Abstract
Low back pain (LBP) has been associated with altered body sway during quiet standing, but the pattern of results is inconsistent. The purpose of this meta-analysis is to examine the effects of vision (eyes open, eyes closed) and changing the support surface (foam surface, firm surface) on postural sway during quiet standing in individuals with chronic LBP (cLBP). Five electronic databases were searched on March 27th, 2022. Of 2,856, 16 studies (n = 663) were included. Across all conditions, we found a positive and medium effect size (g = 0.77 [0.50, 1.04]) that represented greater body sway in individuals with cLBP. Subgroup analyses revealed medium effects during eyes open conditions (firm surface: g = 0.60 [0.33, 0.87]; foam surface: g = 0.68 [0.38, 0.97]), and large effects during eyes closed conditions (firm surface: g = 0.97 [0.60, 1.35]; foam surface: g = 0.89 [0.28, 1.51]). We quantified effects of self-reported pain and found a moderate effect during eyes closed plus firm surface conditions (Q = 3.28; p = 0.070). We conclude that cLBP is associated with increased postural sway, with largest effect sizes evident when vision is removed and when self-reported pain intensity is higher.
Similar content being viewed by others
Introduction
Low back pain (LBP) is a major public health concern, affecting up to 85% of adults at some point throughout their lifetime1. Although many individuals recover from an acute episode of low back pain, a subset of individuals go on to develop chronic low back pain (cLBP), which is pain lasting for 12 or more weeks2. cLBP leads to increases in disability in normal activity and limitation in the workplace3. The cost of cLBP is estimated to range from $259 million to $71.6 billion per year based on healthcare costs and missed work days4. A major concern for individuals with cLBP is instability in body movements during quiet standing5,6,7 which directly relates to the risk of falling8,9.
Stable balance utilizes sensory input from visual, vestibular, and proprioceptive systems10. During quiet standing, pain-free individuals rely on proprioceptive information not only from the lumbar muscles but also from other postural muscles including those that control the ankle. Individuals with low back pain will often restrict trunk movement in an effort to reduce low back pain7. Over time, this change in postural control reduces reliance on lumbar proprioception5,7, leading to an increase in motion perception threshold11. In the long term, this alteration leads to greater dependence on other sensory information to compensate for the loss of input from lumbar proprioception12. A consequence of this change in the weighting of sensory input becomes most apparent when individuals must control movements in an unpredictable environment. A recent systematic review by Koch and Hänsel5 reported that cLBP is associated with more restricted trunk movement when sensory input is manipulated by altering the stability of the surface or the presence of visual feedback.
One of the most common analytical methods to evaluate postural control is to calculate center of pressure (CoP) excursions during quiet standing. CoP is measured using a force plate and represents the change in the center of mass in the sagittal and frontal planes. Both the velocity of the change and the magnitude of the change can be calculated. There is a growing body of evidence linking cLBP with greater and faster CoP movements during quiet standing on stable surfaces with vision and with no-vision6,13, as well as when standing on an unstable surface with vision14,15 and without vision13. This is consistent with several systematic reviews5,16,17,18, which suggest that LBP leads to a shift in postural control from the lumbar spine to the ankles, and controlling posture at the ankles increases magnitude of sway19. However, other studies have reported smaller and slower CoP movements during quiet standing with vision20,21 and no-vision20. Several studies have also reported no difference in CoP between individuals with and without cLBP22,23,24. Differences in task conditions, pain levels, and CoP calculations may account for ambiguity across studies. A meta-analytic approach offers one solution to resolve findings across studies.
The purpose of the current study is to combine and statistically compare results from studies on cLBP and postural control. First, we compared overall effect sizes of velocity and magnitude of CoP sway between individuals with and without cLBP. Second level meta-analyses were conducted by statistically comparing groups within four conditions that varied as a function of proprioceptive input (Firm vs. Foam surfaces) and visual input (eyes open vs. closed). Finally, we analyzed the effects of movement direction and self-reported pain intensity as moderator variables. We hypothesized that people with cLBP would have greater and faster CoP movement and that effect sizes would be larger when proprioceptive and visual input is reduced.
Methods
This meta-analysis was conducted by following the guidelines of the PRISMA checklist25 (see Table S1 in Supplementary materials 1).
Search strategy
The following five databases were used, PubMed, MEDLINE, CINAHL, SPORTDiscus, and APA PsycArticles, to search for articles published from 1979 to 27th March 2022, using the keywords with parentheses, Boolean operators, and field codes. We used the keywords: (low back pain) and (postural* or stability or balance or postural sway or postural balance or postural stability or CoP or center of pressure) and not (meta* or systematic review or review). The full keywords can be found in Supplementary materials 1. Articles not written in the English language were excluded using the filtering function in each database. To find published articles, JP, who is the first author on the paper, conducted the search twice: the first search was conducted on 1st November 2021 and the second on 27th March 2022.
Study selection
The procedure to select studies was conducted using the following steps. First, duplicate studies were removed using EndNote X9 (Clarivate)26. According to the inclusion and exclusion criteria, a researcher (JP) screened titles and abstracts of all studies, and then two researchers (JP and VN) independently rescreened the studies through the full-text versions. In case of disagreement, the last researcher (SAC) was consulted for the final decision. The final studies were selected in our meta-analysis. We included studies that: (1) recruited cLBP subjects with pain duration of greater than or equal to 3 months, (2) recruited adult population ranged from 18 to 65 years, (3) compared a cLBP group and healthy control group (HC), (4) used cross-sectional design, and (5) used CoP parameters during bipedal quiet standing. Exclusion criteria were studies that: (1) recruited athletes with cLBP or cLBP subjects who have had surgery for lumbar disc herniation or other diseases, (2) recruited recurrent or episodic LBP subjects who reported semicontinuous LBP or at least one episode of LBP during the previous 3 months, (3) did not include a control group, (4) did not measure CoP variables (e.g., the center of mass, the center of gravity, and the joint angles) to compare postural control between groups, (5) did not investigate postural control during a bipedal quiet standing task (i.e., perturbation tasks, balance-dexterity tasks, anticipation postural control task, one leg standing task, and dual tasks are exclusion criteria), and (6) did not report statistical results for calculating the effect size.
