Abstract
It is unclear if associations between cardiorespiratory fitness (CRF) and cardiometabolic risk factors are independent of degree of obesity, in children with obesity. The aim of this cross-sectional study on 151 children (36.4% girls), 9–17 years, from a Swedish obesity clinic, was to investigate associations between CRF and cardiometabolic risk factors, adjusted for body mass index standard deviation score (BMI SDS), in children with obesity. CRF was objectively assessed with the Åstrand-Rhyming submaximal cycle ergometer test, and blood samples (n = 96) and blood pressure (BP) (n = 84) according to clinical routine. Obesity specific reference values for CRF were used to create CRF levels. CRF was inversely associated with high-sensitivity C-reactive protein (hs-CRP), independent of BMI SDS, age, sex, and height. The inverse associations between CRF and diastolic BP did not remain significant when adjusted for BMI SDS. CRF and high-density lipoprotein cholesterol became inversely associated when adjusted for BMI SDS. Independent of degree of obesity, lower CRF is associated with higher levels of hs-CRP, as a biomarker of inflammation, in children with obesity and regular assessment of CRF should be encouraged. Future research in children with obesity should investigate if low-grade inflammation decreases when CRF is improved.
Similar content being viewed by others
Introduction
In children, obesity is related to an increased risk of all-cause mortality, already in young adulthood1,2. Pediatric obesity affects the present cardiometabolic risk e.g., increased blood pressure, blood lipids, and inflammatory markers3,4,5. Cardiorespiratory fitness (CRF) is an important marker of health in children6 and low levels of CRF are associated with increased cardiometabolic risk factors7,8,9,10. Adiposity, in terms of body mass index (BMI), waist circumference, and body fat percentage, seems to have a greater impact on cardiometabolic risk in contrast to CRF11,12,13 but results are inconsistent regarding if CRF is independently associated with cardiometabolic risk when adjusted for adiposity10,11,12,14. However, some studies suggest that a high CRF is associated with lower cardiometabolic risk, foremost in children with obesity8,13.
Having a CRF equal to or below the lowest quartile or quintile of a reference population is commonly used as a cut-off for low CRF15,16. Most children with obesity have a low CRF relative to body weight and significantly lower CRF compared with a normal pediatric population17, therefore, the classification of high and low CRF based on a normal population is of limited discriminating clinical value. To facilitate the clinical interpretation o CRF among children with obesity our research group has published age- and sex specific reference values for CRF in this group of children18. The reference values are based on CRF assessments, according to the Åstrand-Rhyming submaximal cycle ergometer test19, in 705 Swedish children with obesity and CRF percentiles are presented for both absolute maximal oxygen uptake (VO2max, L/min), and for VO2max relative to body weight (mL/kg/min)18.
To the best of our knowledge, it has not yet been explored if associations between cardiometabolic risk factors and CRF are present when studying solely children with obesity and adjusting for degree of obesity, in terms of BMI standard deviation score (SDS). Further it remains to be established to which extent different levels of the obesity specific reference values for CRF, developed for clinical practice, are associated with cardiometabolic risk factors. Therefore, the aim of the study was to investigate potential associations between CRF, i.e., absolute- and relative VO2max, and cardiometabolic risk factors in children with obesity, and to evaluate how potential associations were affected when adjusted for BMI SDS.
Methods
Study design
This was a cross-sectional study of a cohort of children with obesity, starting treatment at Martina Children’s Hospital, in Stockholm, Sweden. The pediatric obesity clinic was established in August 2018 and assessment of CRF commenced in January 2019. Treatment initiation was preceded by clinical evaluation by staff in the obesity team. According to Swedish regulations, guardians and children received verbal and written information about data collection in the Swedish Childhood Obesity Treatment Register (BORIS)20, and this study was based on data from BORIS. Informed opt-out consent was obtained from legal guardians, i.e., if the legal guardians did not disapprove, data on height, weight, CRF, blood pressure (BP), and blood samples were registered in BORIS by administrative or clinical staff. The study was approved by the Regional Ethical Committee in Stockholm, Sweden no. 2018/1413-31 and all methods were performed in accordance with relevant guidelines and regulations.
