Abstract
This study aimed to evaluate the prognostic significance of carcinoembryonic antigen (CEA) expression in tumor tissues of patients with colorectal cancer (CRC). The cohort included 7,412 patients with CRC from January 2010 to December 2015. Survival outcomes were assessed based on tissue CEA (t-CEA) patterns and intensities. Three-year (76.7% versus 81.3%) and 5-year (71.7% versus 77.6%, p < 0.001) disease-free survival (DFS) rates were significantly (p < 0.001) poorer in patients with a diffuse-cytoplasmic pattern than an apicoluminal pattern. Three-year (79% versus 86.6%) and 5-year (74.6% versus 84.7%) DFS rates were also significantly (p < 0.001) poorer in patients with high than low t-CEA intensity. Three-year (84.6% versus 88.4%) and 5-year (77.3% versus 82.6%) overall survival (OS) rates were significantly (p < 0.001) poorer in patients with diffuse-cytoplasmic than apicoluminal pattern of CEA expression, and both 3-year (86.7% versus 91.2%) and 5-year (80.1% versus 87.7%) OS rates were significantly (p < 0.001) poorer in patients with high than low t-CEA intensity. Multivariate analyses showed that high-intensity t-CEA was independently associated with DFS (p = 0.02; hazard ratio [HR] = 1.233) and OS (p = 0.032; HR = 1.228). Therefore, high-intensity t-CEA is a significant prognostic factor in CRC, independent of serum CEA (s-CEA), and can complement s-CEA in predicting survival outcomes after CRC resection.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is the third most common malignancy worldwide and the second most frequent cause of cancer-related deaths1. In the Republic of Korea (South Korea), CRC is the fourth most common malignancy and the third leading cause of cancer-related deaths2, 3. Despite advances in medical and surgical management, patient survival continues to be reduced by disease recurrence. Prognosis and overall survival (OS) in CRC have been found to correlate with the TNM staging system currently used by the American Joint Committee on Cancer (AJCC) and the International Union Against Cancer (UICC)4. Adverse features predictive of recurrence and poorer outcome after curative operation include tumor differentiation, lymphovascular invasion (LVI), perineural invasion (PNI), margin status, and mismatch repair protein status5,6,7,8.
Serum carcinoembryonic antigen (s-CEA) is a glycoprotein present in the human digestive system, elevated in patients with colon and rectal neoplasms9. This protein is encoded by CEACAM5 and expressed in colorectal epithelial cells, where it functions in cell recognition and intercellular adhesion10,11,12. In CRC, CEA expressed following the disruption of normal tissue structure and the loss of polarization of neoplastic cells is secreted into the blood stream, eventually resulting in an increase in s-CEA concentration13. Although s-CEA level is neither sufficiently sensitive nor specific as a screening tool for CRC, it plays an important role in surveillance after surgical resection14. In addition, s-CEA can be targeted in cancer imaging and active immunotherapy15.
Elevated preoperative s-CEA concentration, defined as > 5 ng/ml or more than two-fold higher than the normal cut-off value, is significantly associated with poorer overall and higher cancer-specific mortality in CRC patients16,17,18,19,20,21. Because preoperative s-CEA concentration > 5 ng/ml is an independent prognostic factor for poor OS, chemotherapy or intensive follow-up strategies should be considered, particularly in patients with negative lymph node metastasis, if preoperative s-CEA level is > 10 ng/ml8, 19, 22, 23. Recently, s-CEA has been targeted in tumor imaging, using recombinant vaccinia CEA (rV-CEA), or for active immunotherapy with recombinant adenovirus 5 (CEA/MUC1/Brachyury)24.
In addition to measuring preoperative s-CEA concentration, the expression of tissue CEA (t-CEA) can be immunohistochemically assessed in colorectal mucosa and tumor tissues. t-CEA is rarely expressed in normal colorectal mucosa but is consistently found in colorectal neoplasms, with different expression patterns and intensities15, 25. t-CEA expression patterns have been described as apicoluminal (AL), diffuse-cytoplasmic (DC), or a combination of the two. The DC pattern and high levels of expression have been associated with tumor aggressiveness, including LVI26. Studies have suggested that the DC pattern is associated with higher preoperative s-CEA levels, higher rates of lymph node and liver metastases, and higher recurrence and lower survival rates than the AL pattern26,27,28,29, although contradictory findings have also been reported30.