Data extraction
To calculate the pooled effect size, all the CoP outcomes were extracted from included studies. CoP data was classified according to body sway and body sway velocity to reduce the heterogeneity. Body sway included the sway amplitude, displacement, area, and dispersion, while body sway velocity variables represent the velocity of the CoP18. Both parameters were collected as means with their standard deviation and sample size. If a study reported a median and interquartile range, those values were converted into a mean and standard deviation based on the Quantile estimation method27 to calculate an effect size. In the case that a study did not report group level descriptive data, effect sizes were calculated using a combination of sample size, mean differences, p-values, and t-values. If a study reported a standard error, the standard error was converted into a standard deviation based on the corresponding sample size. Furthermore, we extracted demographic information (i.e., sex, age, height, and weight) and pain related characteristics when available (i.e., pain intensity, pain duration, and disability). Two authors, JP and VN, extracted and checked data.
Effect size
Hedges’ g was used to calculate effect sizes of each studies’ outcome. The rationale for using Hedges’ g is that it is less affected by the impact of sample size compared to Cohens’ d28. The Hedges’ g is interpreted based on 3 stages: (1) values from 0.2 to 0.49 represent a small effect size; (2) values from 0.5 to 0.79 represent a medium effect size; (3) values greater than or equal to 0.8 represent a large effect size.
Meta-analyses
We first calculated overall effect sizes for group differences in body sway and body sway velocity during quiet standing across all experimental conditions (i.e., vision and proprioception). Next, we calculated effect sizes for group differences in body sway and body sway velocity across different experimental conditions: (1) on a firm surface with eyes open (Firm with EO), (2) a firm surface with eyes closed (Firm with EC), (3) on a foam surface with eyes open (Foam with EO) and (4) on a foam surface with eyes closed (Foam with EC). In each experimental condition we also assessed moderator variables based on direction of body sway and body sway velocity (anterior–posterior [AP], the medial–lateral [ML], and both [AP-ML] directions) and pain (low and high). For the levels of pain intensity, the results from a visual analogue scale (VAS) or numerical rating scale (NRS) were extracted. In the case where an included study reported pain scores based on the range from 0 to 100, their scores were converted to a value from 0 to 10. Pain intensity was then classified into two levels using the median value across all studies (4.73): a higher level of LBP is equal to and more than the median, which we labeled ‘High’, whereas a lower level of LBP is less than the median and is labeled as ‘Low’.
Quality assessment
The quality assessment tool (QAT) for observational cohort and cross-sectional studies29 was used to evaluate the quality of each study included in the meta-analysis. The QAT contains fourteen questions to access internal validity. The details on the questions are described in the supplementary material (Table S3). All the questions were rated as being positive (“Yes”), negative (“No”), cannot determine (“CD”), not applicable (“AP”), or not reported (“NR”). Two authors, named as JP and VN, rated the quality of studies. The disagreements were discussed until consensus was reached. In cases where JP and VN could not reach an agreement, they discussed it with SAC. The quality of every study was evaluated by “Good”, “Fair”, and “Poor.”
Data analysis
The Comprehensive Meta-Analysis software package version 3 (CMA; BioStat, Englewood, New Jersey) was used to calculate effect sizes and 95% confidence intervals using a random effect model for each analysis. If a study included in the meta-analysis had multiple CoP outcomes, we calculated multiple effect sizes corresponding to CoP outcomes and then averaged those effect sizes into a single effect size per study according to body sway or body sway velocity and standing conditions. For instance, Caffaro et al.13 reported 12 different CoP outcomes of body sway that were different according to experimental conditions and directions (See the Supplementary material 2). When the meta-analysis for body sway was conducted, the CMA program calculated 12 effect sizes from 12 CoP results and then averaged all effect sizes into a single effect, which was 0.77 (see the first row in Fig. 2a). An I2 statistic and prediction interval were used to assess the heterogeneity for each analysis. I2 values higher than 75% represent high heterogeneity, and the prediction interval (PI) represents the range of true effect sizes for 95% of all comparable studies30. To assess publication biases for each analysis, Egger’s regression test was first used31. The significant level for the bias was set to 0.05. If publication bias was found through Egger’s regression test, Duval and Tweedie’s Trim and Fill32 was conducted to reassess publication bias using a funnel plot which can test the symmetry of effect sizes of included studies. Asymmetric funnel plots suggest that publication bias may exist. Using the trim and fill approach, some data was imputed to make the figure look symmetric, and then the changed overall effect size including the imputed data was compared to the previous overall effect size without imputed data. Unless 95% confidence intervals of both overall effect sizes were overlapped, there is an effect of publication bias. For subgroup analyses, Q-statistics were used to assess the moderator effects of movement direction (AP, ML, and AP-ML) and pain intensity (Low and High). The significant level for Q-statistics was set to 0.10. Effect sizes for sublevels of variables (e.g., AP, ML, AP-ML, Low, and High) were statistically analyzed. Significant p-values were set at a level of 0.05, corrected using the false discovery rate (FDR). Meta- or subgroup analyses with only one study were excluded33.
GRADE assessment
Grading of recommendations, assessment, development, and evaluations (GRADE) was used to evaluate the evidence certainty based on five subcategories: ‘Risk of bias’, ‘Inconsistency’, ‘Indirectness’, ‘Imprecision’, and ‘Publication bias’. The ratings for those categories were ‘No serious’, ‘Serious’, and ‘Very serious’. The level of evidence certainty was downgraded if the meta-analysis (i) included 25% of studies with poor quality assessed by QAT (1 level of Risk of bias) or all studies with poor quality (2 level of Risk of bias), (ii) showed higher than 50% of I2 (1 level of Inconsistency)—I2 values have been used previously to evaluate inconsistency in CoP outcomes34,35—(iii) did not include direct evidence related to the main question (1 level of Indirectness), (vi) included less than the optimal sample size (n = 244) calculated based on 5% of margin of error, 95% confidence intervals, and total sample size (n = 663; 1 level of Imprecision), and (v) showed publication bias resulting from Egger’s regression and trim and fill test (1 level of Publication bias). Combining all the ratings, the certainty of the evidence in meta-analyses were interpreted based on four levels: ‘High’, ‘Moderate’, ‘Low’, and ‘Very low certainty’. The high certainty of evidence represents high confidence that the true effect is close to the effect of the estimate, while very low certainty represents very little confidence that the true effect is close to the effect of the estimate. Initial ratings began at a low level since all studies included in the meta-analyses were observational studies. However, the level can be graded up (i) if the pooled Hedges’ g was higher than or equal to 0.8, (ii) evidence of a dose–response relation was found, and (iii) if all the bias and/or confounds were plausible36,37.