Participants
Eligible participants were 9–17 years old and had their first appointment between January 2019 and August 2021. Additional inclusion criteria were having obesity according to the International Obesity Task Force (IOTF)21, completing a submaximal cycle ergometer test19 with a heart rate (HR) ≥ 120 beats per minute (bpm) at the end of the test, and having collected blood samples or BP within 45 days of the performed cycle ergometer test. Children with genetic syndromes, diabetes, heart- or blood disorders or with diagnosed thyroid disease were excluded. Further, children using central nervous system stimulants, long-acting beta2-agonists, or short-acting beta2-agonists (the latter the same day as performing the cycle ergometer test) were not eligible for inclusion. Since this was an explorative study no sample size calculation was conducted.
Measures and outcomes
At the first appointment to a physiotherapist, anthropometric measures and CRF were assessed. Height was measured without shoes to the nearest 0.1 cm using a stadiometer (Hyssna, Sweden) and weight was measured in light clothing to the nearest 0.1 kg (Seca 899, Germany). Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared (kg/m2). Calculation of BMI standard deviation score (SDS) was performed and categorization of degree of obesity (obesity and severe obesity) was based on the IOTF criteria21.
CRF, in terms of absolute and relative VO2max, was assessed by the Åstrand-Rhyming submaximal cycle ergometer (Monark 874E, Sweden) test19. HR was registered every minute by a chest-worn HR monitor (Polar T31, Sweden). Absolute VO2max (L/min) was estimated from workload and the mean HR. If HR differed more than five bpm between minute five and six, the test was prolonged until a constant level was reached. Since oxygen uptake differs in boys and girls, and maximal HR varies with age, absolute VO2max was adjusted for sex and age19, with a factor of 1.1 for participants 15 years and younger22, and according to Andersson23 for older participants. Relative VO2max (mL/kg/min) was calculated by dividing absolute VO2max (converted to mL/min) with weight. The Åstrand-Rhyming nomogram does not enable estimation of VO2max if the mean HR exceeds 140 or 148 bpm (boys and girls respectively) on the minimum workload of 50W/300 kpm, or > 170 bpm for higher workloads (both sexes).
At the first appointment to a pediatrician, BP was measured with an automatic BP monitor (Omron M2, Japan). National guidelines on measuring BP are provided by the Swedish Pediatric Society. A sex-, age-, and height- adjusted reference24 was applied when calculating standard deviations scores (SDS) for systolic- and diastolic BP (SBP and DBP). The same reference was used for calculating hypertension, defined as having either a DBP or SBP above the 95th percentile. Most children with obesity ≥ 9 years old were advised to provide blood samples before the first appointment to the pediatrician. Blood samples were collected at the participants’ local health care centers after nightly fast, and all blood sample analyses were conducted by Swedish laboratories with official authorization. Blood samples included parameters following the clinical routine i.e., alanine aminotransferase (ALT), fasting glucose, fasting insulin, glycated hemoglobin A1c (HbA1c), low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, total cholesterol, triglycerides, and high-sensitivity C-reactive protein (hs-CRP). Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as (fasting glucose [mmol/L] x fasting insulin [µU/L])/22.5.
Elevated ALT was defined as > 0.37 µkat/L in girls and > 0.44 µkat/L in boys25. Lipid profile was categorized as having low HDL cholesterol (< 1.0 mmol/L), elevated LDL cholesterol (≥ 2.8 mmol/L), elevated total cholesterol (≥ 4.4 mmol/L), and elevated triglycerides (≥ 0.8 mmol/L for 9-year-old participants and ≥ 1.0 mmol/L in participants ≥ 10 years)26. Low-grade inflammation (LGI) was defined as a hs-CRP > 3 to ≤ 10 mg/L27. A CRP > 10 mg/L was considered as an ongoing high-grade inflammation28 and therefore excluded from analyses (n = 1). Impaired fasting glucose (IFG) was defined as a fasting glucose ≥ 6.1 mmol/L29.