Few studies to date have assessed the associations of t-CEA expression pattern and intensity with long-term survival outcomes in patients with CRC. The present study evaluated the relationships between t-CEA expression and long-term survival in patients with CRC.
Results
Clinicopathological characteristics of patients and tumors
During the study period (January 2010 to December 2015), 10,566 patients underwent colorectal resection at Asan Medical Center. The 7412 included patients consisted of 4343 (58.6%) men and 3069 (41.4%) women, of mean age 61.7 ± 11.4 years. Of these patients, 5092 (68.7%) were diagnosed with colon cancer and 2320 (31.3%) with rectal cancer. Preoperative s-CEA level was high (> 6 mg/ml) in 1690 (23.2%) patients. Advanced tumor stages (III and IV) were found in 3505 (47.3%) patients. Most patients (6853, 92.5%) had well- and moderately differentiated adenocarcinoma, whereas 556 (7.5%) had unfavorable differentiation. LVI and PNI were identified in 2695 (36.4%) and 1855 (25.1%) patients, respectively.
Tissue carcinoembryonic antigen expression
Immunohistochemical analysis of t-CEA expression showed that resected tumor tissue of 5004 (67.5%) patients had the AL pattern and 2408 (32.5%) had the DC pattern. The DC pattern was significantly associated (p < 0.001) with factors associated with poor patient prognosis, including elevated preoperative s-CEA concentration, advanced TNM stage, unfavorable tumor histology, and LVI/PNI. High-intensity t-CEA expression was observed in tumor samples from 6629 (89.4%) patients and low intensity expression in 783 (10.6%) patients. High-intensity expression correlated significantly with elevated preoperative s-CEA concentration, advanced TNM stage, and LVI/PNI (p < 0.001, Table 1).
Recurrence and survival outcomes
After a mean follow-up time of 86.1 ± 33.0 months, 985 (13.3%) patients experienced tumor recurrence, including 74 (7.5%) with locoregional and 839 (85.2%) with systemic recurrences. Of the 839 patients who experienced systemic recurrence, 547 (65.2%) experienced recurrences to the liver, lungs, and/or distant nodes. Other systemic recurrences included peritoneal metastasis in 109 (13.0%) patients; recurrences to other organs, including the ovaries, brain, bones, adrenal glands, and spleen in 28 (3.4%); and multiple routes in 85 (10.1%).
Overall recurrence rates were significantly greater in patients with the DC than the AL pattern (p = 0.001) and in patients with high than low t-CEA expression intensity (p < 0.001). In patients with systemic (distant) recurrences, the DC pattern tended to show greater peritoneal metastasis than the AL pattern (15.9% versus 11.3%; Table 2).
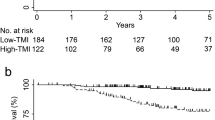
The mean ± SD DFS in the overall patient cohort was 77.5 ± 37.6 months, and the mean ± SD OS was 82.8 ± 33.0 months. Univariate analyses showed that 3- and 5-year DFS and OS rates differed significantly between groups of patients with different t-CEA patterns and intensities (p < 0.001; Fig. 1). For example, the 3- and 5-year DFS rates were 76.7% and 71.7%, respectively, in patients with the DC pattern, and 81.3% and 77.6%, respectively, in patients with the AL pattern. Similarly, the 3- and 5-year OS rates were 84.6% and 77.3%, respectively, in patients with the DC pattern, and 88.4% and 82.6%, respectively, in patients with the AL pattern. Survival rates were also significantly lower in patients with high than low intensity t-CEA expression. For example, the 3- and 5-year DFS rates were 79% and 74.6%, respectively, in patients with high-intensity expression, compared with 86.6% and 84.7%, respectively, in patients with low intensity expression. Similarly, the 3- and 5-year OS rates were 86.7% and 80.1%, respectively, in patients with high-intensity expression, compared with 91.2% and 87.7%, respectively, in patients with low intensity expression. Kaplan–Meier survival analysis according to each TNM stages was performed. In stage III patients, significant poor DFS and OS rate was shown in the high-intensity t-CEA expression patients (Supplementary Fig. 3). In stage II patients, high-intensity t-CEA was shown to have significant poor DFS but not OS rate (Supplementary Fig. 2). In stage 0 ~ I and IV, t-CEA did not show significant correlation with survival outcomes (Supplementary Figs. 1 and 4).