Results
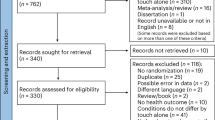
Fig. S1 in supplementary material 1 shows the PRISMA flow chart for the first search. Figure 1 shows the PRISMA flow chart for the second and final search. The search strategies initially yielded 2856 relevant articles, and one additional article38 extracted from a systematic review17. Among them, 842 duplicates were removed, leaving a total of 2014 articles. After investigating titles and abstracts, 1922 articles were excluded. From the remaining 92 articles, we excluded an additional 76 articles based on the criteria outlined in Fig. 1. Additional information on why studies were excluded from the meta-analyses is shown in Table S2. A total of 16 articles were included in this meta-analysis.
Demographic data
Table 1 represents demographic information for cLBP and HC groups for each article. The bottom three rows in the Total section of Table 1 indicate the summed and averaged demographic information on the overall population of cLBP, HC, and total individuals respectively. The first row in the Total section of Table 1 shows demographic information on cLBP group. Total sample size of the cLBP group was 345 (Female: 153; Male: 98; no information on sex: 94). The average age of cLBP individuals was 35.4 ± 8.2 years. The mean height was 169.3 ± 5.9 cm, and weight was 69.9 ± 8.5 kg. Intensity of low back pain was measured using VAS and NRS. Mean pain intensity was 4.8 ± 1.1 (2.5–6.7). The duration of cLBP ranged from 3 to 139 months. The second row in the Total section of Table 1 represents the demographic information on the HC group. The total healthy sample size was 318 (Female: 115; Male: 63; no information on sex: 140). The mean of age in the HC group was 32.7 ± 6.4 years. The height was 167.8 ± 4.4 cm, and weight was 65.7 ± 6.7 kg. The total pooled population (last row) was 663 (Females: 268; Males: 161; no mention of sex: 234). Average age was 34.1 ± 7.3 years (18–61 years). Average height was 168.6 ± 5.2 cm (160–174.9 cm) and average weight was 67.8 ± 7.8 kg (55.2–77.6 kg).
Study characteristics
Table 2 shows information extracted from the 16 included studies, including which questionnaires/scales were implemented, the methods used to collect CoP data, the types of quiet standing tasks, and whether significant differences were found between cLBP and HC groups. Across all studies, pain intensity was most often assessed using a VAS and disability was most often assessed using the Oswestry disability index (ODI). Three studies did not report pain intensity22,23,40. Eight studies reported disability for pain using ODI15,21,22,23,39,41,42,43, and four studies used the Roland Morris disability questionnaire (RM)13,14,20,24. Four studies did not assess disability6,38,40,44. Anxiety, cognitive function, and health condition questionnaires are also included in Table 2 if included in the corresponding study. For the measurement of CoP variables, the sampling rate ranged from 40 to 1500 Hz. Eight studies used 100 Hz13,14,20,21,22,24,38,42, and two studies did not report the sampling rates23,44. A Butterworth filter was used for all studies with cut-off frequencies ranging from 2.5 to 35 Hz and eight studies not reporting cut-off frequencies used14,15,23,38,39,40,41,44. The duration of each trial between studies varied from 10 to 120 s. Six articles used 30 s6,14,20,38,39,41, and one study did not report trial duration44. Most studies (5 studies) used three trials of quiet standing13,20,22,23,39, and three articles did not report the number of trials6,15,42. A total of seven studies gave subjects instructions for quiet standing tasks15,21,22,23,41,42,43. Among them, five studies instructed subjects to stand as still as possible15,21,23,41,43. Two studies instructed subjects to relax22 and to be comfortable42 during quiet standing. The remaining nine studies did not report their instruction for subjects6,13,14,20,24,38,39,40,44. For task conditions, CoP movement was measured on a firm surface with eyes open in 16 studies6,13,14,15,20,21,22,23,24,38,39,40,41,42,43,44 and on a firm surface with eyes closed in 13 studies6,13,14,15,20,22,23,38,39,40,41,42,44. On a foam surface, four studies assessed CoP movement with eyes open13,14,15,44 and four studies measured CoP with eyes closed13,14,15,44. The last column of Table 2 shows the significant differences between cLBP and HC groups. Twelve studies found CoP movement was greater or faster in cLBP group as compared to HC group6,13,14,15,20,21,22,38,39,40,41,44, while one study found that CoP movement was smaller or slower in cLBP group than HC group21. Four studies did not find significant between group differences23,24,42,43.
Data handling
From 16 studies, we extracted 111 combinations of means, standard deviation, standard errors, sample sizes, p-values, and/or t-values. A total of 58 effect sizes were calculated from 11 studies using a combination of means, standard deviations, and sample sizes14,15,20,22,24,38,39,40,41,42,44. A total of 20 effect sizes were calculated from two studies by converting standard errors to standard deviations. A total of five effect sizes were calculated from one study that reported medians and interquartile ranges43. In this case, we converted those values to means and standard deviations based on the Quantile estimation method27. Four studies did not report means, standard deviations, or both6,21,38,44. In this case, we extracted sample size and other statistical values—independent t-values21 and p-values6,38,44—and calculated 28 effect sizes using combinations of these values. Among 111, 81 effect sizes calculated from 15 studies6,13,14,15,20,21,22,23,24,38,39,40,41,43,44 were body sway outcomes, and 30 effect sizes calculated from nine studies6,13,20,21,23,24,38,42,43 were body sway velocity outcomes. Additional data extracted from each study is shown in Supplementary material 2.
Pooled effect sizes of CoP movement for body sway and body sway velocity
Figure 2a shows body sway effect sizes between cLBP and HC groups. A total of 15 effect sizes were calculated from 15 studies. Within each study, effect sizes were averaged across all task conditions. Black squares represent effect sizes for each study and the whiskers represent the confidence interval. These values are also shown on the right of the Fig. 2a for each study. For example, the Caffaro et al.13 study had an effect size of 0.77, with a confidence interval of 0.47 – 1.07. The pooled effect size is shown at the bottom of Fig. 2a, and was large, positive, and significant, revealing a greater amplitude of CoP body sway in the cLBP group as compared to the HC group (p < 0.001, g = 0.77 [0.50, 1.04]). The I2 value was 83.60% and PI was −0.29 to 1.82 (Table S4). Figure 2b shows body sway velocity effect sizes. The pooled effect size of 0.21 (−0.03, 0.45), was not significant, and heterogeneity was 46.95% (I2) and PI was − 0.43 to 0.85 (Table S4).