Statistical analysis
Continuous data are presented with mean and standard deviation (SD) or with median and interquartile range (IQR) based on data distribution. Categorical variables are presented with frequency and/or percentage. Comparisons of two groups (included vs. subgroups of excluded children; valid estimation of VO2max vs. HR too high for estimating VO2max) were analyzed with the Student’s t-test, the Mann–Whitney U test, or the Chi-square test. For all other analyses, solely participants with a valid estimation of VO2max were studied.
To investigate associations between cardiometabolic risk and CRF (i.e., absolute- and relative VO2max), linear regressions were conducted with cardiometabolic risk factors as dependent variables, and absolute VO2max (L/min) or relative VO2max (mL/kg/min) as independent variables. CRF was adjusted for age, sex, and height (Model 1) as well as BMI SDS, age, sex, and height (Model 2). HOMA-IR, fasting insulin, hs-CRP, ALT, HDL, and triglycerides were logarithmically transformed to meet the assumptions of linear regression. For linear regressions the unstandardized beta is presented.
Participants were categorized into three CRF levels based on reference values for children with obesity, according to sex and age group18. Differences of biochemical markers and BP between the three groups were analyzed with one-way analysis of variance (ANOVA) with the Tukey’s post-hoc test, or the Kruskal Wallis test with the Bonferroni post-hoc test. To compare proportions of the outcome variables between the three groups, Chi-square test was applied. If significant, pairwise comparisons of predictive margins, adjusted with the Bonferroni method were further conducted to identify the difference between two groups. One-way analysis of covariance (ANCOVA), with Bonferroni correction, was used to compare differences in logarithmically transformed hs-CRP between the three CRF groups, adjusted for BMI SDS. All p-values for comparing proportion, mean, and rank (Tables 2 and 3) were adjusted for multiple testing by multiplying the uncorrected p-values with number of tests. A p-value of < 0.05 was considered as statistically significant. IBM SPSS version 28 (IBM SPSS Armonk, NY, USA) was used for all calculations except for pairwise comparisons of predictive margins where STATA version 16 (StataCorp, College Station, TX, USA) was used.
Results
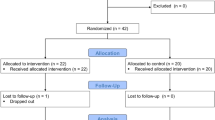
A flowchart for identification of inclusion and exclusion is presented in Fig. 1. Of the 151 included subjects, with a mean age of 13.1 (SD 1.9) years, 36.4% were girls. Of the participants with data on migrant background (86%), 61% had one or two parents born outside Scandinavia, and 47% came from countries outside Europe. Asthma was present in 15 subjects (10%) and 14 participants (9%) were diagnosed with attention deficit hyperactivity disorder or attention deficit disorder without ongoing pharmaceutical treatment. A valid estimation of VO2max was present in 81% (n = 122) of the subjects. of which blood samples were taken in 96 individuals and BP in 84 subjects. Of these participants, 58 children had available data on both blood samples and BP and the other children are unique for each group (blood samples vs BP, Fig. 1). The median (IQR) time between the cycle ergometer test and blood samples were 0 (20) days, and 0 (32) days for measured BP.
Linear regressions (Table 1) showed that Log10 hs-CRP was inversely associated with relative VO2max (β = − 0.022, p = 0.013) and absolute VO2max (β = − 0.238, p = 0.015) when adjusted for BMI SDS (Model 2). In other words, an increment in relative VO2max by 1 mL/kg/min is associated with a 4.9% lower hs-CRP, and for absolute VO2max an increase by 1 L/min is associated with a 42.2% lower hs-CRP value. Model 2 showed inverse associations between Log10 HDL and relative VO2max (β = − 0.005, p = 0.005), and absolute VO2max (β = − 0.053, p = 0.026), but no significant associations were found in Model 1. Log10 fasting insulin, Log10 HOMA-IR, Log10 ALT, fasting glucose, HbA1c, Log10 triglycerides, LDL-, and total cholesterol were not significantly associated with CRF in any of the adjustment models. Of participants with data on BP (n = 84), a significant inverse association between relative VO2max and DBP SDS in Model 1 did not remain significant in Model 2. No significant associations could be detected between CRF and SBP SDS.