Multivariate analyses showed that high-intensity tissue CEA expression was independently associated with DFS (p = 0.02; HR = 1.233) and OS (p = 0.032; HR = 1.228). t-CEA expression pattern was not a prognostic factor in the multivariable analysis (Table 3).
Subgroup analyses were performed in patients assorted by both t-CEA expression intensity and preoperative s-CEA level. In patients with low preoperative s-CEA, those with high-intensity t-CEA expression had significantly poorer DFS (p < 0.001) and OS (p = 0.002) rates than patients with low intensity t-CEA expression. Similarly, among patients with high preoperative s-CEA, high-intensity t-CEA expression had significantly poorer DFS (p = 0.015) and OS (p = 0.025) rates than low intensity t-CEA expression (Fig. 2).
Kaplan–Meier analyses comparing disease-free survival and overall survival in patients with low preoperative s-CEA and low intensity t-CEA expression, low preoperative s-CEA level and high-intensity t-CEA expression, high preoperative s-CEA and low intensity t-CEA expression, and high preoperative s-CEA and high-intensity t-CEA expression.
Discussion
CEA acts as a metastatic potentiator through both homophilic and heterophilic binding31. CEA actively participates in the immune-related tumor microenvironment through a MHC class I-independent inhibitory pathway that mediates homophilic CEA interactions or heterophilic interactions of CEA with CEACAM124. The expression of CEA, especially on the cell membrane, as in patients with the DC pattern, interferes with the signaling of DR5 by direct interaction through the PELPK sequence of the CEA hemophilic binding domain, reducing caspase-8 activity and anoikis32. This biological behavior of CEA, along with the close correlation of t-CEA expression with LVI/PNI, suggests that t-CEA expression may play a significant role in a pre-metastatic niche establishing a potential tumor microenvironment (TME). Curative surgical resection may therefore be the most efficient method of removing the primary tumor as well as the TME.
High preoperative s-CEA level is prognostic of poor survival in patients with CRC16,17,18,19. The present study found that t-CEA expression intensity and pattern correlated significantly with preoperative s-CEA level. Although many previous studies have reported a lack of correlation between preoperative s-CEA levels and t-CEA expression29, 30, 33, 34, one study confirmed this relationship35. These discrepancies may be due to differences in categorization of t-CEA expression patterns and intensities, and the small populations sizes (30–517 patients) in these studies.
Of the 7412 patients included in the present study, only 100 (1.3%) showed inverse relationships between t-CEA expression intensities and preoperative s-CEA levels. Low t-CEA expression intensity in patients with high preoperative s-CEA levels may be explained by factors unrelated to malignancy, including the wide range of normal preoperative s-CEA concentrations among healthy people, the effects of age and benign conditions, the high variability of liver metabolic rates, and the long half-life of glycoproteins. These findings may also be explained by the movement over time of CEA molecules from tissue to blood.
In agreement with previous studies, the present study showed that both high-intensity t-CEA expression and the DC pattern were significantly associated with higher tumor recurrence rates29, 34. Preoperative s-CEA concentration is also related to higher recurrence rates, as confirmed in the present study. The ability of t-CEA expression intensity to predict recurrence was especially noticeable among patients with low preoperative s-CEA levels, with patients having high-intensity t-CEA expression showing significantly higher rates of recurrence regardless of low preoperative s-CEA level.
This study showed that both elevated preoperative s-CEA and high-intensity t-CEA expression28, 34 were independently prognostic of poorer DFS. When divided into four subgroups based on both preoperative s-CEA level and t-CEA expression intensity, DFS was worse in groups with high-intensity t-CEA expression regardless of preoperative s-CEA levels. Taken together, these findings suggest that t-CEA expression intensity plays a complementary role as an adjunctive measurement of preoperative s-CEA level. The intensity of t-CEA expression may therefore be a reliable and accurate measure of patient prognosis beginning at an early stage of treatment.