Forest plots for body sway and body sway velocity. Note. This forest plot represents meta-analysis results for body sway (a) and body sway velocity (b). The leftmost column lists the name of studies included in the meta-analysis, and the rightmost column lists effect sizes and 95% confidence intervals for the studies. The effect sizes were calculated into Hedges’ g which is interpreted based on 3 stages: (1) values from 0.2 to 0.49 represent a small effect size; (2) values from 0.5 to 0.79 represent a medium effect size; (3) values greater than or equal to 0.8 represent a large effect size. The black squares represent the locations of the effect sizes for each study, and the whiskers represent the confidence intervals for each study. If a confidence interval is not overlapped with a zero value which is indicated as a dot line, there was a significant effect on body sway/body sway velocity between chronic low back pain (cLBP) and healthy control (HC) groups. In instances where a significant effect size is positive, individuals with cLBP in the study showed bigger magnitude of body sway or body sway velocity compared to HC group. The black diamonds represent pooled effect sizes across studies. For Fig. 2a, the pooled effect size was 0.77, showing a significant, positive, and large effect on body sway. It suggests that individuals with cLBP have greater magnitude of body sway compared to HCs. In contrast, for Fig. 2b, no significant effect on body sway velocity was found.
Subgroup analyses for body sway
Quiet standing on a firm surface with eyes open
Figure 3a shows effect sizes for body sway on a firm surface with eyes open (EO). All the effect sizes were positive reflecting greater body sway in the cLBP group. The medium and significant pooled effect size (p < 0.001, g = 0.60 [0.33, 0.87]) is represented by the red diamond. The I2 value was 60.62%, and the PI was from − 0.32 to 1.51 (Table S4). No effect of movement direction was found (Q = 2.53, p = 0.282, I2 = 60.62%, PI [−0.47, 1.63]). The red squares in Fig. 3a represent effect sizes for AP, ML, and AP-ML directions. A large and significant effect was found in the AP direction (p = 0.002, g = 0.85 [0.32, 1.38], I2 = 81.50%, PI [−0.93, 2.64]) and a medium and significant effect was found in the AP-ML direction (p < 0.001, g = 0.60 [0.33, 0.88], I2 = 52.93%, PI [−0.20, 1.41]). No significant moderator effect was found for pain intensity (Q = 0.31, p = 0.578, I2 = 64.46%, PI [−0.32, 1.65]). The pink circles in Fig. 3a represent effect sizes for the low and high pain levels. Both pain levels showed medium and significant effects (Low: p = 0.002, g = 0.61 [0.26, 0.95], I2 = 32.92%, PI [−0.31, 1.52]; High: p = 0.002, g = 0.77 [0.29, 1.26], I2 = 75.96%, PI [−0.77, 2.32]).
Subgroup analyses for body sway. Note. Those forest plots indicate the results of subgroup analyses for body sway according to quiet standing conditions. The leftmost column represents moderate variables: conditions, directions (i.e., anterior–posterior [AP], medial–lateral [ML], and both [AP-ML] directions) to measure center of pressure (CoP), and pain intensity (Low and High pain). The second column represents the statistical results: k, the number of studies; PI, prediction interval; I2, heterogeneity; p, p-values; Q, heterogeneity for moderate variables. The third column represents forest plots for each subgroup analysis: diamonds stand for pooled effect sizes for quiet standing conditions (the firm surface with eyes open [EO; dark red] and eyes closed [EC; dark yellow] and the foam surface with EO [dark green] and EC [dark blue]); the squares stand for the pooled effect sizes for results of CoP directions (AP, ML, and AP-ML); the circles stand for the pooled effect sizes for pain intensity (Low and High). All whiskers represent confidence intervals for each analysis. The rightmost column lists effect sizes and confidence intervals, which were calculated into Hedges’ g. Figure 3a displays results for the firm surface with EO condition (red colors). Figure 3b represents results on quiet standing on the firm surface with EC (yellow and orange colors). Figure 3c represents results on quiet standing on the foam surface with EO (green colors). Figure 3d represents results on quiet standing on the foam surface with EC (blue colors). *p < .05; †pQ-test < .10.
Quiet standing on a firm surface with eyes closed
Figure 3b indicates effect sizes for body sway on a firm surface with eyes closed (EC). All the effect sizes were again positive revealing with greater sway in the cLBP group. The large and significant pooled effect size (p = 0.001, g = 0.97 [0.60, 1.35]) is represented by the orange diamond. The I2 value was 75.66%, and the PI was −0.35 to 2.30 (Table S4). No effect of movement direction was found (Q = 0.67; p = 0.716, I2 = 71.49%, PI [−0.23, 1.88]). The yellow squares represent sway in the AP, ML, and AP-ML directions. Large and significant effects were found for the AP and AP-ML directions (AP: p = 0.001, g = 0.89 [0.40, 1.37], I2 = 73.40%, PI [−0.65, 2.42]; AP-ML: p = 0.001, g = 0.94 [0.47, 1.41], I2 = 79.88%, PI [−0.63, 2.51]). A medium and significant effects was found for the ML direction, (p = 0.001, g = 0.70 [0.30, 1.11], I2 = 51.92%, PI [−0.52, 1.93]). A significant moderator effect was found for pain intensity (Q = 3.28; p = 0.070, I2 = 80.43%, PI [−0.69, 1.97]), with the large effect (p = 0.001, g = 1.46 [0.68, 2.25], I2 = 87.44%, PI [−1.44, 4.37]) driven by greater body sway in individuals with high pain compared to controls (i.e., the lighter yellow circle in Fig. 3b). In the low pain level represented by the darker yellow circle in Fig. 3b, a medium and significant effect was found (p = 0.001, g = 0.66 [0.28, 1.04], I2 = 38.16%, PI [−0.66, 1.97]).
