Abstract
Rubella virus infection during pregnancy has several effects on the developing fetus. However, little is known about the epidemiology of the infection in Ethiopia. A cross-sectional study was conducted to assess the seroprevalence of rubella virus infection on consecutive 299 pregnant women attending antenatal care clinics in public health facilities in Halaba Town, Southern Ethiopia. Structured questionnaires were used to collect information on socio-demographic and reproductive characteristics. Venous blood samples were collected, and sera were tested for anti-rubella IgM and IgG using the enzyme-linked immunosorbent assay. Anti-rubella IgG and IgM were detected in 265 (88.6%) and 15 (5.0%) of 299 participants, respectively. Pregnant women in their first trimester [crude odds ratio (cOR) = 4.26; 95% CI (1.47, 12.4)] were at increased risk of having anti-rubella IgM compared to those in their second and third trimesters. Urban residents [cOR = 4.06; 95% CI (1.94, 8.47)] were with a higher percentage of IgG positivity compared to rural residents. Anti-rubella IgG positivity was higher in housewives [cOR = 2.94; 95% CI (1.07, 8.04)] compared to self-employed women. Our findings showed a high prevalence of rubella virus exposure, and considerable percentages of recent infection and susceptible women to contracting the infection, emphasizing the importance of congenital rubella syndrome in the research area.
Similar content being viewed by others
Introduction
Rubella is a contagious infection caused by the rubella virus that mostly affects children and young adults. Postnatal rubella is commonly acquired through the respiratory route and causes a self-limiting illness, which is mainly characterized by a low-grade fever and skin rash1. Infection during pregnancy may result in congenital rubella infection (CRI), with outcomes ranging from subclinical infection, miscarriage, and stillbirth to congenital rubella syndrome (CRS). Defects associated with CRS include blindness, deafness, mental retardation, and congenital heart diseases2. The risk of CRS ranges from 10 to 90%, with a higher risk for infection occurring during early gestational age (< 12 weeks), and a lower risk for infection after 20 weeks of gestation3.
With the expanded introduction of rubella-containing vaccine (RCV) into countries’ childhood immunization programs, a significant reduction in the burden of infection has been reported, from 670,894 in 2000 to 26,006 in 2018. As of January 2020, 173 (89%) of the 194 World Health Organization (WHO) member countries used rubella immunizations in their national programs4; Ethiopia has not yet made the vaccines available. According to estimates, there are 24 and 112 CRS cases per 100,000 live births in urban Addis Ababa and rural Ethiopia, respectively5.
In African nations, case-based surveillance for measles has been utilized to identify cases of rubella, with blood samples screened for anti-rubella immunoglobulin (Ig)M, if anti-measles IgM tests were negative6. Analysis of such surveillance national data in Ethiopia showed a 12.1% prevalence of anti-rubella IgM from 2004 to 20097 and 15.3% from 2009 to 20158. Case-based surveillance should be supplemented with serosurveys to better understand the epidemiology of rubella infection in a defined population. For instance, despite an increased risk of CRI in Ethiopia, where rubella outbreaks are frequent, there is scarce information available on the infection status of pregnant women9,10. Thus, the current study aimed to determine the seroprevalence of rubella virus infection and its distribution by socio-demographic and reproductive characteristics among antenatal clinic clients of Halaba Town public health facilities in southern Ethiopia.
Methods
Study design, setting, and period
A facility-based cross-sectional study was conducted in Halaba Town, which is 245 kms south of Addis Ababa and 90 kms from Hawassa City, the capital of the Southern Nations, Nationalities, and Peoples’ Region (SNNPR) and the Sidama Region. The town has five Kebeles (the smallest administrative units in Ethiopia) and two public health facilities (one General Hospital and one Health Centre). An estimated 39,507 people live in the town; 51% of which were females, and 23.2% of the females were in the reproductive age group. The town’s population density was four persons per hectare, and the average family size was about six11. The study was conducted from March to April 2021.