Several ambiguous results from the present and previous studies require further explanation. Although Kaplan–Meier analysis showed that the DC pattern was associated with significantly poorer DFS and OS, these correlations were not statistically significant on multivariate analyses. Although the DC pattern is indicative of CEA distribution in the cytoplasm and may be associated with poorer prognosis, multivariable analysis showed that only high-intensity t-CEA expression was significantly associated with poorer survival outcomes. Also, unfavorable histologic differentiation was significantly associated with DC expression pattern, whereas high-intensity t-CEA expression was significantly associated with favorable tumor differentiation. A previous study also found that t-CEA expression intensity was higher in well differentiated than poorly differentiated colorectal adenocarcinomas33. Further research is needed to explain these phenomena.
This study is limited by its non-randomized design and the retrospective nature of the data. For example, the inability to measure t-CEA in patients with unresectable CRC required excluding this group of patients. The results of this study suggested that advanced CRC stages are associated with high intensity or the DC pattern of t-CEA expression. Some discrepancies with previous studies may be caused by differences in pathologic interpretations. For example, determination of t-CEA expression patterns is subjective, as these evaluations are related to the depth of CEA distribution. Regarding this issue, due to the substantially large number of patients included in this study, the reliability of t-CEA results from the pathologists could not be assessed. However, t-CEA expression pattern has been analyzed for over a decade in the present tertiary medical center and experience has accumulated to form a consensus, resulting in consistent pathologic reports.
High intensity of tissue carcinoembryonic antigen is a significant prognostic factor in patients with colorectal cancer, independent of serum carcinoembryonic antigen. Tissue carcinoembryonic antigen can be used complementary with serum carcinoembryonic antigen to predict survival outcomes after colorectal cancer resection.
Methods
Data collection and study design
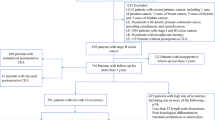
The medical records of 10,566 patients diagnosed with CRC who underwent resection between January 2010 and December 2015 at Asan Medical Center (Seoul, South Korea) were reviewed retrospectively. Patients with inflammatory bowel disease, familial adenomatous polyposis, and hereditary nonpolyposis colorectal cancer were excluded. Also excluded were patients with missing data on t-CEA expression, those who received neoadjuvant therapy, patients with synchronous malignancies other than CRC, patients with metachronous or recurrent CRC, and those who died within 90 days of resection. Patients with incomplete data due to loss to follow-up were excluded. Unresectable stage IV patients who received palliative resection of the primary tumor due to complications related to tumor (obstruction, bleeding, ischemia) were included. Thus, a total of 7412 patients was enrolled in this study (Fig. 3).
The study protocol was approved by the Institutional Review Board at Asan Medical Center (approval number: 2021–0908), which waived the requirement for informed consent due to the retrospective design of this study. This study was conducted according to the Declaration of Helsinki36.
Evaluation of CEA
t-CEA distribution in CRC tissue was assessed immunohistochemically, using 1:1600 mouse monoclonal, clone CEA31, catalog No.236 M-96, CELL MARQUE, CALIFONIA, USA. The distribution patterns in neoplastic tissue were categorized into two patterns (Fig. 4), with the AL pattern defined as CEA immunoreactivity along the cytoplasmic membrane and the DC pattern as homogeneous staining within the cytoplasm including the cytoplasmic membrane. Samples showing both the AL and DC patterns were categorized as DC. The intensity of CEA immune staining was classified as weak, moderate, or strong, depending on the proportion of tumor cells presenting more than moderate staining intensity (i.e. < 25%, 25–50%, or > 50%, respectively). Staining was performed in one whole slide section and intratumoral heterogeneity was not shown in most cases. Weak staining was scored as low intensity, whereas moderate and strong were scored as high intensity. Preoperative s-CEA concentrations > 6 ng/ml were defined as high according to the criteria of Asan Medical Center, in which routine modality of detecting s-CEA is by radioimmunoassay (RAI).