Quiet standing on a foam surface with eyes open
Figure 3c shows effect sizes for body sway on a foam surface with EO. All effect sizes were positive in favor of cLBP group. A medium and significant pooled effect size was found (p < 0.001, g = 0.68 [0.38, 0.97]) which is represented by the green diamond. I2 value was 7.55%, and the PI was −0.05 to 1.41 (Table S4). No effect of movement direction (Q = 2.85; p = 0.240, I2 = 0%, PI = NaN) was found. The green squares in Fig. 3c indicate AP, ML, and AP-ML, showing significant effects: AP had the medium effect size (p = 0.003, g = 0.53 [0.23, 0.84], I2 = 0%, PI = NaN); ML had the small effect size (p = 0.022, g = 0.45 [0.09, 0.81], I2 = 0%, PI = NaN); AP-ML represented by the lighter square had a large effect size (p < 0.001, g = 0.84 [0.51, 1.18], I2 = 0%, PI = NaN). A moderator effect of pain intensity (Q = 0.05; p = 0.827, I2 = 7.55%, PI [−0.14, 1.49]) was not found. The green circles in Fig. 3c represent medium and significant effects for both pain levels (Low: p = 0.003, g = 0.66 [0.26, 1.05], I2 = 0%, PI = NaN; High: p = 0.025, g = 0.74 [0.09, 1.39], I2 = 59.65%, PI = NaN).
Quiet standing on a foam surface with eyes closed
Figure 3d represents effect sizes for body sway on a foam surface with EC. All effect sizes were positive in favor of cLBP group. The large and significant pooled effect size (p = 0.004, g = 0.89 [0.28, 1.51]) is represented by the blue diamond. The I2 value was 77.42%, and the PI was −1.82 to 3.61 (Table S4). No moderator effect of movement direction was found (Q = 3.66; p = 0.161, I2 = 69.04%, PI [−0.52, 1.64]). The blue squares represent AP, ML, and AP-ML. The ML plane had a small and significant effect (p = 0.028, g = 0.47 [0.11, 0.83], I2 = 0%, PI = NaN), and the AP-ML plane had a large and significant effect (p = 0.020, g = 1.20 [0.49, 1.90], I2 = 74.28%, PI [− 7.03, 9.42]). We found no evidence of a moderator effect of pain intensity (Q = 0.70; p = 0.402, I2 = 77.42%, PI [− 1.87, 3.13]). The blue circles in Fig. 3d indicate low and high pain levels. A significant medium effect of the low pain level was found (p = 0.020, g = 0.59 [0.20, 0.97], I2 = 0%, PI = NaN) represented by the darker blue circle.
Subgroup analyses for body sway velocity
Figure 4 indicates effect sizes for body sway velocity. No significant pooled effect size or moderator effect was found as shown in Fig. 4a and 4b.
Subgroup analyses for body sway velocity. Note. Those forest plots indicate the results of subgroup analyses for body sway velocity according to quiet standing conditions. The leftmost column represents moderate variables: conditions, directions (i.e., anterior–posterior [AP], medial–lateral [ML], and both [AP-ML] directions) to measure center of pressure (CoP), and pain intensity (self-reported Low and High pain). The second column represents the statistical results: k, the number of studies included in each subgroup analysis; PI, prediction interval; I2, heterogeneity for each subgroup analysis; p, p-values for each subgroup analysis; Q, heterogeneity for moderate variables. The third column represents forest plots for each subgroup analysis: diamonds stand for pooled effect sizes for quiet standing conditions (the firm surface with eyes open [EO] and eyes closed [EC]); the squares stand for the pooled effect sizes for results of CoP directions (AP, ML, and AP-ML); the circles stand for the pooled effect sizes for pain intensity (Low and High). All whiskers stand for confidence intervals for each analysis. The rightmost column represents effect sizes and confidence intervals, which were calculated into Hedges’ g. Figure 4a indicates results on the condition on firm surface with EO (red colors). Figure 4b represents results on quiet standing on the firm surface with EC (yellow and orange colors). *p < .05; †pQ-test < .10.
Publication bias
Publication biases were assessed using Egger’s regression and Trim and Fill analysis. Table S5 in the supplementary material 1 represents the statistical results of Egger’s regression test. The last column shows p-values for all meta- and subgroup analyses. Significant biases were found in the three conditions of body sway: the firm with EC (p = 0.033) and foam with EO (p = 0.006) and EC (p = 0.033). Those analyses were reassessed using the Trim and Fill analysis.
Fig. S2 in the supplementary material 1 represents the results of the Trim and Fill analyses. The circles in the funnel plots represent standard errors by Hedges’ g of each study. The white filled diamond under the x-axis represents the overall 95% confidence interval for each meta-analysis. For instance, Fig. S2a shows the funnel plot for the body sway meta-analysis when collapsing across all conditions within each study. Each study is represented by an open circle. The absence of a filled black circle means that no imputation had to be conducted for this meta-analysis. Open circles were evident for the firm surface with EO (Fig. S2b) and firm surface with EC (Fig. S2c). Fig. S2d and e show the funnel plots for body sway on a Foam surface with EO and EC. Black circles are included in each plot. Each black circle represents one imputed study, which makes the distribution of the values symmetric. The black diamond on the x-axis represents the mean confidence interval when including the imputed data. When the white and black diamonds overlap, we infer that publication bias is not affected. This was evident in each case. Figs. S2f–h show funnel plots for body sway velocity meta-analyses. Although black dots are shown in each case, overlap between the white and black diamond suggests no evidence of publication bias in either case.
Assessment of the quality in studies
The results of QAT for every study are presented in Table S3. Five studies were evaluated as being good quality13,14,39,41,43, and six studies were of fair quality15,20,21,22,23,24. Five studies were rated as poor quality6,38,40,42,44.
GRADE assessment
GRADE was used to assess the evidence quality and the results are shown in Table 3. The first column in Table 3 lists the different outcome measures and conditions, and the second column represents the number of studies included in the corresponding meta-analysis as well as the total sample size and CoP variables included. The third to seventh columns indicate the ratings for subcategories of GRADE. The eight column (“other”) shows decisions on publication bias and other considerations that can upgrade the quality of evidence, which is followed by estimate of outcome values. The final column represents the final decision for rating evidence certainty. As a result, the evidence of CoP outcomes on Firm with EC in body sway, CoP outcome in body sway velocity, and CoP outcome on Firm with EO in body sway velocity were rated as low certainty. The rest of evidence was rated as very low certainty.
Discussion
Compared with controls, individuals with cLBP showed increased postural sway during quiet standing. Subgroup analyses revealed larger effect sizes when vision was removed and when self-reported pain intensity was higher but only when subjects completed the task on a firm surface. In contrast, we did not find strong support for an effect of cLBP on postural sway velocity.
