectasis and TB-destroyed lungs resulting in cough, sputum, and dyspnea.
In the TORCH study, fluticasone propionate with long-acting β2-agonists salmeterol vs. placebo showed an increased risk of pneumonia (HR 1.64; 95% CI 1.33–2.02; p < 0.001), a similar effect to fluticasone propionate alone (HR 1.53; 95% CI 1.24–1.89; p < 0.001), contrasting with salmeterol alone (HR 1.09; 95% CI 0.87–1.37, p = 0.465)10. More patients using an ICS-containing regimen had severe pneumonia compared to either salmeterol or placebo.
In the INSPIRE study, the patient group treated with fluticasone propionate/salmeterol showed a significantly higher risk of pneumonia than those treated with tiotropium (HR 1.94; 95% CI 1.19–3.17, p = 0.008)14. This is consistent with our results that ICS increases the risk of pneumonia compared to patients who used LAMA. However, the INSPIRE study is limited by the protocol’s unclear prior definition due to the lack of prediction of excessive occurrence of pneumonia during treatment.
In the post hoc analysis of the 4-year UPLIFT® trial, COPD patients who used fluticasone propionate had a higher risk of pneumonia than patients who did not use ICS (HR 1.33; 95% CI 1.00–1.75; p = 0.046)15. The risk of pneumonia-related hospitalization was also greater in patients treated with fluticasone propionate compared with other or no ICS. However, there was no significant difference in pneumonia and pneumonia-related hospitalization risk between patients treated with ICS other than fluticasone and those without ICS15. This suggests that fluticasone is associated with pneumonia more than any other ICS.
In the PATHOS study, the rate of pneumonia and admission to hospital and mortality related to pneumonia were higher in patients treated with fluticasone/salmeterol compared with budesonide/formoterol16. Our analysis, based on real-world data, had results consistent with the above clinical trial. The use of ICS/LABA was also a risk factor associated with pneumonia. When ICS/LABA was divided into a group containing fluticasone and a group containing ICS other than fluticasone, only ICS/LABA with fluticasone was a risk factor for pneumonia.
Yang et al. performed a meta-analysis of 25 double-blind clinical trials (including 49,982 patients) using ICS as an intervention drug and non-ICS treatment as a control group17. ICS treatment was significantly associated with an increased risk of pneumonia in COPD patients. In a subgroup analysis based on ICS type, fluticasone increased the risk of pneumonia regardless of high-, medium-, or low-doses. Contrastingly, budesonide did not increase the risk of pneumonia, irrespective of dose17.
A study based on real-world clinical practice from the UK's Clinical Practice Research Datalink compared the efficacy and safety of treatment with ICS/LABA or LAMA in COPD patients aged > 55 years from 2002 to 201518. ICS/LABA was associated with a higher risk of pneumonia than LAMA and showed an exceptionally higher risk when fluticasone was used as ICS. In a real-world, observational study comparing the efficacy and safety of ICS/LABA and a LABA/LAMA combination instead of LAMA monotherapy, the incidence of pneumonia was also high when ICS/LABA was used19. However, the study did not analyze the pneumonia risk according to the type of ICS.
Lee et al. also analyzed the relationship between ICS and pneumonia risk in patients with COPD using data from the Korean NHIS20. In this study, all patients were classified into ICS and non-ICS users based only on the inclusion of ICS, regardless of the ingredients of the inhaler. Pneumonia was significantly related to ICS use, and the pneumonia risk increased as the cumulative dose of ICS increased. However, the daily dose of ICS was not associated with pneumonia20. And, as in other studies, fluticasone propionate (HR 1.79; 95% CI 1.70–1.89; p < 0.0001) and fluticasone furoate (HR 1.80; 95% CI 1.61–2.01; p < 0.0001) showed a higher risk of pneumonia than other ICS such as budesonide (HR 1.44; 95% CI 1.35–1.54; p < 0.0001).