Statistical analysis
Primary outcomes were disease-free survival (DFS) and OS. DFS was defined as the interval between the date of surgery and the date of cancer recurrence or death from any cause. OS was defined as the interval between the date of surgery to the date of death from any cause or the end of the study. Categorical variables were analyzed using chi-square tests. Continuous variables were expressed as mean ± standard deviation and compared using Student’s t-tests. Survival was analyzed using the Kaplan–Meier method and compared by log-rank test. Confounding factors, including sex, age, preoperative s-CEA concentration, tumor location (colon versus rectum), tumor differentiation, LVI, PNI, and tumor stage, were adjusted using multivariable analysis (Cox proportional hazards model). All statistical analyses were performed using IBM SPSS® version 26.0 (IBM, Armonk, NY), with p < 0.05 considered statistically significant.
Data availability
The data that support the findings of this study are available from the corresponding author on request.
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
Annual report of cancer statistics in Korea in 2016, National Cancer Center Registry. http://www.ncc.re.kr/indexEn.ncc. Accessed 1 July 2021.
Kim, M. H., Park, S., Yi, N., Kang, B. & Park, I. J. Colorectal cancer mortality trends in the era of cancer survivorship in Korea: 2000–2020. Ann. Coloproctol. 38, 343–352 (2022).
Amin, M. B. et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 67, 93–99 (2017).
Eom, T. et al. Prognostic factors affecting disease-free survival and overall survival in T4 colon cancer. Ann. Coloproctol. 37, 259–265 (2021).
Kim, Y. I. et al. Prognostic impact of extranodal extension in rectal cancer patients undergoing radical resection after preoperative chemoradiotherapy. Clin. Colorectal Cancer 20, e35–e42 (2021).
Kim, Y. I. et al. Clinical implication of perineural and lymphovascular invasion in rectal cancer patients who underwent surgery after preoperative chemoradiotherapy. Dis. Colon Rectum 65, 1325–1334 (2022).
Lee, Y. et al. Effect of adjuvant chemotherapy on elderly stage II high-risk colorectal cancer patients. Ann. Coloproctol. 37, 298–305 (2021).
Gold, P. & Freedman, S. O. Specific carcinoembryonic antigens of the human digestive system. J. Exp. Med. 122, 467–481 (1965).
Thompson, J. A., Grunert, F. & Zimmermann, W. Carcinoembryonic antigen gene family: Molecular biology and clinical perspectives. J. Clin. Lab. Anal. 5, 344–366 (1991).
Au, F. C., Stein, B. S., Gennaro, A. R. & Tyson, R. R. Tissue CEA in colorectal carcinoma. Dis. Colon Rectum 27, 16–18 (1984).
Jessup, J. M. et al. Adhesion to carcinoembryonic antigen by human colorectal carcinoma cells involves at least two epitopes. Int. J. Cancer 55, 262–268 (1993).
Hammarström, S. The carcinoembryonic antigen (CEA) family: Structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 9, 67–81 (1999).
Hardiman, K. M. et al. The American society of colon and rectal surgeons clinical practice guidelines for the surveillance and survivorship care of patients after curative treatment of colon and rectal cancer. Dis. Colon Rectum 64, 517–533 (2021).
Tiernan, J. P. et al. Carcinoembryonic antigen is the preferred biomarker for in vivo colorectal cancer targeting. Br. J. Cancer 108, 662–667 (2013).
Becerra, A. Z. et al. Evaluating the prognostic role of elevated preoperative carcinoembryonic antigen levels in colon cancer patients: Results from the National Cancer Database. Ann. Surg. Oncol. 23, 1554–1561 (2016).
Thirunavukarasu, P. et al. Effect of incorporation of pretreatment serum carcinoembryonic antigen levels into AJCC staging for colon cancer on 5-year survival. JAMA Surg. 150, 747–755 (2015).
Ozawa, H. et al. Incorporation of serum carcinoembryonic antigen levels into the prognostic grouping system of colon cancer. Int. J. Colorectal Dis. 32, 821–829 (2017).
Kim, C. W. et al. Elevation of preoperative s-CEA concentration in stage IIA colorectal cancer can also be a high risk factor for stage II patients. Ann. Surg. Oncol. 20, 2914–2920 (2013).
Park, K. S., Hong, Y. K., Choi, Y. J. & Kang, J. G. Clinicopathologic characteristics of early-onset colorectal cancer. Ann. Coloproctol. 38, 362–369 (2022).
