Abstract
Patients with device detected atrial high-rate episodes (AHRE) have an increased risk of MACE. The R2CHA2DS2-VASc, CHADS2, R2CHADS2 and CHA2DS2-VASc score have been investigated for predicting major adverse cardiovascular events (MACE) in different groups of patients. We aimed to evaluate the R2CHA2DS2-VASc score in combination with AHRE ≥ 6 min for predicting MACE in patients with dual-chamber PPM but no prior atrial fibrillation (AF). We retrospectively enrolled 376 consecutive patients undergoing dual-chamber PPM implantation and no prior AF. The primary endpoint was subsequent MACE. For all patients in the cohort, CHADS2, R2CHADS2, CHA2DS2-VASc, R2CHA2DS2-VASc scores and AHRE ≥ or < 6 min were determined. AHRE was recorded as a heart rate > 175 bpm (Medtronic) or > 200 bpm (Biotronik) lasting ≥ 30 s. Multivariate Cox regression analysis with time-dependent covariates was used to determine the independent predictors of MACE. ROC-AUC analysis was performed for CHADS2, R2CHADS2, CHA2DS2-VASc, and R2CHA2DS2-VASc scores and then adding AHRE ≥ 6 min to the four scores. The median age was 77 years, and 107 patients (28.5%) developed AHRE ≥ 6 min. After a median follow-up of 32 months, 46 (12.2%) MACE occurred. Multivariate Cox regression analysis showed that R2CHA2DS2-VASc score (HR, 1.485; 95% CI, 1.212–1.818; p < 0.001) and AHRE ≥ 6 min (HR, 2.125; 95% CI, 1.162–3.887; p = 0.014) were independent predictors for MACE. The optimal R2CHA2DS2-VASc score cutoff value was 4.5 (set at ≥ 5), with the highest Youden index (AUC, 0.770; 95% CI, 0.709–0.831; p < 0.001). ROC-AUC analysis of the four risk scores separately combined with AHRE ≥ 6 min all showed better discriminatory power than the four scores alone (All Z-statistic p < 0.05). In patients with PPM who develop AHRE ≥ 6 min, it is crucial to perform risk assessment with either four scores to further stratify risk for MACE.
Similar content being viewed by others
Introduction
The increased use of cardiac implantable electronic device (CIED) such as dual chamber permanent pacemakers (PPM) or internal cardiac defibrillators (ICDs) can detect episodes of atrial tachyarrhythmias, including atrial tachycardia, atrial flutter and atrial fibrillation (AF) in patients with an atrial lead. These tachycardia episodes, commonly asymptomatic, are known as atrial high-rate episodes (AHRE), also called subclinical AF1,2. The rate criterion for AHRE varies in different studies and is > 175 bpm on current guideline, and there is a wide range of duration cut-offs, from 10 to 20 s to > 24 h1. AHRE were reported in 10–35% in studies including patients without known AF2,3. Previous studies have reported that AHRE ≥ 5–6 min increase the risk of clinical AF4 and ischemic stroke3,4. The increased risk of major adverse cardiovascular events (MACE), especially myocardial infarction has also been reported in patients with AF5,6 but only few studies in those with AHRE. The recent studies have demonstrated AHRE are associated with increased risk of cardiovascular death7, and MACE8. A higher burden leads to a higher risk of thrombo-embolism, heart failure and MACE8,9,10, indicating that dual chamber PPMs should be interrogated regularly to identify AHRE, and these patients should undergo further risk assessment for MACE.
CHADS2 and CHA2DS2-VASc scores are used for stroke risk stratification in patients with AF and the risk scores decide the subsequent use of oral anticoagulant according to current guideline1. R2CHADS2 and R2CHA2DS2-VASc scoring system adding 2 points for renal dysfunction to CHADS2 and CHA2DS2-VASc scores had a better performance to stratify thromboembolic risk in patients with AF11,12. These scores are composed of parameters which are known as atherosclerotic risk factors for cardiovascular events. In addition to risk assessment for stroke, these scores have been proposed to predict cardiovascular mortality and all-cause death in different sets of population, including patients with high cardiovascular risk13, chest pain14, coronary artery disease15, acute coronary syndrome12,16,17,18,19, heart failure20, sick sinus syndrome21 and patients undergoing transcatheter aortic valve replacement22. The predictive ability of the R2CHA2DS2-VASc score on MACE occurrence in patients with dual chamber PPMs and no prior AF and the combination of AHRE ≥ 6 min and R2CHA2DS2-VASc score have not yet been studied.
Accordingly, we investigated the performance of R2CHA2DS2-VASc score in comparison to other risk scales, including CHADS2, CHA2DS2-VASc and R2CHADS2 scores, and also elucidated the predictive value of AHRE ≥ 6 min in combination with R2CHA2DS2-VASc score and other scores for MACE occurrence. The novelty of this study is that there is no previous research to examine the discriminating ability of R2CHA2DS2-VASc and the other three scores in combination with AHRE ≥ 6 min to predict MACE.
Methods
Consecutive patients ≥ 18 years of age who underwent dual-chamber PPM implantation (Medtronic, Minneapolis, MN, USA or Biotronik, Berlin, Germany) in the Cardiology Department of National Cheng Kung University Hospital from January 2014 to April 2021 were retrospectively included.