Study population
Pregnant women who visited the antenatal clinics at the General Hospital and Health Centre in the town during the study period constituted the study population. Pregnant women older than 18 years were included. However, those who were critically ill at the time of enrolment and refused to participate in the study were excluded.
Sample size and sampling technique
The sample size was determined using a single population proportion formula (n = z2 p (1 − p)/d2), assuming an anti-rubella IgG seroprevalence of 86.3% among pregnant women based on a recent report from Hawassa9, aiming for a 95% confidence level with 4% precision. Assuming a non-response rate of 5%, the final sample size was estimated to be 299. Consecutive pregnant women were enrolled from the health facilities until the target sample size is obtained.
Data collection techniques
Socio-demographic and reproductive characteristics
Nurses with a 4-years degree gathered data on socio-demographic characteristics (age, residence, marital status, women’s educational and occupational status, and estimated monthly income) as well as reproductive characteristics (gestational age, gravidity, parity, and histories of stillbirth and spontaneous abortion) using pretested and structured questionnaire.
Serological analysis
Venous blood samples (about 5 ml) were collected from every study participant, and sera were stored at − 20 °C for a month. Samples were transported to the Public Health Laboratory Institute of SNNPR using a cold box, and stored at − 70 °C until tested. All samples were tested for anti-rubella IgM and IgG using enzyme-linked immunosorbent assay (ELISA) test kits. The Anti-Rubella Virus Glycoprotein (IgM) (EUROIMMUN Medical Laboratory Diagnostics AG, Lubeck, Germany) and the Rubella IgG test kits (DIALAB Diagnostics, Wiener Neudorf, Austria) were used and performed according to the respective manufacturer’s instructions.
Definitions
Past exposure to rubella virus infection: pregnant women whose blood is tested positive for anti-rubella IgG; thus, protective immunity against the infection.
Recent rubella virus infection: pregnant women who tested positive for IgM antibody.
Data analysis
Data were double-entered into EpiData version 3.112 and analyzed using SPSS version 20 (IBM Corp., Armonk, NY). Descriptive statistics results were presented using percentages. Binary logistic regression analysis was used to assess the association between socio-demographic and reproductive characteristics and anti-rubella IgM and IgG serostatus. Variables found to have a p value < 0.05 were considered significant differences.
Ethics approval and consent to participate
Ethical approval was obtained from the Institutional Review Board (IRB) (Ref.No: IRB/091/13) of Hawassa University College of Medicine and Health Sciences. Prior to data collection, a supportive letter was obtained from the Halaba Zone Health Department. Study participants were given adequate information regarding the purpose, risk, benefit, and confidentiality of the study. Participation was voluntary, and informed written consent was obtained from each participant. All of the acquired data were linked to code numbers. Positive laboratory results were communicated to the ANC clinics for possible management. All methods were performed in accordance with the relevant guidelines and regulations.
Results
Sociodemographic characteristics
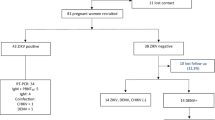
Of 308 pregnant women approached during the study period, 9 (2.9%) women were excluded because they refused to participate. Thus, 299 (97.1%) participated in the study. All of the excluded women were from the health centre. The age of participants ranged from 18 to 40 years, with a mean age of 25.4 years (standard deviation, 3.94 years). Half of the participants, 152 (50.8%), were within the age group of 21–30 years, and 210 (70.2%) were urban dwellers. Of the study participants, 131 (43.8%) had no formal education, 247 (82.6%) were housewives, and 118 (39.5%) had an estimated monthly income of ≥ 2001 Ethiopian Birr (Table 1).
Reproductive health-related characteristics
Of 299 participants, 83 (27.8%) were in their first trimester of pregnancy, 169 (56.5%) were multigravida, and 27 (9.0%) had a history of spontaneous abortion (Table 2).
Seroprevalence of rubella virus infection
Among 299 pregnant women, 15 (5%, 95% CI 2.5–7.5%) were positive for anti-rubella IgM and 265 (88.6%, 95% CI 85.0–92.2%) were positive for anti-rubella IgG. Pregnant women with no IgM or IgG to rubella infection accounted for 11.4%. Of the 299 participants, 11 (3.7%) were found to be positive for both IgM and IgG antibodies.