When averaging data across all experimental conditions within each study, we found strong evidence that cLBP is associated with greater postural sway during quiet standing. Our findings suggest that cLBP is associated with altered postural control7,45. Several explanations may account for this change in control including reduced proprioceptive acuity46, restrictive trunk movement5, and protective trunk muscle strategies47,48. Reduced proprioception means that larger movements must be made for a change in internal estimate of CoP position to be updated11. Once perceived, a correction in the opposite direction may also then be disproportionality large22. Restrictive trunk movement and co-contractions across trunk muscles may also contribute to changes in posture by shifting control from the trunk to the ankles. LBP has been associated with co-activation of left and right gluteus medius muscles49,50, sustained activation of back and abdominal muscles during quiet standing43, leading to a restriction in trunk movement reflective of a protective strategy12,47. In addition to changes in trunk muscle activation, a shift in motor control away from the trunk has also been evidenced, with the ankle being most commonly observed5,19,51,52. This shift in control to the ankle can be problematic given that perturbation studies show that trunk control is most effective for maintaining postural equilibrium53, with the ankles contributing minimally to posture. Taken together, the greater CoP sway in cLBP evident in our findings may result from altered proprioception and restrictive and protective trunk strategies, which combine to shift postural control to the ankles.
Previous systematic reviews on back pain and postural sway have reported inconsistent results5,18,54. In the current meta-analysis twelve out of 16 articles reported greater CoP sway in cLBP compared to healthy controls. The difference in findings between previous reviews and the present meta-analysis may be due to different inclusion criteria for the back pain group. Our meta-analysis included studies that recruited individuals with cLBP, while previous reviews included individuals with both recurrent and cLBP5,16,18. Smaller CoP movements have been reported in LBP studies (e.g., Mok et al.55 and Salavati et al.56), these were excluded from our meta-analysis because they did not meet our inclusion criteria for cLBP. Models of motor adaptation have been proposed for muscular pain7,57,58, such that motor strategies are altered based on pain chronicity. For instance, the progression from acute to chronic neck-shoulder pain is associated with a progressive decrease in variability of trunk movement with a corresponding increase trunk stiffness57. In the context of LBP, it is plausible, therefore, that acute LBP is associated with an increase in variability of trunk movement and a decrease in trunk stiffness as individuals search for optimal solutions to minimize the pain. This could potentially lead to reduced postural sway. As chronicity develops, a progressive decrease in variability and increase in stiffness would lead to an increase in postural sway. We therefore suggest that distinguishing between acute, recurrent and chronic phases of LBP is critical when assessing the impact of low back pain on postural sway.
Removing vision during quiet standing increased effect sizes by ~ 0.2 irrespective of which surface individuals were standing on. Effect sizes were similar for both eyes open conditions (0.64 and 0.68) and both eyes closed conditions (0.98, 0.89). Changing the surface on which subjects were standing had a minimal impact on effect size. If a deficit in proprioception is a dominant factor in altered postural control in cLBP, then one would have expected effect sizes to be largest in the eyes closed plus foam surface condition. This was not the case in our data, suggesting that the change in surface had a minimal impact. We offer three explanations. First, the number of studies contributing to the foam condition was smaller as compared to the firm condition, and second, altered proprioceptive feedback in cLBP may have reached a ceiling in the eyes open plus firm surface condition (effect size ~ 0.6), such that changing the surface to foam had a minimal additional effect. Other factors, such as deconditioning in cLBP59, pain-related fear, and catastrophizing60, may also be associated with greater sway, but we did not assess these factors in the current study. In contrast to the surface condition, changing visual input had a larger impact on effect size. Our finding is consistent with other studies in chronic pain that have manipulated vision61,62,63. For instance, individuals with cLBP show greater variability in stride time and minimum foot clearance during gait when vision was removed61. Other evidence comes from individuals with chronic neck pain62,63. Underestimating the extent of neck rotation using virtual reality led to greater movement prior to the onset of pain62. During standing, greater body sway was found in individuals with chronic neck pain compared to controls in eyes open condition, with the between group difference becoming larger when vision was removed63.
With eyes closed, we also found evidence that effect sizes were greatest when comparing controls and individuals who self-reported higher as compared to lower pain intensity. This effect was not evident in the eyes open conditions, suggesting that vision may mask or compensate for the effect of pain intensity on postural sway. Our findings are consistent with other studies that have found that individuals who report higher pain intensity (VAS ≧ 5) show greater CoP sway compared to individuals with lower pain intensity (VAS = 1.47)64 and controls14,38,39,41. Importantly, individuals with lower LBP intensity (VAS < 2) were found to have smaller and slower CoP sway compared to healthy controls55,56, which suggests that pain and intensity in addition to pain chronicity should be considered when associating cLBP with postural sway.
We note several limitations of this meta-analysis. First, we restricted our analysis to studies which recruited individuals with cLBP since previous evidence revealed that there is a different impact of the LBP disease stages on a postural control strategy7,57,58. Our findings, therefore, can only generalize to individuals with cLBP. Second, we did not consider the impact of specific vs. non-specific cLBP on postural sway. Third, we identified 16 studies that included an eyes-open condition, but the number of studies included in the different analyses and sub-analyses decreased. Fourth, the certainties of the outcomes included in our meta-analysis were rated as low or very low. In part this is because all included studies were observational studies and by default these are initially rated as low certainty. A high magnitude of heterogeneity across CoP outcomes also contributed to the low or very low ratings. High heterogeneity in body sway has been noted in previous systematic reviews16,17,18. We move the field forward by demonstrating that a small portion of the heterogeneity can be accounted for by pain intensity. Nevertheless, we acknowledge that even when accounting for pain intensity and CoP direction, there was still a considerable amount of variance unexplained. Other factors such as different sampling rates, filters, trial durations, number of trial repetitions, CoP equations, and instructions for the quiet standing tasks may help explain this variance, but our sample size was not large enough to address these potential factors. As additional studies are published, future meta-analyses can address these important factors. Nevertheless, we adhered to strict inclusion guidelines and our findings do suggest that vision and pain intensity play important roles in the impact of cLBP on postural sway.
Conclusion
Impact of this meta-analysis is that cLBP is associated with greater sway, which likely comes from a visual occlusion and a change in control strategy. Interventions that target both of these factors should therefore be explored.