Ethical considerations
The protocol for this cohort study was reviewed and approved by the ethics committee of National Cheng Kung University Hospital and conducted according to the guidelines of the International Conference on Harmonization for Good Clinical Practice (B-ER-108–278).
Data collection and definitions23
Patients’ medical histories and data regarding comorbidities and echocardiographic parameters were collected from medical records for retrospective evaluation. Diabetes mellitus was defined as the presence of symptoms and random plasma glucose concentration ≥ 200 mg/dL, fasting plasma glucose concentration ≥ 126 mg/dL, 2-h plasma glucose concentration ≥ 200 mg/dL from a 75-g oral glucose tolerance test, or use of medication for diabetes mellitus. Hypertension was defined as in-office systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg or use of antihypertensive medication. Dyslipidemia was defined as low-density lipoprotein ≥ 140 mg/dL, high-density lipoprotein < 40 mg/dL, triglycerides ≥ 150 mg/dL, or use of medication for dyslipidemia. Chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 for at least 3 months23. Previous AF was defined as any documented AF on 12-lead electrocardiography (ECG) or Holter recordings ≥ 30 s before the date of implantation of PPM. The primary endpoint for this study was the occurrence of MACE after the date of PPM implantation, including ST elevation myocardial infarction (MI), non-ST elevation MI, unstable angina, or death (cardiac or noncardiac). We excluded stroke from the components of MACE because the CHA2DS2-VASc and R2CHA2DS2-VASc scoring systems have been validated to predict the incidence of stroke. For each outcome, only the first event of that outcome in a given patient was included. For the composite outcome, only the first event in a given patient was included.
AHRE was detected by a CIED as a heart rate > 175 bpm (Medtronic) or > 200 bpm (Biotronik) and at least 30 s of atrial tachyarrhythmia recorded by the devices on any day during the study periods23. Atrial sensitivity was programmed to 0.3 mV with bipolar sensing of Medtronic devices and 0.2 mV with bipolar sensing of Biotronik devices. AHRE electrograms extracted from the devices via telemetry at each office visit (every 3–6 months) were reviewed by at least one experienced electrophysiologist, who considered the possibility that AHRE included lead noise or artifacts, far-field R-waves, or paroxysmal supraventricular tachycardia and who visually confirmed AF in the detected AHRE. The duration of detected AHRE was recorded and we divided the patients into two groups according to whether detected AHRE duration was more than 6 min or not.
Scoring system assessments
The CHADS2 score1 ranges from 0 to 6. A history of heart failure, hypertension, or diabetes mellitus and age ≥ 75 years are calculated as 1 point; prior stroke, transient ischemic attack (TIA) or thromboembolism are each calculated as 2 points. The CHA2DS2-Vasc score1 ranges from 0 to 9. Patients are given 1 point for history of heart failure, hypertension, diabetes, or vascular disease; age 65–74 years; and female sex and 2 points for age ≥ 75 years and prior stroke, transient ischemic attack, or systemic thromboembolism. The R2CHADS2 score11 ranges from 0 to 8, in which 1 point is assigned for a history of heart failure, hypertension, or diabetes mellitus and age ≥ 75 years, and 2 points are assigned for prior stroke, transient ischemic attack, or systemic thromboembolism and chronic kidney disease (CKD), which is defined as an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 for at least 3 months.
The R2CHA2DS2-VASc score ranges from 0 to 11, in which 1 point is assigned for history of heart failure, hypertension, diabetes, or vascular disease; age 65–74 years, and female sex, and 2 points are assigned for CKD, age ≥ 75 years and prior stroke, transient ischemic attack, or systemic thromboembolism.
Statistical analysis
Categorical variables are presented as percentages, and continuous variables are presented as the mean and standard deviation for normally distributed values or as the median and interquartile interval (IQI) for nonnormally distributed values. Normal distribution of continuous variables was assessed using the Kolmogorov–Smirnov method. Pearson’s chi-square test or Fisher's exact test was used to determine differences in baseline characteristics for categorical variables and differences between the risk scores groups. A two-sample Student’s t test or the Mann–Whitney U test was used to analyze continuous variables. Survival was estimated using the Kaplan–Meier method, and differences in survival were evaluated using the log-rank test. Multivariate Cox regression analysis with time-dependent covariate was used to identify variables associated with MACE occurrence, reported as hazard ratios (HR) with 95% confidence intervals (CI). Parameters with a p < value 0.05 in the univariate analysis were entered into multivariate analysis, but variables already considered by the R2CHA2DS2-VASc score were not evaluated separately in any multivariate Cox regression analysis regardless of their significance in the univariate analysis. Previous studies have used Cox regression analysis to investigate the relationship of AHRE and stroke, CV mortality and MACE3,7,8. The receiver-operating characteristic (ROC) area under the curve (AUC) of the CHA2DS2-VASc, CHADS2, R2CHADS2, R2CHA2DS2-VASc score, and the associated 95% confidence intervals (CI) were evaluated for association with future MACE after PPM implantation. The optimal cutoff values with the highest Youden index were chosen based on the results of ROC analysis and used to evaluate the associated values of the R2CHA2DS2-VASc score for determining MACE. To further examine discriminatory power of the risk assessment model, we performed ROC-AUC analysis of combined AHRE and the risk scores. We used DeLong test24, a nonparametric approach to the comparison of the area under two or more ROC curves, to compare the performances of the four scores and different models. For all comparisons, p < 0.05 was considered statistically significant. All data were analyzed using SPSS statistical package version 23.0 (SPSS Inc. Chicago, IL, USA).