Tables 1 and 2 show the associations of participants’ socio-demographic and reproductive characteristics with seropositivity for anti-rubella IgM and IgG antibodies. In bivariate logistic regression analyses, pregnant women in their first trimester [crude odds ratio (cOR) = 4.26; 95% CI (1.47, 12.4)] had higher odds of having anti-rubella IgM compared to those in their second and third trimesters taken together. A higher proportion of pregnant women with anti-rubella IgG was found among urban residents [cOR = 4.06; 95% CI (1.94, 8.47)] than rural residents. Compared to self-employed women, housewives were more likely to have anti-rubella IgG positivity (cOR = 2.94; 95% CI (1.07, 8.04)). Further, multigravida women [cOR = 3.41; 95% CI (1.30, 8.92)] had a higher likelihood of having anti-rubella IgG positivity compared to grand multigravida women.
Discussion
In these serological analyses of rubella infection in pregnant women, we found anti-rubella IgM positivity of 5%, indicating a recent rubella virus infection (or re-infection), and anti-rubella IgG positivity of 88.6%, indicating past exposure to rubella virus infection with development of protective immunity. While a recent rubella infection was more frequently detected among pregnant women in their first trimester, past rubella virus infection was more likely among urban residents, housewives, and multigravida women.
The observed proportion of anti-rubella IgM positivity in this study was comparable with pooled seroprevalence of rubella among pregnant women in sub-Saharan Africa (89.0%) including a single report from southern Ethiopia (86.3%)9. Compared to our finding, slightly lower proportions were reported in studies in China (83.3%)13 and India (83.4 and 82.3%)14,15, as also in northern Ethiopia (79.5%)10. Despite the observed variability in the prevalence of rubella infections, studies generally showed high proportions of exposure to rubella infections among pregnant women in various geographical regions where a rubella vaccination has not been introduced or has recently been introduced. Most children aged under 15 years in Africa develop immunity as a result of natural infection1. The observed seronegativity of anti-rubella IgG (11.4%) in our study indicates an intermediate level of susceptibility (10–20%) and a medium risk of CRS in the study setting16. Our study showed a higher proportion of seronegativity compared to the WHO threshold of < 5% in a childbearing-age woman17, which was below the target to be achieved in Africa by 203018.
Our finding of seroprevalence of IgM was higher compared to a result from southern Ethiopia (2.1%)9. However, the pooled prevalence of recent rubella infection in sub-Saharan Africa (5.1%) was consistent with our current observation, as also reported recently from Cameroon (5.5% and 5%)19,20. On the other hand, the proportion of IgM positivity was lower than that reported in northern Ethiopia (9.5%)10 and Nigeria 7.8–38.8%21,22,23. The observed difference in proportions of IgM positivity among the studies might be due to variable endemicity of rubella, testing methods, and population density.
A higher proportion of IgM positivity among pregnant women who were in their first trimester was consistent with studies from Ethiopia24 and Tanzania25, and it is a major concern in light of an increased risk of developing CRS with decreasing gestational age3. Our finding of the higher proportion of anti-rubella IgG positivity among urban dwellers compared to rural ones was consistently reported by several studies in African countries21,26,27,28. The possible reason might be crowded living conditions in urban populations increase the risk of rubella transmission.
Our study has the strength of being one of few such investigations of rubella virus infection among pregnant women in Ethiopia. Findings from this study should inform decision-makers on the prevalence of recent rubella virus infection and the status of susceptibility to rubella virus infection to plan intervention efforts aimed at reducing the incidence of CRS. However, the study faced some limitations. Since participants were recruited from health facilities, findings may not be compared to all pregnant women in the study area. Further, our study might not have been powered adequately to identify socio-demographic and reproductive characteristics associated with rubella infection. The study participants were interviewed in health facilities by health workers, and data collection relied on participants’ self-reports, which might be subject to social desirability, recall, and information bias.