It is also true that ICS, particularly fluticasone, makes a significant difference in lowering acute exacerbations in COPD patients. In the IMPACT trial, once-daily single-inhaler triple therapy with fluticasone furoate/umeclidinium/vilanterol resulted in a significantly lower rate of moderate or severe COPD exacerbations and better lung function and health-related quality of life than dual therapy with fluticasone furoate/vilanterol or the dual bronchodilator umeclidinium/vilanterol among patients with symptomatic COPD and a history of exacerbations21. Although the incidence of AE COPD is almost ten times higher than that of pneumonia, the GOLD strategy currently discourages using ICS in patients with repeated pneumonia episodes or blood eosinophil counts less than 100 cells/μL or a history of mycobacterial infection. Therefore, the decision to use ICS in patients with COPD must always be made carefully, and caution is required during use.
Several studies, including ours, showed that fluticasone increased the risk of pneumonia even more so than other ICS16,22. This may be caused by fluticasone's lipid-solubility and slow dissolution properties, implying that it remains in the airway lining fluid longer compared to other ICS causing local immunosuppression16,22.
Our study had several strengths compared to other observational studies in a real-world setting. The analysis was performed using a nationwide population-based cohort, the NHID from the NHIS (including 98% of the population of South Korea) and had a long-term observational period. Pneumonia, pneumonia-related hospitalization, outpatient pneumonia, and pneumonia-related death were systematically analyzed according to risk by incidence rate, frequency, hazard ratio, and probability estimates based on time to the first event. Compared to LAMA, subgroups showing high pneumonia risk among patients using ICS/LABA were analyzed. These subgroup analyses included age; sex; income level; COPD risk group based on exacerbation history; history of pneumonia; and comorbidities. The analysis of these high-risk group, large sample size, and longer duration is novel and has not been seen in previous studies.
There were several limitations to our study. First, considering the inclusion and exclusion criteria, the sample does not represent the entire COPD population. Second, the data used are not very recent. Third, there is a lack of rationale regarding the criteria for prescribing ICS/LABA or LAMA. Fourth, because our study was based on NHIS data, pneumonia events were identified only by diagnostic codes, and radiologic findings or severity analyses were not performed. Fifth, the study could not include a control group of non-treatment subjects. Sixth, no analysis was performed on the probability of pneumonia by comparing ICS/LABA with and without fluticasone using the Kaplan–Meier curve, nor the frequency of use of each type of ICS or the pneumonia risk for each ICS type other than fluticasone. Finally, we did not analyze the pneumonia risk according to the dose of ICS.
Conclusions
The use of ICS/LABA in COPD patients was associated with a higher risk of pneumonia than LAMA monotherapy. Particularly, the risk of pneumonia was high when fluticasone was used as ICS. Chronic respiratory comorbidities, a history of previous pneumonia and GOLD lower-risk group, were also at high risk for pneumonia. Thus, it is recommended that ICS use be avoided in COPD patients with high pneumonia risk.
Data availability
The datasets generated during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- ASD:
-
Absolute standardized difference
- CI:
-
Confidence interval
- COPD:
-
Chronic obstructive pulmonary disease
- FDC:
-
Fixed dose combination
- GOLD:
-
Global Initiative for Chronic Obstructive Lung Disease
- HIV:
-
Human immunodeficiency virus
- HR:
-
Hazard ratio
- ICD-10:
-
International Classification of Disease-10
- ICS:
-
Inhaled corticosteroid
- LABA:
-
Long-acting β2-agonist
- LAMA:
-
Long-acting muscarinic antagonist
- mCCI:
-
Modified Charlson Comorbidity Index
- NHI:
-
National Health Index
- NHID:
-
National Health Information Database
- NHIS:
-
National Health Insurance Service
- PS:
-
Propensity score
- PYs:
-
Person-years
- SD:
-
Standard deviation
References
Vogelmeier, C. F. et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am. J. Respir. Crit. Care Med. 195, 557–582. https://doi.org/10.1164/rccm.201701-0218PP (2017).