Ethical approval
Approved by the Institutional Review Board of National Cheng Kung University Hospital (B-ER-108–278).
Results
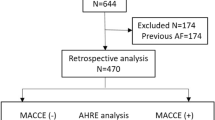
Between January 1, 2014, and April 2021, a total of 511 consecutive patients who underwent dual-chamber PPM implantation at National Cheng Kung University Hospital were recruited. Patients with previous AF (n = 135) were excluded. The final analysis included 376 patients, of whom 46 (12.2%) had experienced MACE.
The median follow-up period was 32 months after dual-chamber PPM implantation. Table 1 shows the patients’ baseline demographic and clinical characteristics according to the presence or absence of MACE. The median age was 77 (69–84) years, and 56.1% of participants were male. The median BMI was 24.8 kg/m2, and most patients were not obese. The brands of PPMs were Medtronic (58.5%) and Biotronik (41.5%). The most common indication for PPM implantation was sinus node dysfunction (66.0%), followed by atrioventricular block (34.0%) (Table 1). The overall median percentages of atrial pacing and ventricular pacing were 41.1% and 7.1%, respectively. High percentages of diabetes (50.5%), hypertension (91%), and dyslipidemia (87.5%) suggested a relatively high risk of MACE for the entire study cohort. One hundred thirty-seven patients (36.4%) used antiplatelet therapy, 99 patients (26.3%) took beta-blockers, 146 patients (38.9%) took RAAS inhibitors, and 131 patients (34.8%) took statins at baseline. The total number of MACE was 46 (12.2%). The median R2CHA2DS2-VASc score was 4 (range, 3–6), the median CHA2DS2-VASc score was 3 (range, 2–4), the median CHADS2 score was 3 (range, 2–3), and the median R2CHADS2 score was 3 (range, 2–5). One hundred seven (28.5%) patients had AHRE ≥ 6 min. Twenty five of the 107 (23.3%) patients with AHRE ≥ 6 min had MACE. In those without AHRE ≥ 6 min, 21 of 269 (7.8%) patients had MACE, which was significantly lower (p < 0.05).
Univariate analysis and multivariate Cox regression analysis to identify independent predictors of MACE
Univariate analysis revealed that MACE occurrence was significantly associated with a history of diabetes mellitus, prior myocardial infarction, heart failure, chronic kidney disease, worse LVEF, higher mitral E/e’, larger left atrial (LA) diameter, worse RV systolic function, higher CHA2DS2-VASc, CHADS2, R2CHA2DS2-VASc, R2CHADS2 score and AHRE ≥ 6 min (Table 1). In the multivariate Cox regression analysis, we did not include components of the R2CHA2DS2-VASc score such as chronic kidney disease, heart failure, diabetes mellitus, and prior myocardial infarction. The R2CHA2DS2-VASc score was an independent predictor of MACE in multivariate Cox regression analysis (HR, 1.485; 95% CI, 1.212–1.818; p < 0.001). AHRE ≥ 6 min was another stronger independent predictor of MACE occurrence (HR, 2.125; 95% CI, 1.162–3.887; p = 0.014). A larger LA diameter was associated with a trend toward increased MACE occurrence (HR, 1.550; 95% CI, 0.933–2.572, p = 0.090).
ROC-AUC determination of R2CHA2DS2-VASc score cutoff values for factors predictive of future MACE and survival analysis
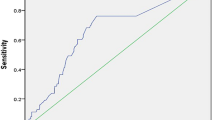
The ROC analysis of the R2CHA2DS2-VASc score showed that the optimal cutoff value for predicting the occurrence of MACE was 4.5 according to the highest Youden index (sensitivity, 76.1%; specificity, 65.8%; AUC, 0.770; 95% CI, 0.709–0.831; p < 0.001; Fig. 1). In practice, the cutoff value would be set at ≥ 5. The other AUC values were as follows: CHA2DS2-VASc score: 0.757, 95% CI = 0.688–0.826, p < 0.001; R2CHADS2 score: 0.748, 95% CI = 0.681–0.815, p < 0.001; and CHADS2 score: 0.727, 95% CI = 0.659–0.796, p < 0.001, which represented acceptable discriminating ability of MACE prediction. We compared AUC values of each two different risk scores using Z-statistic. The corresponding comparisons were as follows: CHADS2 versus R2CHADS2 (Z-statistic: 0.909, p > 0.05), CHADS2 versus CHA2DS2-VASc (Z-statistic: 0.799, p > 0.05), CHADS2 versus R2CHA2DS2-VASc (Z-statistic: 1.284, p > 0.05), R2CHADS2 versus R2CHA2DS2-VASc (Z-statistic: 0.965, p > 0.05), R2CHADS2 versus CHA2DS2-VASc (Z-statistic: 0.22, p > 0.05), and CHA2DS2-VASc versus R2CHA2DS2-VASc (Z-statistic: 0.537, p > 0.05), demonstrating that there was no statistically significant differences in the AUC values among the four scores. The event numbers and rates of the four scores were listed in Table 2.