Conclusion
The high proportion of pregnant women with exposure to the rubella virus, particularly in urban areas, indicates the endemicity of the infection in the Ethiopian context. The observed proportion of recent infection, particularly in pregnant women in their first trimester, may reflect the significance of CRS in the study area. Our findings call for interventions to reduce the risk of CRS, including vaccinating susceptible women of childbearing against the rubella virus and introducing vaccines to the routine childhood immunization programme.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author, upon reasonable request and with the Institutional Review Board of the Hawassa University College of Medicine and Health Sciences. Restrictions apply to the availability of these clinical data, which caregivers had consented for the collected information to be used for our research study only, and so are not publicly available.
References
Goodson, J. L. et al. Rubella epidemiology in Africa in the Prevaccine Era, 2002–2009. J. Infect. Dis. 204, S215–S225 (2011).
Patel, M. K. et al. The epidemiology of rubella, 2007–18: An ecological analysis of surveillance data. Lancet Glob. Health 8, e1399–e1407. https://doi.org/10.1016/s2214-109x(20)30320-x (2020).
Centers for Diseases Control and Prevention. Manual for the Surveillance of Vaccine-Preventable Diseases. Rubella, https://www.cdc.gov/vaccines/pubs/surv-manual/chpt14-rubella.html (2012).
Plotkin, S. A. Rubella eradication: Not yet accomplished, but entirely feasible. J. Infect. Dis. 224, S360–S366. https://doi.org/10.1093/infdis/jiaa530 (2021).
Vynnycky, E. et al. Using seroprevalence and immunisation coverage data to estimate the global burden of congenital rubella syndrome, 1996–2010: A systematic review. PLoS ONE 11, e0149160 (2016).
World Health Organization. Global Vaccine Action Plan. Priority Country Reports on Progress Towards GVAP-RVAP Goals. Annex to the GVAP Secretariat Annual Report, https://reliefweb.int/report/world/global-vaccine-action-plan-priority-country-reports-progress-towards-gvap-rvap-goals (2017).
Mitiku, K. et al. The epidemiology of rubella disease in Ethiopia: Data from the measles case-based surveillance system. J. Infect. Dis. 204, S239–S242 (2011).
Getahun, M. et al. Epidemiology of rubella virus cases in the pre-vaccination era of Ethiopia, 2009–2015. BMC Public Health 16, 1168 (2016).
Tamirat, B., Hussen, S. & Shimelis, T. Rubella virus infection and associated factors among pregnant women attending the antenatal care clinics of public hospitals in Hawassa City, Southern Ethiopia: A cross-sectional study. BMJ Open 7, e016824. https://doi.org/10.1136/bmjopen-2017-016824 (2017).
Wondimeneh, Y. et al. Rubella virus infections and immune status among pregnant women before the introduction of rubella vaccine in Amhara Regional State, Ethiopia. Int. J. Infect. Dis. 76, 14–22. https://doi.org/10.1016/j.ijid.2018.07.024 (2018).
Abuka Abebo, T. & Jember Tesfaye, D. Postnatal care utilization and associated factors among women of reproductive age Group in Halaba Kulito Town, Southern Ethiopia. Arch. Public Health 76, 9. https://doi.org/10.1186/s13690-018-0256-6 (2018).
Lauritsen, J. M. & Bruus, M. EpiData (Version 3.1). A Comprehensive Tool for Validated Entry and Documentation of Data (The EpiData Association, 2004).
Meng, Q. et al. Rubella seroprevalence among pregnant women in Beijing, China. BMC Infect. Dis. 18, 130–130. https://doi.org/10.1186/s12879-018-3032-x (2018).
Muliyil, D. E. et al. Sero-prevalence of rubella among pregnant women in India, 2017. Vaccine 36, 7909–7912. https://doi.org/10.1016/j.vaccine.2018.11.013 (2018).
Shanmugasundaram, D. et al. Burden of congenital rubella syndrome (CRS) in India based on data from cross-sectional serosurveys, 2017 and 2019–20. PLoS Negl. Trop. Dis. 15, e0009608. https://doi.org/10.1371/journal.pntd.0009608 (2021).
