Abstract
Infectious keratitis refers to a group of corneal disorders in which corneal tissues suffer inflammation and damage caused by pathogenic infections. Among these disorders, fungal keratitis (FK) and acanthamoeba keratitis (AK) are particularly severe and can cause permanent blindness if not diagnosed early and accurately. In Vivo Confocal Microscopy (IVCM) allows for imaging of different corneal layers and provides an important tool for an early and accurate diagnosis. In this paper, we introduce the IVCM-Keratitis dataset, which comprises of a total of 4001 sample images of AK and FK, as well as non-specific keratitis (NSK) and healthy corneas classes. We use this dataset to develop multiple deep-learning models based on Convolutional Neural Networks (CNNs) to provide automated assistance in enhancing the diagnostic accuracy of confocal microscopy in infectious keratitis. Densenet161 had the best performance among these models, with an accuracy, precision, recall, and F1 score of 93.55%, 92.52%, 94.77%, and 96.93%, respectively. Our study highlights the potential of deep learning models to provide automated diagnostic assistance for infectious keratitis via confocal microscopy images, particularly in the early detection of AK and FK. The proposed model can provide valuable support to both experienced and inexperienced eye-care practitioners in confocal microscopy image analysis, by suggesting the most likely diagnosis. We further demonstrate that these models can highlight the areas of infection in the IVCM images and explain the reasons behind their diagnosis by utilizing saliency maps, a technique used in eXplainable Artificial Intelligence (XAI) to interpret these models.
Similar content being viewed by others
Introduction
The cornea is the transparent, outermost layer of the eye, covering the iris, pupil, and anterior chamber, and it plays a crucial role in protecting internal eye structures and in light refraction1. This area can be damaged through injury, external particles, or pathogenic microorganisms, causing inflammation and swelling in the cornea, which is referred to as keratitis2. Infectious keratitis is the swelling and damage of the cornea caused by infectious organisms such as bacteria, viruses, fungi, or parasites, and may lead to various serious complications or blindness without early diagnosis and treatment3. Fungal keratitis (FK) is the most devastating and the leading cause of vision loss, with one million cases of blindness caused by FK worldwide, every year4,5. Acanthamoeba keratitis (AK) is a rare but highly severe form of keratitis caused by the parasitic acanthamoeba genus of amoebae, which is often associated with the use of soft contact lenses6. Diagnosis of AK often proves challenging and delayed, and the disease is difficult to treat with conventional medications, which can lead to permanent visual impairment or blindness7.
In Vivo Confocal Microscopy (IVCM) is an emerging non-invasive tool for capturing high-definition cross-sectional images of eye structures, and is of great assistance for early diagnosis of different types of keratitis. It offers significant advantages in terms of accuracy and sensitivity of diagnosis, particularly in detecting deep stromal infections that are not easily diagnosed through corneal scraping. However, this comes at the cost of an increased workload on medical staff and the need for specialized training to interpret IVCM images8,9.
Deep learning (DL) is a class of advanced machine learning techniques, in which convolutional neural networks (CNN) can be used to develop sophisticated artificial intelligence (AI) systems to process high-definition data, such as images, quickly and accurately10. In image processing applications, CNNs have multiple convolutional layers capable of capturing high-level features of the image into multiple abstraction levels for processing which improves accuracy and versatility, at the cost of requiring a large amount of annotated data11. In recent years, the emergence of DL and CNN has proven tremendously useful in medical data analysis and medical image recognition12,13,14. In ophthalmology, multiple DL-based AI systems have been developed for diagnosis of glaucoma and macular degeneration15,16. While DL-based systems have proven useful in various medical diagnosis applications, the development of DL-based systems for keratitis, especially in IVCM imaging technology, has been lackluster. Although some studies have developed DL models for the diagnosis of keratitis using slit-lamp biomicroscopy, research on diagnostic AI models using IVCM images remains limited17,18,19. Researchers have developed models on datasets with a limited number of either data samples (as in17 with 137 images) or classes (as in18 with two classes of healthy and FK images introduced) or patients (as in19 based on a small sample of six patients).