Receiver operating characteristic analysis of the R2CHA2DS2-VASc score in patients with permanent pacemakers with subsequent major cardiovascular events. The R2CHA2DS2-VASc score: optimal cutoff value with the highest Youden index, 4.5; sensitivity, 76.1%; specificity, 65.8%; AUC, 0.770; 95% CI, 0.709–0.831; p < 0.001. The other C-statistics: CHA2DS2-VASc score 0.757, 95% CI = 0.688–0.826, p < 0.001; R2CHADS2 score 0.748, 95% CI = 0.681–0.815, p < 0.001; CHADS2 score 0.727, 95% CI = 0.659–0.796, p < 0.001. There are no statistically significant differences in the C-indexes among the four scores.
Preliminarily, we divided our patients into three risk groups according to the R2CHA2DS2-VASc score: low risk (R2CHA2DS2-VASc score: 0–1), intermediate risk (score: 2–4) and high risk (score: 5–11). The MACE rates were 0%, 5.9% and 23.6%, respectively.
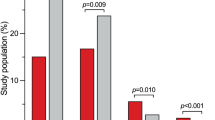
Kaplan–Meier survival curves showed a significant decrease in cumulative rates of freedom from MACE (log-rank p < 0.001) as the R2CHA2DS2-VASc score increased according to the three risk categories (Fig. 2). In the three groups, we further analyzed the occurrence of MACE in patients with AHRE ≥ or < 6 min (Fig. 3a,b). The MACE rates in patients with AHRE < 6 min were as follows: low risk (0%), intermediate risk (2.2%) and high risk (17.8%) (p < 0.001). The MACE rates in patients with AHRE ≥ 6 min were higher: low risk (0%), intermediate (15.7%), and high risk (36.2%) (p = 0.013). There were significant differences in MACE occurrence among the three groups regardless of AHRE ≥ 6 or < 6 min. There were also significant differences between the event rates of the intermediate-risk group with AHRE ≥ 6 min (15.7%) and the intermediate-risk group with AHRE < 6 min (2.2%), and between the high-risk group with AHRE ≥ 6 min (36.2%) and the high-risk group with AHRE < 6 min (17.8%), respectively. Kaplan–Meier survival curves showed a significant decrease in cumulative rates of freedom from MACE (log-rank p < 0.05) in high-risk group and intermediate-risk group with AHRE ≥ 6 min (Fig. 3c).
The major cardiovascular event rate significantly increased with increasing R2CHA2DS2-VASc scores, whether in patients with (A) AHRE ≥ or (B) < 6 min. (C) Kaplan–Meier curves depicting the cumulative survival rates free from major cardiovascular events with respect to the R2CHA2DS2-VASc score and AHRE ≥ or < 6 min, log-rank p < 0.05.
ROC-AUC determination of combined AHRE and the four scores for predicting future MACE
The patients with intermediate and high risk according to R2CHA2DS2-VASc score had higher risk for MACE rates in the presence of AHRE ≥ 6 min (15.7% vs. 2.2% and 36.2% vs. 17.8%, respectively), indicating that combination of the score and AHRE may enhance the discrimination ability. We performed further ROC analysis of combined AHRE and the four scores for predicting MACE. The results showed significantly higher AUC values as compared to each score alone (Table 3). As listed in Fig. 4, the combined AHRE ≥ 6 min and R2CHA2DS2-VASc score demonstrated higher AUC value (0.804, 95%CI: 0.761–0.843) than R2CHA2DS2-VASc score alone (0.770, 95%CI: 0.724–0.812) (Z-statistic = 3.265, p = 0.0011) and AHRE (Z-statistic = 3.074, p = 0.0021), suggestive of better discriminating ability to predict MACE. The risk stratification models, R2CHA2DS2-VASc score plus AHRE ≥ 6 min and CHA2DS2-VASc score plus AHRE ≥ 6 min had numerically higher AUC values of 0.804 and 0.810, respectively, indicating excellent discrimination. The corresponding comparisons of AUC values of the four combined risk stratification models (CHADS2 plus AHRE ≥ 6 min, R2CHADS2 plus AHRE ≥ 6 min, CHA2DS2-VASc plus AHRE ≥ 6 min, and R2CHA2DS2-VASc plus AHRE ≥ 6 min) with each other by using DeLong test showed no statistically significant difference between the models (All p > 0.05). The event rates of the combination of AHRE for each score were listed in Table 4.
Receiver operating characteristic analysis of the R2CHA2DS2-VASc score in combination with AHRE ≥ 6 min for predicting major cardiovascular adverse events. The AUC values of R2CHA2DS2-VASc score in combination with AHRE ≥ 6 min, R2CHA2DS2-VASc score and AHRE are 0.804 (95% CI = 0.761–0.843, p < 0.001), 0.770 (95% CI = 0.724–0.812, p < 0.001) and 0.641 (95% CI = 0.590–0.689, p < 0.001). The comparisons of each other are R2CHA2DS2-VASc score in combination with AHRE ≥ 6 min versus R2CHA2DS2-VASc score (Z-statistic: 3.265, p = 0.0011), R2CHA2DS2-VASc score in combination with AHRE ≥ 6 min versus AHRE (Z-statistic: 3.074, p = 0.0021), R2CHA2DS2-VASc score versus AHRE (Z-statistic: 2.126, p = 0.0335).