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
References
Volinn, E. The epidemiology of low back pain in the rest of the world. Spine 22, 1747–1754 (1997).
Hoy, D. et al. The global burden of low back pain: Estimates from the global burden of disease 2010 study. Ann. Rheum Dis. 73, 968–974 (2014).
Pitcher, M. H., von Korff, M., Bushnell, M. C. & Porter, L. Prevalence and profile of high-impact chronic pain in the United States. J. Pain 20, 146–160 (2019).
Zemedikun, D. T., Kigozi, J., Wynne-Jones, G., Guariglia, A. & Roberts, T. Methodological considerations in the assessment of direct and indirect costs of back pain: A systematic scoping review. PLoS ONE 16, 1–17 (2021).
Koch, C. & Hänsel, F. Non-specific low back pain and postural control during quiet standing-A systematic review. Front. Psychol. 10, 586 (2019).
Nogueira, J. F. et al. Posturography comparison and discriminant analysis between individuals with and without chronic low back pain. J. Manip. Physiol. Ther. 43, 469–475 (2020).
Meier, M. L., Vrana, A. & Schweinhardt, P. Low back pain: The potential contribution of supraspinal motor control and proprioception. Neuroscientist 25, 583–596 (2019).
Leveille, S. G. et al. Chronic musculoskeletal pain and the occurrence of falls in an older population. JAMA – J. Am. Med. Assoc. 302, 2214–2221 (2009).
Rosa, N. M. B. et al. Risk of falls in Brazilian elders with and without low back pain assessed using the physiological profile assessment: Bace study. Braz. J. Phys. Ther. 20, 502–509 (2016).
Allum, J. H. J., Bloem, B. R., Carpenter, M. G., Hulliger, M. & Hadders-Algra, M. Proprioceptive control of posture: A review of new concepts. Gait Posture 8, 214–242 (1998).
Lee, A. S., Cholewicki, J., Reeves, N. P., Zazulak, B. T. & Mysliwiec, L. W. Comparison of trunk proprioception between patients with low back pain and healthy controls. Arch. Phys. Med. Rehabil. 91, 1327–1331 (2010).
Hodges, P. W. & Tucker, K. Moving differently in pain: A new theory to explain the adaptation to pain. Pain 152, 90–98 (2011).
Caffaro, R. R. et al. Postural control in individuals with and without non-specific chronic low back pain: A preliminary case-control study. Eur. Spine J. 23, 807–813 (2014).
Caña-Pino, A. et al. Energy spectral density as valid parameter to compare postural control between subjects with nonspecific chronic low back pain vs healthy subjects: A case-control study. Musculoskelet. Sci. Pract. 53, 102370 (2021).
Yahia, A. et al. Evaluation of the posture and muscular strength of the trunk and inferior members of patients with chronic lumbar pain. Joint Bone Spine 78, 291–297 (2011).
Ruhe, A., Fejer, R. & Walker, B. Center of pressure excursion as a measure of balance performance in patients with non-specific low back pain compared to healthy controls: A systematic review of the literature. Eur. Spine J. 20, 358–368 (2011).
Berenshteyn, Y., Gibson, K., Hackett, G. C., Trem, A. B. & Wilhelm, M. Is standing balance altered in individuals with chronic low back pain? A systematic review. Disabil. Rehabil. 41, 1514–1523 (2019).
Mazaheri, M., Coenen, P., Parnianpour, M., Kiers, H. & van Dieën, J. H. Low back pain and postural sway during quiet standing with and without sensory manipulation: A systematic review. Gait Posture 37, 12–22 (2013).
Brumagne, S., Janssens, L., Knapen, S., Claeys, K. & Suuden-Johanson, E. Persons with recurrent low back pain exhibit a rigid postural control strategy. Eur. Spine J. 17, 1177–1184 (2008).
da Silva, R. A. et al. People with chronic low back pain have poorer balance than controls in challenging tasks. Disabil. Rehabil. 40, 1294–1300 (2018).
Lafond, D. et al. Postural control during prolonged standing in persons with chronic low back pain. Gait Posture 29, 421–427 (2009).
Popa, T., Bonifazi, M., Della Volpe, R., Rossi, A. & Mazzocchio, R. Adaptive changes in postural strategy selection in chronic low back pain. Exp. Brain Res. 177, 411–418 (2007).
della Volpe, R. et al. Changes in coordination of postural control during dynamic stance in chronic low back pain patients. Gait Posture 24, 349–355 (2006).
Shigaki, L. et al. Effects of holding an external load on the standing balance of older and younger adults with and without chronic low back pain. J. Manipulative Physiol. Ther. 40, 284–292 (2017).
Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J. Clin. Epidemiol. 62, W65 (2009).
Bramer, W. M., Giustini, D., de Jong, G. B., Holland, L. & Bekhuis, T. De-duplication of database search results for systematic reviews in endnote. J. Med. Libr. Assoc. 104, 240–243 (2016).
McGrath, S. et al. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat. Methods Med. Res. 29, 2520–2537 (2020).
Lin, L. & Aloe, A. M. Evaluation of various estimators for standardized mean difference in meta-analysis. Stat. Med. 40, 403–426 (2021).
National Institutes of Health. National institutes of health quality assessment tool for observational cohort and cross-sectional studies. Preprint at (2016).
Borenstein, M. Research Note: In a meta-analysis, the I2 index does not tell us how much the effect size varies across studies. J. Physiother. 66, 135–139 (2020).
Egger, M., Smith, G. D., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 315, 629–634 (1997).
Duval, S. & Tweedie, R. Trim and fill: A simple funnel-plot-based method. Biometrics 56, 455–463 (2000).
Higgins, J. P. T. et al. Cochrane handbook for systematic reviews of interventions (John Wiley & Sons, 2019).
Quijoux, F. et al. Center of pressure characteristics from quiet standing measures to predict the risk of falling in older adults: A protocol for a systematic review and meta-analysis. Syst. Rev. 8, 1–9 (2019).
Nunes, G. S. et al. Is postural control affected in people with patellofemoral pain and should it be part of rehabilitation? A systematic review with meta-analysis. Sports Med. Open https://doi.org/10.1186/s40798-022-00538-4 (2022).
Schünemann, H. J. et al. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. J. Clin. Epidemiol. 111, 105–114 (2019).