The aim of this study is to present a more advanced deep learning model that provides automated diagnostic assistance for different types of infectious keratitis, offering a comprehensive approach to diagnosis when interpreting IVCM images, both for experienced and inexperienced eye-care practitioners. We consider the classification of four types of IVCM images in our study, consisting of fungal keratitis (FK), acanthamoeba keratitis (AK), non-specific keratitis (NSK), and healthy images. NSK refers to other types of inflammations or injuries not induced by fungal, amoebic or bacterial organisms (further details in "Dataset" section). To achieve this, we introduce the IVCM-Keratitis dataset, consisting of 4001 IVCM images along with their official diagnoses. We trained multiple recent DL models and optimized them to find the best model with the highest performance. To the best of our knowledge, this is the first attempt to develop an automated keratitis deep learning model using IVCM images at this scale and class diversity. Furthermore, this is the first model capable of detecting AK cases using IVCM images.
However, although there have been some efforts to interpret DL models in the emerging field of eXplainable Artificial Intelligence (XAI)20, DL models developed using IVCM images are still treated as a black box. We also demonstrate the interpretability of deep learning models for IVCM images and its utility to eye-care practitioners in making decisions faster and with more confidence. We used saliency maps21 as a tool to show the areas in the images that a DL model would consider significant for its diagnosis.
Materials and methods
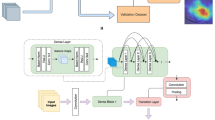
Figure 1 shows the overall pipeline of our study including data collection, training, and evaluation processes. All building blocks will be discussed in the next subsections.
Dataset
To conduct this study, a full ethical approval was obtained from the ethics committee of the Research Institute for Ophthalmology and Vision Science, Shahid Beheshti University of Medical Sciences, Tehran, Iran (ethical approval code number #IR.SBMU.ORC.REC.1389.20). This retrospective study followed the tenets of the declaration of Helsinki regulations.
The IVCM-Keratitis dataset introduced and used in this paper was gathered from a data pool of IVCM images collected from hundreds of patients who visited the Confoscan unit of the Central Eye Bank of Iran between January 2008 and September 2018. These images were captured using the confocal scan unit ConfoScan 3.0 (Nidek Technology, Padova, Italy).
All the investigated IVCM images had already been confirmed by the microbiologic smear/culture results. Their corresponding clinical features were also consistent with the gold standard of microbiologic smear and cultures (see Supplementary Fig. S1 and Supplementary Fig. S2)22.
In order to select the confocal scan images that best represented the diagnosis, two physician-scientists (MA and SMM) independently reviewed all the IVCM images taken during the patients’ visits, and excluded the images that were unfocused, too dark/bright, or out of focus.
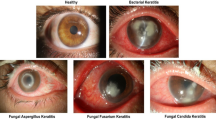
The IVCM-Keratitis dataset consists of 4001 IVCM images of size 768 × 576 pixels in JPEG format including 1391 AK, 897 FK, 1004 NSK, and 743 healthy images. The dataset is randomly split into 3000 training and 1001 test images. Sample images of each class are shown in Fig. 2.
Sample IVCM images of each class showing their different confocal microscopic features. (a) Acanthamoeba keratitis, showing acanthamoeba cyst (black arrows) and trophozoite (white arrows) along with inflammatory cells. (b) Fungal keratitis, showing fungal filaments (black arrows). (c) Healthy cornea, showing normal epithelium along with subepithelial nerves (white arrows). (d) Non-specific keratitis, showing hyper reflective structures mainly consisting of inflammatory cells.
The IVCM-Keratitis dataset is made publicly available for research use in the Figshare repository in23.
Data processing
Preprocessing
Images were first converted from RGB to grayscale and then normalized to have pixel values between zero and one as shown in Eq. (1).
Here, \(y\) represents the normalized grayscale pixel values and \({x}_{r}\), \({x}_{g}\), and \({x}_{b}\) represent the pixel values of red, green, and blue channels, respectively.
After that, the mean and variance of the whole dataset were calculated, and each image was standardized relative to the dataset’s mean and standard deviation, as demonstrated in Eqs. (2)–(4).