Discussion
The main findings of our study
The R2CHA2DS2-VASc score, and AHRE ≥ 6 min are significantly and independently associated with MACE in patients who with dual-chamber PPMs and no prior AF. The optimal cutoff value for the R2CHA2DS2-VASc score was ≥ 5 for predicting MACE. The other three risk score had acceptable discriminating ability to predict MACE and R2CHA2DS2-VASc score had numerically higher AUC values than others without statistically significant differences. Combination of AHRE ≥ 6 min and R2CHA2DS2-VASc score had an excellent and a better discriminatory power than R2CHA2DS2-VASc score alone or AHRE to predict MACE occurrence. The patients with R2CHA2DS2-VASc scores ≥ 5 and detected AHRE ≥ 6 min had the highest MACE rate (36.2%) at a median follow-up of 32 months. The other three risk scores in combination of AHRE ≥ 6 min also had significantly better discrimination ability to predict MACE. The AUC values were higher in R2CHA2DS2-VASc score and CHA2DS2-VASc score plus AHRE ≥ 6 min numerically. The results illustrated that when AHRE ≥ 6 min is added to the above four scores, the predictive ability would be enhanced significantly. These findings suggest that further risk assessment with these scores, can further identify the highest risk for MACE in patients with AHRE ≥ 6 min, which may allow early management and prevent MACE.
The performance of R2CHA2DS2-VASc score as compared to previous studies
The recent studies have shown that R2CHA2DS2-VASc score have better predictive value for long-term outcomes in different groups of patients, including those with high cardiovascular risk, chest pain, and ACS12,13,14,17. In those studies, the reported AUC values of ROC analysis were all above 0.7 for the R2CHA2DS2-VASc score, indicating acceptable discrimination power. The optimal cutoff values for predicting clinical outcomes in those studies were set at ≥ 4 for the R2CHA2DS2-VASc score, and each 1-point increase in the R2CHA2DS2-VASc score was associated with a 31–53% increase in the risk of clinical outcomes13,14,17. In our study, the cutoff value was different and set ≥ 5 and each 1-point increase in the R2CHA2DS2-VASc score was associated with a 48% increase in the risk of MACE in patients with dual-chamber PPM, which was comparable to the previous studies in different sets of patients. The area under ROC curve of R2CHA2DS2-VASc score to predict MACE in our study is numerically higher than the reported values12,13,14,17.
The relationship between AHRE and thrombo-embolism events and possible mechanisms
The rate of AHRE in our study was 28.5%, consistent with previous reported rates (10–30%). In spite of the fact that patients with detected AHRE are at significantly increased risk for stroke, the temporal relationship between device detected AHRE and stroke has not been well established25,26,27. It is possible that AHRE are simply a marker of a population at risk for cardio-embolic events28. Miyazawa et al. also demonstrated that longer AHREs more frequently occurred in patients at higher risk of thromboembolism (CHADS2 score ≥ 3)29. Pastori et al.30 reported that inflammatory markers (high CRP and leukocyte count), are factors in association with AHRE. The mechanism of stroke in patients with implantable devices and detected AHRE may be related to the presence of atherosclerotic risk factors, arterial plaque rupture, inflammation, other than cardio-embolism27,28. 5. The proposed mechanisms of MACE in patients with AF were systemic inflammation with prothrombotic state; concomitant presence of classic atherosclerotic risk factors including hypertension, diabetes and dyslipidemia associated with platelet activation; and episodes of high ventricular rates leading to supply and demand mismatch and subsequent type 2 MI5,30. The increase in cardiovascular risk in patients with AHRE is in line with patients with AF, strengthening the hypothesis that AHRE, also called subclinical AF, and clinical AF are a clinical continuum7,8. Thus, the mechanisms of MACE in patients with AF possibly play an important role in patients with AHRE. Previous study7 demonstrated that patients with PPM and multiple atherosclerotic risk factors (CHA2DS2-VASc score > 2), device-detected AHRE can further predict the cardiovascular death and all-cause mortality. Of note, we found that combining R2CHA2DS2-VASc score, consisting of more atherosclerotic risk factors, with AHRE ≥ 6 min as a risk assessment model can further stratify MACE risk (Figs. 3 and 4). The ROC-AUC analysis confirmed the significantly a better predictive value. To the best of our knowledge, the current study is the first to demonstrate that the R2CHA2DS2-VASc score and other three scores combined with AHRE ≥ 6 min have discriminatory power to predict MACE occurrence and the AUC values are statistically higher than the original four risk scores, respectively, in patients with dual-chamber PPM and no prior AF. Kaplan et al.31 had shown that there was an interaction between device-detected AF burden and CHA2DS2-VASc score. The stroke and systemic embolic rates increase across 1%/y in patients with CHA2DS2-VASc score of 3–4 with > 6 min of AF burden and those with CHA2DS2-VASc score of 2 with > 23.5 h of AF burden. The results of the study31 and ours emphasized the prognostic importance (MACE and stroke) of clinically relevant AHRE in these special population with continuous arrhythmia burden monitoring.