Nakamura, Y. et al. The prognosis and recurrence pattern of right- and left-sided colon cancer in stage II, stage III, and liver metastasis after curative resection. Ann. Coloproctol. 37, 326–336 (2021).
Yeh, C. Y. et al. Preoperative carcinoembryonic antigen elevation in colorectal cancer. Hepatogastroenterology 58, 1171–1176 (2011).
Huang, S. H. et al. Preoperative carcinoembryonic antigen as a poor prognostic factor in stage I-III colorectal cancer after curative-intent resection: A propensity score matching analysis. Ann Surg Oncol 26, 1685–1694 (2019).
Nachmias, B. et al. Manipulation of NK cytotoxicity by the IAP family member Livin. Eur. J. Immunol. 37, 3467–3476 (2007).
Tormo, B. R. et al. CEA in colonic adenocarcinomas and precancerous lesions. An immunohistochemical study with a novel monoclonal antibody. Apmis 97, 1073–1080 (1989).
Kim, J. C. et al. Genetic and pathologic changes associated with lymphovascular invasion of colorectal adenocarcinoma. Clin. Exp. Metastasis 22, 421–428 (2005).
Tokunaga, N. et al. Immunohistochemical localization of carcinoembryonic antigen as a predictor of lymph node status in submucosa-invasive colorectal carcinoma. Dis. Colon Rectum 38, 842–847 (1995).
Kim, J. C. et al. Distribution of carcinoembryonic antigen and biologic behavior in colorectal carcinoma. Dis. Colon Rectum 42, 640–648 (1999).
Zedan, S. et al. Study of carcinoembryonic antigen tissue expression in colorectal cancer. Coloproctology 23, 88–93 (2001).
Nazato, D. M. et al. Prognostic value of carcinoembryonic antigen distribution in tumor tissue of colorectal carcinoma. Arq. Gastroenterol. 46, 26–31 (2009).
Kim, J. C. et al. Carcino-embryonic antigen may function as a chemo-attractant in colorectal-carcinoma cell lines. Int. J. Cancer 82, 880–885 (1999).
Samara, R. N., Laguinge, L. M. & Jessup, J. M. Carcinoembryonic antigen inhibits anoikis in colorectal carcinoma cells by interfering with TRAIL-R2 (DR5) signaling. Cancer Res. 67, 4774–4782 (2007).
Saito, G. et al. Relation between carcinoembryonic antigen levels in colon cancer tissue and serum carcinoembryonic antigen levels at initial surgery and recurrence. Oncology 91, 85–89 (2016).
Tong, G. et al. The role of tissue and serum carcinoembryonic antigen in stages I to III of colorectal cancer-A retrospective cohort study. Cancer Med. 7, 5327–5338 (2018).
Park, J. W., Chang, H. J., Kim, B. C., Yeo, H. Y. & Kim, D. Y. Clinical validity of tissue carcinoembryonic antigen expression as ancillary to serum carcinoembryonic antigen concentration in patients curatively resected for colorectal cancer. Colorectal Dis. 15, e503-511 (2013).
Association, W. M. Declaration of Helsinki Ethical. Principles for medical research involving human subjects. Jahrbuch für Wissenschaft und Ethik 14, 233–238 (2009).
Acknowledgements
The authors thank Dr. J. Milburn Jessup and the late Dr. On Ja Kim for their sincere devotion to our CEA research.
Author information
Authors and Affiliations
Contributions
K.Y.I. and A.F.A. wrote the main manuscript text, prepared figures, analyzed data, and contributed equally to this research. K.J. provided pathological insights and R.J.-S. reviewed statistics. Y.Y.S., P.I.J., and L.S.-B. provided data. K.C.W. and K.J.C. supervised the project. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aldilaijan, A.F., Kim, Y.I., Kim, C.W. et al. Clinical implication of tissue carcinoembryonic antigen expression in association with serum carcinoembryonic antigen in colorectal cancer. Sci Rep 13, 7616 (2023). https://doi.org/10.1038/s41598-023-34855-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-34855-9
This article is cited by
-
scFv biofunctionalized nanoparticles to effective and safe targeting of CEA-expressing colorectal cancer cells
Journal of Nanobiotechnology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.