Here, \({y}_{mn}^{i}\) represents the value of the pixel located at the \({m}{th}\) row and \({n}{th}\) column of the \({i}{th}\) normalized grayscale image of size M by N. \(\mu \) and \(\sigma \) are the mean and standard deviation of our dataset \(D\), which has a size of \(|D|\).
Augmentation
Since deep learning (DL) usually requires a large amount of annotated data, data augmentation methods have been devised to expand the size of the training set. During augmentation, a transformation is applied to the data samples, resulting in new data samples with the same label. Image augmentation methods include mirroring, translation, blurring, zooming, rotation, and others. These methods have been shown to increase a model’s prediction accuracy24.
In this paper, we have selected four augmentation methods: color jittering, horizontal flips, vertical flips, and light Gaussian blur. Although data augmentation is typically performed before training, in our study, we have implemented data augmentation dynamically during the training process. For each training epoch, there is a probability of transformation (we set it to 40% in our study) for each image before it is fed into the training process. This approach allows the same image to appear differently during training, effectively augmenting the training set without artificially increasing its size. Moreover, this method enables deep learning models to be trained on large datasets without memory issues.
Model development
Model types
In recent years, DL has grown rapidly with the range of available models greatly expanding. In this study, we trained eight different CNN models that have been extensively used in image classification in recent years, and compared their performance. These models include Densenet161, Densenet12125, Resnet152, Resnet10126, Resnext101, Resnext5027, Cspresnet5028, Vgg19, Vgg16, and Vgg1329. We did not use the pre-trained weights available for these models, and instead, trained them from scratch with random weight initialization. Specifically, we initialized the linear layers using a uniform distribution within the range of [− 1/|W|, 1/|W|], where |W| represents the output size of the linear layer. For the convolutional layers, we used Xavier initialization30.
Training
To find the best hyperparameters for each model, such as batch size and learning rate, we performed fivefold cross-validation31. In this process, we randomly split the training set into five folds and trained the network on four folds while evaluating its performance on the remaining one-fold. We repeated this process for five rounds to test all the folds, and then calculated the average performance over all the rounds. We trained the model for 10 epochs in each round. Finally, we selected the hyperparameters that led to the best cross-validation performance as the best hyperparameters for the corresponding model.
Using the best hyperparameters for each model, we trained each model for 250 epochs using the training set. We also employed a weighted random sampler during training to deal with any imbalances in the dataset. This sampler samples smaller classes more frequently than larger classes, ensuring that samples from smaller classes appear as many times as samples from larger classes during training.
Evaluation
To evaluate the performance of our trained models, we used the test set. We report the performance of our models by calculating several performance metrics, including accuracy, precision, recall, specificity, and F1 score. These performance metrics are defined for a two-class classification problem (positive and negative) as follows in Eqs. (5)–(9).
Here, \(ACC\), \(PPV\), \(TPR\), \(TNR\), and \(F1\) represent accuracy, positive predictive value (precision), true positive rate (recall/sensitivity), true negative rate (specificity), and F1 score, respectively. \(P\) represents the number of positive samples, and \(N\) represents the number of negative samples. \(TP\) (True Positive) is the number of positive samples that are correctly classified, \(FP\) (False Positive) is the number of samples that are wrongly predicted as a positive class, and \(TN\) (True Negative) and \(FN\) (False Negative) are the equivalent of \(TP\) and \(FP\) for the negative class.
To address the imbalance in the sizes of different classes in the dataset, we calculated the weighted average of these metrics across all classes, using the size of each class as its corresponding weight. Specifically, for each class, samples from that class were considered positive samples, and samples from all other classes were considered negative samples.
Results
The performance of all 10 trained models is summarized in Table 1. To maintain brevity, we only included the F1 score in this table as it provides a more comprehensive measure of the models’ performance, calculating the harmonic mean of the sensitivity/recall and precision values. Please refer to Supplementary Table S1 for additional details on the performance of the models by other metrics.
Of all the 10 trained models, Densenet161 achieved the best performance with respect to all the metrics, with a weighted average F1 score of 93.53% (Table 1). We summarized the predictions of Densenet161 on the test set in the confusion matrix in Fig. 3. Each cell in the matrix represents the number of samples that have the corresponding predicted and true label. As shown, NSK class has been the most challenging to classify correctly, as it can exhibit similarities with the healthy, AK, and FK classes.