Lozano, R. et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2095–2128. https://doi.org/10.1016/s0140-6736(12)61728-0 (2012).
Mannino, D. M. & Buist, A. S. Global burden of COPD: Risk factors, prevalence, and future trends. Lancet 370, 765–773. https://doi.org/10.1016/s0140-6736(07)61380-4 (2007).
Anzueto, A. & Miravitlles, M. The role of fixed-dose dual bronchodilator therapy in treating COPD. Am. J. Med. 131, 608–622. https://doi.org/10.1016/j.amjmed.2017.12.018 (2018).
Vedel-Krogh, S., Nielsen, F. S., Lange, P., Vestbo, J. & Nordestgaard, B. G. Blood eosinophils and exacerbations in chronic obstructive pulmonary disease: The Copenhagen general population study. Am. J. Respir. Crit. Care Med. 193, 965–974 (2016).
Vedel-Krogh, S., Nordestgaard, B. G., Lange, P., Vestbo, J. & Nielsen, S. F. Blood eosinophil count and risk of pneumonia hospitalisations in individuals with COPD. Eur. Respir. J. https://doi.org/10.1183/13993003.00120-2018 (2018).
Singh, D. et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: The GOLD science committee report 2019. Eur. Respir. J. https://doi.org/10.1183/13993003.00164-2019 (2019).
Lee, C. M. et al. Inhaled corticosteroid-related tuberculosis in the real world among patients with asthma and COPD: A 10-year nationwide population-based study. J. Allergy Clin. Immunol. Pract. 7, 1197-1206 e1193. https://doi.org/10.1016/j.jaip.2018.10.007 (2019).
Yang, I. A., Clarke, M. S., Sim, E. H. & Fong, K. M. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD002991.pub3 (2012).
Crim, C. et al. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur. Respir. J. 34, 641–647. https://doi.org/10.1183/09031936.00193908 (2009).
Tashkin, D. P. & Strange, C. Inhaled corticosteroids for chronic obstructive pulmonary disease: What is their role in therapy?. Int. J. Chron. Obstruct. Pulmon. Dis. 13, 2587–2601. https://doi.org/10.2147/COPD.S172240 (2018).
Burgel, P. R. et al. Real-life use of inhaled corticosteroids in COPD patients versus the GOLD proposals: A paradigm shift in GOLD 2011?. Eur. Respir. J. 43, 1201–1203 (2014).
Park, J. H. et al. Risk for pneumonia requiring hospitalization or emergency room visit according to delivery device for inhaled corticosteroid/long-acting beta-agonist in patients with chronic airway diseases as real-world evidence. Sci. Rep. 9, 12004. https://doi.org/10.1038/s41598-019-48355-2 (2019).
Calverley, P. M. A. et al. Reported pneumonia in patients with COPD: Findings from the INSPIRE study. Chest 139, 505–512. https://doi.org/10.1378/chest.09-2992 (2011).
Tashkin, D. P. et al. Concomitant inhaled corticosteroid use and the risk of pneumonia in COPD: A matched-subgroup post hoc analysis of the UPLIFT(R) trial. Respir. Res. 19, 196. https://doi.org/10.1186/s12931-018-0874-0 (2018).
Janson, C. et al. Pneumonia and pneumonia related mortality in patients with COPD treated with fixed combinations of inhaled corticosteroid and long acting beta2 agonist: Observational matched cohort study (PATHOS). BMJ 346, f3306. https://doi.org/10.1136/bmj.f3306 (2013).
Yang, M., Du, Y., Chen, H., Jiang, D. & Xu, Z. Inhaled corticosteroids and risk of pneumonia in patients with chronic obstructive pulmonary disease: A meta-analysis of randomized controlled trials. Int. Immunopharmacol. 77, 105950. https://doi.org/10.1016/j.intimp.2019.105950 (2019).