Zhang, Y. et al. GRADE guidelines: 20. Assessing the certainty of evidence in the importance of outcomes or values and preferences—inconsistency, imprecision, and other domains. J. Clin. Epidemiol. 111, 83–93 (2019).
Mann, L., Kleinpaul, J. F., Pereira Moro, A. R., Mota, C. B. & Carpes, F. P. Effect of low back pain on postural stability in younger women: Influence of visual deprivation. J. Bodyw. Mov. Ther. 14, 361–366 (2010).
Wang, H. et al. Impaired static postural control correlates to the contraction ability of trunk muscle in young adults with chronic non-specific low back pain: A cross-sectional study. Gait Posture 92, 44–50 (2022).
Hamaoui, A., Do, M. C. & Bouisset, S. Postural sway increase in low back pain subjects is not related to reduced spine range of motion. Neurosci Lett 357, 135–138 (2004).
Zhang, C. et al. Pain catastrophizing is related to static postural control impairment in patients with nonspecific chronic low back pain: A cross-sectional study. Pain Res Manag 2020, 9629526 (2020).
Abbasi, S., Rojhani-Shirazi, Z., Shokri, E. & García-Muro San José, F. The effect of kinesio taping on postural control in subjects with non-specific chronic low back pain. J. Bodyw. Mov. Ther. 22, 487–492 (2018).
Ringheim, I., Austein, H., Indahl, A. & Roeleveld, K. Postural strategy and trunk muscle activation during prolonged standing in chronic low back pain patients. Gait Posture 42, 584–589 (2015).
Sundaram, B., Doshi, M. & Pandian, J. S. Postural stability during seven different standing tasks in persons with chronic low back pain – A cross-sectional study. Indian J. Physiother. Occup. Ther. 6, 22–28 (2012).
van Dieën, J. H., Peter Reeves, N., Kawchuk, G., van Dillen, L. R. & Hodges, P., PW Motor control changes in low back pain: Divergence in presentations and mechanisms. J. Orthop. Sports Phys. Therapy 49, 370–379 (2019).
Tong, M. H. et al. Is there a relationship between lumbar proprioception and low back pain? A systematic review with meta-analysis. Arch. Phys. Med. Rehabil. 98, 120-136.e2 (2017).
Griffioen, M. et al. Identification of intrinsic and reflexive contributions to trunk stabilization in patients with low back pain: A case–control study. Eur. Spine J. 29, 1900–1908 (2020).
Laird, R. A., Gilbert, J., Kent, P. & Keating, J. L. Comparing lumbo-pelvic movement in people with and without back pain: A systematic review. BMC Musculoskelet. Disord. 15, 1–13 (2014).
Nelson-Wong, E., Gregory, D. E., Winter, D. A. & Callaghan, J. P. Gluteus medius muscle activation patterns as a predictor of low back pain during standing. Clin. Biomech. 23, 545–553 (2008).
Nelson-Wong, E. & Callaghan, J. P. Is muscle co-activation a predisposing factor for low back pain development during standing? A multifactorial approach for early identification of at-risk individuals. J. Electromyogr. Kinesiol. 20, 256–263 (2010).
Claeys, K., Brumagne, S., Dankaerts, W., Kiers, H. & Janssens, L. Decreased variability in postural control strategies in young people with non-specific low back pain is associated with altered proprioceptive reweighting. Eur. J. Appl. Physiol. 111, 115–123 (2011).
Claeys, K. et al. Young individuals with a more ankle-steered proprioceptive control strategy may develop mild non-specific low back pain. J. Electromyogr. Kinesiol. 25, 329–338 (2015).
Shumway-Cook, A. & Woollacott, M. H. Motor control: Translating research into clinical practice (Lippincott Williams & Wilkins, 2007).
Ruhe, A., Fejer, R. & Walker, B. The test-retest reliability of centre of pressure measures in bipedal static task conditions - A systematic review of the literature. Gait Posture 32, 436–445 (2010).
Mok, N. W., Brauer, S. G. & Hodges, P. W. Hip strategy for balance control in quiet standing is reduced in people with low back pain. Spine 29, 107–112 (2004).
Salavati, M. et al. Effect of dual-tasking on postural control in subjects with nonspecific low back pain. Spine 34, 1415–1421 (2009).
Madeleine, P. On functional motor adaptations: From the quantification of motor strategies to the prevention of musculoskeletal disorders in the neck-shoulder region. Acta Physiol. 199, 1–46 (2010).
Hodges, P. W., Cholewicki, J. & Van Dieën, J. H. Spinal control: The rehabilitation of back pain: State of the art and science (Elsevier health sciences, London, 2013).
Verbunt, J. A. et al. Disuse and deconditioning in chronic low back pain: Concepts and hypotheses on contributing mechanisms. www.EuropeanJournalPain.com.
Christe, G. et al. Relationship between psychological factors and spinal motor behaviour in low back pain: A systematic review and meta-analysis. Pain 162, 672–686 (2021).
Hamacher, D., Hamacher, D., Krowicki, M. & Schega, L. Gait variability in chronic back pain sufferers with experimentally diminished visual feedback: A pilot study. J. Mot. Behav. 48, 205–208 (2016).
Harvie, D. S. et al. Bogus visual feedback alters onset of movement-evoked pain in people with neck pain. Psychol. Sci. 26, 385–392 (2015).
Hsu, W. L. et al. Fatigue changes neck muscle control and deteriorates postural stability during arm movement perturbations in patients with chronic neck pain. Spine J. 20, 530–537 (2020).
Sipko, T. & Kuczyński, M. Intensity of chronic pain modifies postural control in low back patients. Eur. J. Pain 17, 612–620 (2013).
Acknowledgements
This work was supported in part by the National Institutes of Health [R01AG076082].
Author information
Authors and Affiliations
Contributions
J.P. and S.A.C. designed this research. J.P. searched the literature. J.P., V.N., and S.A.C. selected and evaluated studies. J.P. and V.N. extracted data from the studies. J.P. and S.A.C. analyzed the extracted data. J.P., R.H., and S.A.C. substantially contributed to writing and checking the manuscripts.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, J., Nguyen, V.Q., Ho, R.L.M. et al. The effect of chronic low back pain on postural control during quiet standing: A meta-analysis. Sci Rep 13, 7928 (2023). https://doi.org/10.1038/s41598-023-34692-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-34692-w
This article is cited by
-
Poor lumbar spine coordination in acute low back pain predicts persistent long-term pain and disability
European Spine Journal (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.