In order to increase the model utility as an aide for practitioners, we implemented a model interpretation method called saliency map, also known as pixel attribution, or gradient-based attribution. A saliency map is generated by calculating the absolute value of the gradient of the output class of a trained model with respect to the input image. This gradient tells us how much the change of any pixel value of the input image will change the classification probabilities on the output classes. In other words, the gradient of each pixel of the input image can tell us how much the model relies on that pixel for prediction, or how important that pixel is to the classification output. By visualizing this gradient, we can see the areas that the model more focused on for its predictions; therefore, giving practitioners more information about how the model made its predictions, which then can be pondered to judge the validity of the predictions. Saliency maps have the advantage of being fast to compute and having the same resolution as the input image21.
From our analysis of saliency maps to identify the areas that the DL model deems important for diagnosis, we observed that it mainly pays focuses on significant cell structures, such as pathogens and inflammatory cells, while deliberately ignoring the irrelevant structures, such as image artifacts and isolated cells from other layers of ocular surface or tear film (see Fig. 4). However, since there are no peculiar structures in normal, healthy corneal images, the model extends its attention to the entire image. Additionally, as far as we observed, in the test set, the corneal nerve in normal images was never confused with fungal filaments, which can sometimes be misidentified by confocal practitioners, resulting in errors in diagnosis (see Fig. 5).
IVCM image samples along with their saliency maps and their overlaid images, displaying different confocal microscopic features. (a) Acanthamoeba keratitis, showing acanthamoeba trophozoites (white arrows) and cysts (black arrows), as well as inflammatory cells; (b) Fungal keratitis, featuring fungal hyphae (white arrows) and inflammatory cells. Notably, the model correctly ignores superficial cells (black arrows).
IVCM image samples along with their saliency maps and their overlaid images, displaying different confocal microscopic features. (a) Normal cornea, with the model barely considered central corneal nerve (white arrow) as a significant structure, as the saliency map is spread out to the whole image; (b) Non-specific keratitis, with the saliency map hardly focusing on any particular structure.
Discussion
Amongst the various forms of keratitis, acanthamoeba keratitis (AK) and fungal keratitits (FK) remain the most challenging, as early detection is crucial for effective treatment and better outcomes. Despite advances in laboratory tests, microbiological examinations are time-consuming and have low sensitivity. Although molecular tests are sensitive, they are expensive and require advanced equipment, making image-based diagnosis a more viable option for faster, more accurate, and less demanding diagnoses32. However, IVCM image diagnosis of keratitis requires specialized training, which most eye-care practitioners lack9. In addition, the time-consuming process of assessing large numbers of IVCM images can lead to delays and inaccuracies in diagnoses33. To address this, we introduce IVCM-Keratitis, a large dataset of IVCM images of different types of keratitis, including the less prevalent AK. We also propose a framework for AI-assisted diagnosis of these types of keratitis with high accuracy, using recent Deep Learning (DL) models. Our study shows the interpretability power of these models in indicating the location of the infection in the IVCM images.
During the process of capturing IVCM image, hundreds of images are taken from various layers of the cornea, but most of them are of poor quality or show healthy cells, with only a few displaying infectious structures34. This means that physicians would have to manually examine a large number of images containing healthy cells to find the ones that show infections. By training DL models to recognize different types of keratitis and healthy images, these models can quickly sort through a large number of IVCM images. The models can recognize and exclude healthy images, providing eye-care practitioners with images that may show infectious structures, along with the model’s diagnosis and its confidence level. This process can significantly reduce the workload of ophthalmologists and IVCM technicians. Since these models can be trained on NSK images, they can identify difficult images that require accurate diagnosis, which can then be presented to experienced specialists for further evaluation. Having a model that not only provides a diagnosis with its corresponding confidence level but also highlights the areas in the image that led to its diagnosis, can greatly reduce the time needed to assess IVCM images and help eye-care practitioners reach a more accurate conclusion. Although the proposed DL model offers significant benefits in terms of enhancing the accuracy and efficiency of infectious keratitis diagnosis, it is important to emphasize that this model is not intended to replace the experience of skilled eye-care practitioners. Instead, our objective is to provide an additional resource to support their existing expertise and ease the pressure of workload and time constraints they face.