World Health Organization. Guidelines for Surveillance of Congenital Rubella Syndrome and Rubella., https://apps.who.int/iris/bitstream/handle/10665/66104/WHO-VandB-99.22-eng.pdf?sequence=1 (1999).
Nardone, A. et al. Comparison of rubella seroepidemiology in 17 countries: Progress towards international disease control targets. Bull. World Health Organ 86, 118–125. https://doi.org/10.2471/blt.07.042010 (2008).
World Health Organization. Business Case for WHO Immunization Activities on the African Continent 2018–2030, https://www.afro.who.int/publications/business-case-who-immunization-activities-african-continent-2018-2030 (2018).
Michel, N. et al. Seroprevalence of rubella IgM and IgG antibodies and associated risk factors among pregnant women attending antenatal clinic at Bafoussam Regional Hospital, West Region of Cameroon. J. Trop. Dis. https://doi.org/10.4172/2329-891X.1000279 (2018).
Taku, N. A. et al. Seroprevalence of rubella virus antibodies among pregnant women in the Center and South-West regions of Cameroon. PLoS ONE 14, e0225594. https://doi.org/10.1371/journal.pone.0225594 (2019).
Okonko, B. J., Cookey, T. I., Okonko, I. O. & Ogbu, O. Prevalence of rubella IgG antibodies among pregnant women in Rivers State, Nigeria. J. Adv. Med. 32, 49–58 (2020).
Olajide, O. M., Aminu, M., Randawa, A. J. & Adejo, D. S. Seroprevalence of rubella-specific IgM and IgG antibodies among pregnant women seen in a tertiary hospital in Nigeria. Int. J. Women’s Health 7, 75–83 (2015).
Koki, Y. A. et al. Sero-prevalence of rubella virus IgM antibodies among pregnant women attending Muhammadu Abdullahi Wase Specialist Hospital Kano. CAS 2, 141–148 (2014).
Tulu, B., Mekonnen, D., Amsalu, E., Zenebe, Y. & Getahun, M. Rubella virus sero-prevalence and associated factors among non-vaccinated pregnant women in Northwest Ethiopia. Ethiop. J. Health Dev. 3, 32 (2018).
Mirambo, M. M., Aboud, S., Majigo, M., Groβ, U. & Mshana, S. E. Adverse pregnancy outcomes among pregnant women with acute Rubella infections in Mwanza city, Tanzania. Int. J. Infect. Dis. 78, 72–77. https://doi.org/10.1016/j.ijid.2018.10.020 (2019).
Jonas, A. et al. Rubella immunity among pregnant women aged 15–44 years, Namibia. Int. J. Infect. Dis. 49, 196–201 (2016).
Lulandala, L., Mirambo, M. M., Matovelo, D., Gumodoka, B. & Mshana, S. E. Acute rubella virus infection among women with spontaneous abortion in Mwanza City, Tanzania. J. Clin. Diagn. Res. 11, Qc25–Qc27. https://doi.org/10.7860/jcdr/2017/22634.9544 (2017).
Tahita, M. C. et al. Rubella seroprevalence among pregnant women in Burkina Faso. BMC Infect. Dis. 13, 164 (2013).
Acknowledgements
We acknowledge Hawassa University for supporting the study with finance. Furthermore, our acknowledgement goes to Halaba Zone Health Department for the support of the study. We also thank the study participants who agreed to take part in the research as well as the study staff for helping us collect the data.
Author information
Authors and Affiliations
Contributions
B.A., T.S., A.A.A., and S.H. contributed to the design of the study. B.A. participated in the laboratory analysis. All authors contributed to the statistical analyses, interpretation of results and write-up and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Asrat, B., Shimelis, T., Assefa, A.A. et al. Seroprevalence of rubella virus infection among antenatal care clients of Halaba Town public health facilities, southern Ethiopia. Sci Rep 13, 7220 (2023). https://doi.org/10.1038/s41598-023-34444-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-34444-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.