Suissa, S., Dell’Aniello, S. & Ernst, P. Comparative effectiveness of LABA-ICS versus LAMA as initial treatment in COPD targeted by blood eosinophils: A population-based cohort study. Lancet Respir. Med. 6, 855–862. https://doi.org/10.1016/s2213-2600(18)30368-0 (2018).
Suissa, S., Dell’Aniello, S. & Ernst, P. Comparative effectiveness and safety of LABA-LAMA vs LABA-ICS treatment of COPD in real-world clinical practice. Chest 155, 1158–1165. https://doi.org/10.1016/j.chest.2019.03.005 (2019).
Lee, J. H. et al. Risk of pneumonia associated with inhaled corticosteroid in patients with chronic obstructive pulmonary disease: A Korean Population-Based Study. Int. J. Chron. Obstruct. Pulmon. Dis. 15, 3397–3406. https://doi.org/10.2147/COPD.S286149 (2020).
Lipson, D. A. et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N. Engl. J. Med. 378, 1671–1680. https://doi.org/10.1056/NEJMoa1713901 (2018).
Janson, C., Stratelis, G., Miller-Larsson, A., Harrison, T. W. & Larsson, K. Scientific rationale for the possible inhaled corticosteroid intraclass difference in the risk of pneumonia in COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 12, 3055–3064. https://doi.org/10.2147/COPD.S143656 (2017).
Funding
This study was sponsored by Boehringer-Ingelheim Korea Ltd.
Author information
Authors and Affiliations
Contributions
Investigation: Y.K., Y.I.H., K.H.Y., S.E.L., K.Y.J., D.L., Y.B.P., and C.K.R.; Writing-Original Draft: E.G.L.; Formal analysis: Y.K., Y.I.H., K.H.Y., S.E.L., K.Y.J., D.L., Y.B.P., and C.K.R.; Data Curation and Visualization: S.E.L., K.Y.J., D.L.; Conceptualization and Supervision: Y.K., Y.I.H., K.H.Y., S.E.L., K.Y.J., D.L., Y.B.P., and C.K.R. All authors contributed to reviewing and editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
EGL and YIH have no conflict of interest. CKR received consulting and lecture fees from MSD, AstraZeneca, GlaxoSmithKline, Novartis, Takeda, Mundipharma, Boehringer-Ingelheim, TEVA, Sanofi, and Bayer. KHY has conducted clinical trials in Asthma, COPD, and Pneumonia with GlaxoSmithKline, AstraZeneca, Boehringer-Ingelheim, Novartis, Takeda, Nycomed, TEVA, MSD, Mundipharma, Hyundai Ankook, Chongkendang, Hanmi, and Hanlym. He received consulting fees from GlaxoSmithKline, AstraZeneca, Boehringer-Ingelheim, Novartis, Mundipharma, Ankook, Chongkendang, Hanmi, and Hanlym. YBP received consulting and lecture fees from AstraZeneca, GlaxoSmithKline, Novartis, Mundipharma, Boehringer-Ingelheim, and Sanofi. SEL was a former employee of Boehringer-Ingelheim Korea. KYJ was a former employee of Boehringer-Ingelheim Korea. YK received consulting and lecture fees from MSD, GlaxoSmithKline, Boehringer-Ingelheim, and Hanlim.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, E.G., Kim, Y., Hwang, Y.I. et al. Comparison of pneumonia incidence between long-acting muscarinic antagonist and inhaled corticosteroid plus long-acting beta agonist in patients with COPD. Sci Rep 13, 8183 (2023). https://doi.org/10.1038/s41598-023-35223-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-35223-3
This article is cited by
-
Effect of Broncho-Vaxom (OM-85) on the frequency of chronic obstructive pulmonary disease (COPD) exacerbations
BMC Pulmonary Medicine (2023)
-
LABA/LAMA versus LABA/ICS fixed-dose combinations in the prevention of COPD exacerbations: a modeling analysis of literature aggregate data
European Journal of Clinical Pharmacology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.