Nevertheless, we do believe that this model has the potential to serve as an adjunct to the diagnostic process by providing a second opinion for less experienced eye-care practitioners and boosting their confidence in their initial diagnosis. This is particularly important because previous research has shown that inexperienced eye-care practitioners generally have lower sensitivity and specificity rates (59% and 92.7% for AK and 42.9% and 87.5% for FK, respectively)35,36 compared to our proposed model (91.4% and 98.3% for AK and 97.0% and 96.4% for FK, respectively). Therefore, our model has the potential to improve the accuracy of the diagnosis, while also reducing the risk of errors and misdiagnosis, ultimately leading to better patient management.
We believe that incorporating saliency maps as a model interpretability tool, will greatly benefit practitioners and specialists in improving the promptitude and accuracy of their diagnosis when using IVCM. By highlighting the important regions of medical images, these tools can reduce the workload of practitioners and provide a second opinion in the form of an AI model diagnosis with confidence level.
In general, deep learning requires a significant amount of annotated data to achieve high performance. Although augmentation methods can improve the model performance on smaller datasets, models still benefit greatly from having more manually annotated data. However, obtaining a sufficient amount of annotated data can be challenging for rare diseases, which can cause an imbalance in the dataset. As a result, trained models may be biased towards predicting more prevalent diseases. This is the case for AK, which does not have enough samples in IVCM image databases. In this study, we present an almost balanced, high-definition dataset that includes 1391 images of AK. To the best of our knowledge, this is the first time that AK has been included in a diagnostic system using IVCM images.
While our study has a large dataset, it only includes distinct images selected by specialists, which may not fully represent the spectrum of infections encountered in real-world clinical settings. Additionally, poor image quality may pose a challenge for accurate AI-assisted diagnosis, particularly for classifying healthy images. In future studies, we suggest the development of a preliminary DL model to exclude unfocused, poor-quality, or blank images commonly captured by IVCM technology. This model could assist in the diagnosis of clear images showing cellular structures and potentially improve the overall accuracy and speed of diagnosis.
Upon examining the misdiagnosed images, we found that the model may be influenced by hypo-reflectivity caused by interstitial edema and/or exudation, leading to the model overlooking inflammatory cells or pathogens. Additionally, hyper-reflective superficial epithelial cells can be misclassified as acanthamoeba cysts, and dendritic cells in the sub-basal layer of the epithelium, which are a hallmark of infectious keratitis such as AK37, may be mistaken for fungal filaments. However, such misdiagnoses are infrequent and can occur even among expert eye-care practitioners in clinical setting.
In conclusion, while the 96.93% accuracy of our proposed model may not be sufficient as a standalone diagnostic tool, it can still serve as a valuable reference for eye-care practitioners to make quicker and more confident diagnoses by leveraging the model’s predictions and saliency maps that highlight the probable infectious areas in the IVCM images. Moreover, this DL model could be integrated into the confocal scans software in a user-friendly fashion. We believe that future studies using larger IVCM datasets of more diverse infections from various institutions can further enhance model’s performance. To facilitate this effort, we have made the IVCM-Keratitis dataset openly available for future research. With more training data and more advanced techniques, models may still be able to improve further.
Data availability
The datasets generated during and/or analyzed during the current study are available in the Figshare repository, https://figshare.com/articles/dataset/Dataset/19838083.
References
Goldstein, E. B. & Brockmole, J. Sensation & perception. (10th ed). In Cengage Learning, 75–77 (2016)
Keay, L., Stapleton, F. & Schein, O. Epidemiology of contact lens-related inflammation and microbial keratitis: A 20-year perspective. Eye Contact Lens 33(6 Pt 2), 346–363 (2007).
Austin, A., Lietman, T. & Rose-Nussbaumer, J. Update on the management of infectious keratitis. Ophthalmology 124(11), 1678–1689 (2017).