Current evidence and ongoing trials
Previous study demonstrated the CHADS2 and CHA2DS2-VASc risk scores with integration of AF presence/duration/burden have the potential to improve stroke risk stratification in patients with PPM32. Considering stroke prevention, the 2017 European Heart Rhythm Association (EHRA) consensus recommends the consideration of oral anticoagulation (OAC) use for patients with subclinical AF burden > 5.5 h and CHA2DS2-VASc score ≥ 2. While in 2020 ESC guideline, consideration of OAC use is recommended in patients with subclinical AF burden > 24 h and CHA2DS2-VASc score ≥ 2 in male and ≥ 3 in female1. On the other hand, MACE prevention in patients with device detected AHRE still lacks evidence. A observational cohort study from Danish health care registries reported that direct oral anticoagulation (DOAC) were all associated with a significant risk reduction of MI compared with vitamin K antagonist in patients with non-valvular AF33. Two ongoing trial (ARTESiA and NOAH-AFNET 6) will deal with the unmet needs concerning the benefit of apixaban and edoxaban, respectively, for stroke, systemic embolism, or cardiovascular death, as compared with aspirin in patients with AHRE ≥ 6 min34,35. The ARTESiA trial will enroll 4000 high-risk (CHA2DS2-VASc score ≥ 3) patients with CIEDs and at least one AHRE ≥ 6 min. The NOAH-AFNET 6 will recruit 3400 patients aged > 65 years, with one additional CHA2DS2-VASc factor and CIED-detected AHRE ≥ 6 min. These two trials have the potential to inform future guideline on the management of patients with device detected AHRE to prevent thromboembolism. It is noteworthy that the occurrence of myocardial infarction, acute coronary syndrome or cardiovascular death is secondary outcome rather than primary outcome in the two trials. Therefore, if the trials have positive results for thromboprophylaxis in these patients, further large studies are still needed to investigate whether patients with AHRE have net benefit from use of DOAC to prevent MACE occurrence.
Limitations
This study has some limitations. First, this was a single-center, retrospective, observational study that enrolled a relatively small number of patients with dual-chamber PPM and no prior AF in a hospital-based setting. Probably due to small number of patients, there was just numerically but not statistically significantly better discriminatory power of R2CHA2DS2-VASc score than other scores to predict MACE. Given the observational design, the cause-effect relationship among the R2CHA2DS2-VASc score, other risk scores, AHRE and MACE could not be determined. Additionally, confounding factors can’t be ruled out. Second, all patients were Taiwanese people. Therefore, the results may not be applicable to other populations. Further prospective and multicenter studies are needed to validate the results of our study. Third, 41.5% of the patients used Biotronik pacemakers, which only recorded an AHRE when heart rate > 200 bpm. This may underestimate the number of AHREs, as patients with a heart rate of 175–200 bpm wouldn’t be recorded as possible AHRE by the devices. Fourth, the severity of common cardiovascular comorbidity factors (e.g., HbA1c level in diabetic patients, blood pressure in hypertensive patients and lipid profile in dyslipidemia patients) would have an impact on all-cause mortality and were not reported in our study.
Conclusions
In patients with dual-chamber PPM and no prior AF, device-detected AHRE are associated with higher risk for MACE. R2CHA2DS2-VASc score with the cut-off value of 5 can be used to predict MACE in patients with dual chamber PPM and no prior AF. Combination of AHRE and R2CHA2DS2-VASc score to predict MACE has an acceptable discriminatory power, which is comparable to other three risk scores. When adding AHRE ≥ 6 min to the four risk scores, all demonstrate significantly better and comparable discriminatory power to predict MACE. Our study suggests the significant prognostic importance of risk assessment, e.g., R2CHA2DS2-VASc score, in PPM patients with detected AHRE ≥ 6 min. In dual chamber PPM patients with high risk in R2CHA2DS2-VASc score, and with device-detected AHRE ≥ 6 min plus intermediate risk in R2CHA2DS2-VASc score, early management may be warranted to prevent MACE occurrence. Optimization of thrombo-embolism prevention strategy with DOACs in patients AHRE is under investigation. Large cohort studies are in need to address whether DOACs can reduce cardiovascular mortality in patients with AHRE in the future.
Data availability
The datasets generated and analyzed during the current study are not publicly available due to privacy restriction but can be made available from the corresponding author on reasonable request.
Abbreviations
- AF:
-
Atrial fibrillation
- AHRE:
-
Atrial high-rate episodes
- AMI:
-
Acute myocardial infarction
- CIEDs:
-
Cardiac implantable electronic devices; disease
- eGFR:
-
Estimated glomerular filtration rate
- TIA:
-
Transient ischemic attacks
References
Hindricks, G. et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 42, 373–498. https://doi.org/10.1093/eurheartj/ehaa612 (2021).
Bertaglia, E. et al. Atrial high-rate episodes: prevalence, stroke risk, implications for management, and clinical gaps in evidence. Eur. Eur. Pacing Arrhythm. Card. Electrophysiol. J. Work. Groups Card. Pacing Arrhythm. Card. Cell. Electrophysiol. Eur. Soc. Cardiol. 21, 1459–1467. https://doi.org/10.1093/europace/euz172 (2019).
Healey, J. S. et al. Subclinical atrial fibrillation and the risk of stroke. N. Engl. J. Med. 366, 120–129. https://doi.org/10.1056/NEJMoa1105575 (2012).