Alshehri, J. M. et al. Evaluation of corneal cross-linking for treatment of fungal keratitis: Using confocal laser scanning microscopy on an ex vivo human corneal model. Investig. Ophthalmol. Vis. Sci. 57(14), 6367–6373 (2016).
Rodrigues, M. L. & Nosanchuk, J. D. Fungal diseases as neglected pathogens: A wake-up call to public health officials. PLoS Negl. Trop. Dis. 14(2), e0007964 (2020).
Keay, L. et al. Microbial keratitis predisposing factors and morbidity. Ophthalmology 113(1), 109–116 (2006).
Lorenzo-Morales, J., Khan, N. A. & Walochnik, J. An update on Acanthamoeba keratitis: Diagnosis, pathogenesis and treatment. Parasite 22, 10 (2015).
Jalbert, I., Stapleton, F., Papas, E., Sweeney, D. F. & Coroneo, M. In vivo confocal microscopy of the human cornea. Br. J. Ophthalmol. 87(2), 225–236 (2003).
Hau, S. C. et al. Diagnostic accuracy of microbial keratitis with in vivo scanning laser confocal microscopy. Br. J. Ophthalmol. 94(8), 982–987 (2010).
Deng, L. & Dong, Y. Deep learning: Methods and applications. Found. Trends Signal Process. 7(3–4), 197–387. https://doi.org/10.1561/2000000039 (2014).
LeCun, Y., Bengio, Y. & Hinton, G. Deep learning. Nature 521(7553), 436–444 (2015).
Shen, D., Wu, G. & Suk, H. I. Deep learning in medical image analysis. Annu. Rev. Biomed. Eng. 19, 221–248 (2017).
Grassmann, F. et al. A deep learning algorithm for prediction of age-related eye disease study severity scale for age-related macular degeneration from color fundus photography. Ophthalmology 125(9), 1410–1420 (2018).
Chen, X., Xu, Y., Yan, S., Wong, D. W. K., Wong, T. Y. & Liu, J. Automatic feature learning for glaucoma detection based on deep learning. In Medical Image Computing and Computer-Assisted Intervention–MICCAI 2015. MICCAI 2015, vol 9351 https://doi.org/10.1007/978-3-319-24574-4_80 (Springer, 2015).
Kim, S. J., Cho, K. J. & Oh, S. Development of machine learning models for diagnosis of glaucoma. PLoS ONE 12(5), e0177726 (2017).
Kermany, D. S. et al. Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell 172(5), 1122-1131.e9 (2018).
Maruoka, S. et al. Deep neural network-based method for detecting obstructive meibomian gland dysfunction with in vivo laser confocal microscopy. Cornea 39(6), 720–725 (2020).
Lv, J. et al. Deep learning-based automated diagnosis of fungal keratitis with in vivo confocal microscopy images. Ann. Transl. Med. 8(11), 706 (2020).
Zhang, X. Deep learning for three types of keratitis classification based on confocal microscopy images. In Proceedings of the 2020 3rd International Conference on Signal Processing and Machine Learning (SPML 2020), 91–97 https://doi.org/10.1145/3432291.3432310, (Association for Computing Machinery, 2020).
Gunning, D. et al. XAI-explainable artificial intelligence. Sci. Robot. 4(37), 7120 (2019).
Simonyan, K., Vedaldi, A. & Zisserman, A. Deep inside convolutional networks: Visualising image classification models and saliency maps. arXiv preprint 1312.6034 https://doi.org/10.48550/ARXIV.1312.6034 (2013).
Abolhosseini, M., Moshtaghion, S. M., Rezaei Kanavi, M. & Hosseini, S. B. A triad of microscopes for rapid and proper diagnosis of infectious keratitis. Clin. Exp. Optom. 105(3), 333–335 (2022).
Essalat, M., Abolhosseini, M., Le, T. H., Moshtaghion, S. M. & Kanavi, M. R. An interpretable deep learning approach for automatic diagnosis of fungal keratitis and acanthamoeba keratitis based on an introduced database of in vivo confocal microscopic images (Version 1.0.0), https://figshare.com/articles/dataset/Dataset/19838083 (2022).