Mahajan, R. et al. Subclinical device-detected atrial fibrillation and stroke risk: A systematic review and meta-analysis. Eur. Heart J. 39, 1407–1415. https://doi.org/10.1093/eurheartj/ehx731 (2018).
Violi, F., Soliman, E. Z., Pignatelli, P. & Pastori, D. Atrial fibrillation and myocardial infarction: A systematic review and appraisal of pathophysiologic mechanisms. J. Am. Heart Assoc. https://doi.org/10.1161/jaha.116.003347 (2016).
Soliman, E. Z. et al. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern. Med. 174, 107–114. https://doi.org/10.1001/jamainternmed.2013.11912 (2014).
Gonzalez, M. et al. Newly detected atrial high rate episodes predict long-term mortality outcomes in patients with permanent pacemakers. Heart Rhythm 11, 2214–2221. https://doi.org/10.1016/j.hrthm.2014.08.019 (2014).
Pastori, D. et al. Atrial high-rate episodes and risk of major adverse cardiovascular events in patients with cardiac implantable electronic devices. Clin. Res. Cardiol. Off. J. German Card. Soc. 109, 96–102. https://doi.org/10.1007/s00392-019-01493-z (2020).
Uittenbogaart, S. B., Lucassen, W. A. M., van Etten-Jamaludin, F. S., de Groot, J. R. & van Weert, H. C. P. M. Burden of atrial high-rate episodes and risk of stroke: A systematic review. EP Eur. 20, 1420–1427. https://doi.org/10.1093/europace/eux356 (2017).
Nishinarita, R. et al. Burden of implanted-device-detected atrial high-rate episode is associated with future heart failure events—clinical significance of asymptomatic atrial fibrillation in patients with implantable cardiac electronic devices. Circ. J. 83, 736–742. https://doi.org/10.1253/circj.CJ-18-1130 (2019).
Piccini, J. P. et al. Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: validation of the R(2)CHADS(2) index in the ROCKET AF (Rivaroxaban Once-daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) and ATRIA (AnTicoagulation and Risk factors In Atrial fibrillation) study cohorts. Circulation 127, 224–232. https://doi.org/10.1161/circulationaha.112.107128 (2013).
Kiliszek, M. et al. CHA2DS2-VASc and R2CHA2DS2-VASc scores have predictive value in patients with acute coronary syndromes. Pol. Arch. Med. Wewn 125, 545–552. https://doi.org/10.20452/pamw.2965 (2015).
D’Errico, M. M. et al. CHA2DS2-VASc and R2CHA2DS2-VASc scores predict mortality in high cardiovascular risk population. Eur. J. Clin. Invest. 52, e13830. https://doi.org/10.1111/eci.13830 (2022).
Topaz, G. et al. Prediction of acute-coronary-syndrome using newly-defined R(2)-CHA(2)DS(2)-VASc score among patients with chest pain. J. Cardiol. 77, 370–374. https://doi.org/10.1016/j.jjcc.2020.08.013 (2021).
Li, Y., Wang, J., Lv, L., Xu, C. & Liu, H. Usefulness of the CHADS(2) and R(2)CHADS(2) scores for prognostic stratification in patients with coronary artery disease. Clin. Interv. Aging 13, 565–571. https://doi.org/10.2147/cia.S156208 (2018).
Poçi, D. et al. Role of the CHADS2 score in acute coronary syndromes: Risk of subsequent death or stroke in patients with and without atrial fibrillation. Chest 141, 1431–1440. https://doi.org/10.1378/chest.11-0435 (2012).
Węgiel, M. et al. CHA(2)DS(2)-VASc and R(2)-CHA(2)DS(2)-VASc scores predict in-hospital and post-discharge outcome in patients with myocardial infarction. Postepy w Kardiologii Interwencyjnej Adv Interv. Cardiol. 14, 391–398. https://doi.org/10.5114/aic.2018.79869 (2018).
Borovac, J. A. et al. The predictive value of CHA(2)DS(2)-VASc score on in-hospital death and adverse periprocedural events among patients with the acute coronary syndrome and atrial fibrillation who undergo percutaneous coronary intervention: A 10-year national inpatient sample (NIS) analysis. Cardiov. Revasc. Med. Incl. Mol. Interv. 29, 61–68. https://doi.org/10.1016/j.carrev.2020.08.003 (2021).
Chua, S. K., Lo, H. M., Chiu, C. Z. & Shyu, K. G. Use of CHADS2 and CHA2DS2-VASc scores to predict subsequent myocardial infarction, stroke, and death in patients with acute coronary syndrome: Data from Taiwan acute coronary syndrome full spectrum registry. PLoS ONE 9, e111167. https://doi.org/10.1371/journal.pone.0111167 (2014).
Paoletti Perini, A. et al. CHADS2 and CHA2DS2-VASc scores to predict morbidity and mortality in heart failure patients candidates to cardiac resynchronization therapy. Eur. Eur. Pacing Arrhythm. Card. Electrophysiol. J. Work. Groups Card. Pacing Arrhythm. Card. Cell. Electrophysiol. Eur. Soc. Cardiol. 16, 71–80. https://doi.org/10.1093/europace/eut190 (2014).