Mikołajczyk, A. & Grochowski, M. Data augmentation for improving deep learning in image classification problem. In 2018 International Interdisciplinary PhD Workshop (IIPhDW 2018), 117–122, https://doi.org/10.1109/IIPHDW.2018.8388338 (IEEE, 2018)
Huang, G., Liu, Z., Van Der Maaten, L. & Weinberger, K. Q. Densely connected convolutional networks. In 2017 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), 2261–2269, https://doi.org/10.1109/CVPR.2017.243 (IEEE, 2017)
He, K., Zhang, X., Ren, S. & Sun, J. Deep residual learning for image recognition. In 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR 2016), 770–778, https://doi.org/10.1109/CVPR.2016.90 (IEEE,2016)
Wang, C. Y. et al. CSPNet: A new backbone that can enhance learning capability of CNN. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition Workshops. arXiv preprint; 1911.11929. https://doi.org/10.48550/ARXIV.1911.11929 (2019).
Xie, S., Girshick, R., Dollár, P. & Tu, Z. Aggregated residual transformations for deep neural networks. In 2017 IEEE Conference on Computer Vision and Pattern Recognition (CVPR 2017), 5987–5995, https://doi.org/10.1109/CVPR.2017.634 (IEEE,2017)
Simonyan, K. & Zisserman, A. Very deep convolutional networks for large-scale image recognition. arXiv preprint 1409.1556. https://doi.org/10.48550/arxiv.1409.1556. (2014).
Glorot, X. & Bengio, Y. Understanding the difficulty of training deep feedforward neural networks. Proceedings of the Thirteenth International Conference on Artificial Intelligence and Statistics, In Proceedings of Machine Learning Research (PMLR) vol. 9, 249–256. https://proceedings.mlr.press/v9/glorot10a (2010).
Refaeilzadeh, P., Tang, L. & Liu, H. Cross-validation. In Encyclopedia of Database Systems (eds Liu, L. & Özsu, M. T.) (Springer, 2009). https://doi.org/10.1007/978-0-387-39940-9_565.
Kuo, M. T. et al. A deep learning approach in diagnosing fungal keratitis based on corneal photographs. Sci. Rep. 10(1), 14424 (2020).
Niederer, R. L. & McGhee, C. N. Clinical in vivo confocal microscopy of the human cornea in health and disease. Prog. Retin. Eye Res. 29(1), 30–58 (2010).
Patel, D. V. & McGhee, C. N. Quantitative analysis of in vivo confocal microscopy images: A review. Surv. Ophthalmol. 58(5), 466–475 (2013).
Kheirkhah, A. et al. Sensitivity and specificity of laser-scanning in vivo confocal microscopy for filamentous fungal keratitis: Role of observer experience. Am. J. Ophthalmol. 179, 81–89. https://doi.org/10.1016/j.ajo.2017.04.011 (2017) (Epub 2017 Apr 23).
Kheirkhah, A. et al. Factors influencing the diagnostic accuracy of laser-scanning in vivo confocal microscopy for acanthamoeba keratitis. Cornea 37(7), 818–823. https://doi.org/10.1097/ICO.0000000000001507 (2018).
Cruzat, A. et al. Inflammation and the nervous system: The connection in the cornea in patients with infectious keratitis. Investig. Ophthalmol. Vis. Sci. 52(8), 5136–5143. https://doi.org/10.1167/iovs.10-7048 (2011).
Author information
Authors and Affiliations
Contributions
Conceptualization: M.A., M.E., S.M.M., M.R.K. Data curation: M.A., S.M.M., M.R.K. Formal analysis: M.E., T.H.L., M.A., S.M.M., M.R.K. Coding: T.H.L. Code review and guidance: M.E. original draft: T.H.L., M.E., M.A. Writing—review and editing: M.E., M.R.K., T.H.L., M.A., S.M.M. Supervision: M.E., M.R.K.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Essalat, M., Abolhosseini, M., Le, T.H. et al. Interpretable deep learning for diagnosis of fungal and acanthamoeba keratitis using in vivo confocal microscopy images. Sci Rep 13, 8953 (2023). https://doi.org/10.1038/s41598-023-35085-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-35085-9
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.