Svendsen, J. H. et al. CHADS2 and CHA2DS2-VASc score to assess risk of stroke and death in patients paced for sick sinus syndrome. Heart 99, 843–848. https://doi.org/10.1136/heartjnl-2013-303695 (2013).
Kalyoncuoglu, M. & Ozturk, S. Is the newly defined R2CHA2DS2-vasc score a predictor for late mortality in patients undergoing transcatheter aortic valve replacement?. Braz. J. Cardiovasc. Surg. 35, 145–154. https://doi.org/10.21470/1678-9741-2019-0221 (2020).
Chen, J. Y., Chen, T. W. & Lu, W. D. HAT(2)CH(2) score predicts systemic thromboembolic events in elderly after cardiac electronic device implantation. Front. Med. 8, 786779. https://doi.org/10.3389/fmed.2021.786779 (2021).
DeLong, E. R., DeLong, D. M. & Clarke-Pearson, D. L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 44, 837–845 (1988).
Gorenek, B. C. et al. Device-detected subclinical atrial tachyarrhythmias: definition, implications and management-an European Heart Rhythm Association (EHRA) consensus document, endorsed by Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS) and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLEACE). Eur. Eur. Pacing Arrhythm. Card. Electrophysiol. J. Work. Groups Card. Pacing Arrhythm. Card. Cell. Electrophysiol. Eur. Soc. Cardiol. 19, 1556–1578. https://doi.org/10.1093/europace/eux163 (2017).
Brambatti, M. et al. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation 129, 2094–2099. https://doi.org/10.1161/circulationaha.113.007825 (2014).
Daoud, E. G. et al. Temporal relationship of atrial tachyarrhythmias, cerebrovascular events, and systemic emboli based on stored device data: A subgroup analysis of TRENDS. Heart Rhythm 8, 1416–1423. https://doi.org/10.1016/j.hrthm.2011.04.022 (2011).
Camm, A. J. et al. Atrial high-rate episodes and stroke prevention. Eur. Eur. Pacing Arrhythm. Card. Electrophysiol. J. Work. Groups Card. Pacing Arrhythm. Card. Cell. Electrophysiol. Eur. Soc. Cardiol. 19, 169–179. https://doi.org/10.1093/europace/euw279 (2017).
Miyazawa, K. et al. Characteristics of patients with atrial high rate episodes detected by implanted defibrillator and resynchronization devices. Eur. Eur. Pacing Arrhythm. Card. Electrophysiol. J. Work. Groups Card. Pacing Arrhythm. Card. Cell. Electrophysiol. Eur. Soc. Cardiol. 24, 375–383. https://doi.org/10.1093/europace/euab186 (2022).
Pastori, D. et al. Inflammation and the risk of atrial high-rate episodes (AHREs) in patients with cardiac implantable electronic devices. Clin. Res. Cardiol. Off. J. German Card. Soc. 107, 772–777. https://doi.org/10.1007/s00392-018-1244-0 (2018).
Kaplan, R. M. et al. Stroke risk as a function of atrial fibrillation duration and CHA(2)DS(2)-VASc score. Circulation 140, 1639–1646. https://doi.org/10.1161/circulationaha.119.041303 (2019).
Boriani, G. et al. Improving stroke risk stratification using the CHADS2 and CHA2DS2-VASc risk scores in patients with paroxysmal atrial fibrillation by continuous arrhythmia burden monitoring. Stroke 42, 1768–1770. https://doi.org/10.1161/strokeaha.110.609297 (2011).
Lee, C.J.-Y. et al. Risk of myocardial infarction in anticoagulated patients with atrial fibrillation. J. Am. Coll. Cardiol. 72, 17–26. https://doi.org/10.1016/j.jacc.2018.04.036 (2018).
Kirchhof, P. et al. Probing oral anticoagulation in patients with atrial high rate episodes: Rationale and design of the Non-vitamin K antagonist Oral anticoagulants in patients with Atrial High rate episodes (NOAH-AFNET 6) trial. Am. Heart J. 190, 12–18. https://doi.org/10.1016/j.ahj.2017.04.015 (2017).
Lopes, R. D. et al. Rationale and design of the apixaban for the reduction of thrombo-embolism in patients with device-detected sub-clinical atrial fibrillation (ARTESiA) trial. Am. Heart J. 189, 137–145. https://doi.org/10.1016/j.ahj.2017.04.008 (2017).
Acknowledgements
The authors would like to thank AJE for assistance with English editing of the manuscript.
Funding
The authors would like to thank the Ministry of Science and Technology of the Republic of China, Taiwan, for financially supporting this research under contract MOST 110–2218-E-006–017.
Author information
Authors and Affiliations
Contributions
Conception and design: J.-Y.C.; data acquisition: T.-W.C., W.-D.L.; data analysis and interpretation: J.-Y.C.; statistical analysis: J.-Y.C.; drafting and finalizing the manuscript: Y.-P.L., J.-Y.C.; critical revision of the manuscript for important intellectual content: J.-Y.C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, YP., Chen, JY., Chen, TW. et al. Atrial high-rate episodes intensify R2CHA2DS2-VASc score for prognostic stratification in pacemaker patients. Sci Rep 13, 7640 (2023). https://doi.org/10.1038/s41598-023-34784-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-34784-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.