Group comparisons between CRF levels of relative VO2max (mL/kg/min) are presented in Tables 2, 3. Hs-CRP increased for each group with lower CRF, where the group with lowest CRF had a median (IQR) of 4.0 (3.0) mg/L vs 1.5 (2.3) mg/L in the group with highest CRF (p = 0.021).
The one-way ANCOVA (Levene’s test p = 0.04) showed that the significant difference of Log10 hs-CRP between the lowest and the highest CRF levels remained when adjusted for BMI SDS (p = 0.026). The same trend was seen in LGI, but group differences were non-significant (p = 0.063). The lower CRF-level, the higher proportion of severe obesity and mean BMI SDS were found. For the other biomarkers, BP, or descriptive variables no group differences could be detected (Tables 2, 3). For absolute VO2max (L/min), no significant group differences were found for biochemical markers, BP, age, sex, or BMI SDS.
Data on study participants and subgroups of excluded subjects are presented in Table 4. The excluded group without available data on blood samples and BP (n = 37) were younger (p = 0.017) and had lower BMI SDS (p < 0.001) compared with included subjects (n = 151). The excluded subgroup with blood samples and BP assessed more than 45 days from the cycle ergometer test (n = 77) had significantly fewer individuals with a HR too high for estimating VO2max (p = 0.024). The included subjects had a significantly lower mean value of relative VO2max (mL/kg/min) compared with the other groups (p = 0.044 and p = 0.026).
For 29 (19%) of the included participants, HR during the cycle ergometer test was too high to estimate VO2max with the Åstrand-Rhyming nomogram. These individuals were significantly younger compared to other participants (mean (SD) age of 11.6 (1.7) vs. 13.5 (1.8), p < 0.001) and within the group, 22 (76%) individuals were younger than 12 years old. Further, the group with high HR had higher HDL cholesterol levels with a median (IQR) of 1.3 (0.3) vs. 1.2 (0.3), p = 0.005. No other significant group differences could be detected.
Discussion
In this cross-sectional study of children and adolescents with obesity, we found an inverse association between cardiorespiratory fitness and hs-CRP, that was independent of BMI SDS. CRF (mL/kg/min) equal to or below the 25th percentile, according to reference values in children with obesity18, entailed significantly higher levels of inflammation, in terms of increased hs-CRP. These results add to existing literature by investigating the influence of CRF on hs-CRP within children with obesity, including the continuous variable of BMI SDS as an adjustment for the impact of obesity.
Elevated inflammatory markers are associated with obesity-related disease in children5,30. LGI in individuals with obesity is involved in the pathogenesis of multiple severe health conditions such as autoimmune diseases, atherosclerosis, and cardiovascular disease31,32,33. Exercise training has been found to reduce hs-CRP in adults, independent of BMI reduction34. The mechanisms behind the effects of exercise training on LGI are not fully understood, however, several pathways are suggested to be involved, including muscle- and adipose tissue as well as endothelial- and immune cells35. In children, the current literature is mixed regarding if hs-CRP is associated with CRF when adjusted for adiposity10,36,37,38,39, however, previous studies mainly included children with normal weight. Since children with obesity have significantly higher prevalence of low-grade inflammation compared to children with normal weight5 it is important to study children with obesity separately. In a pooled cross-sectional analysis of 1 706 adolescents, Agostinis-Sobrinho et al.40 found that high CRF attenuated the association between hs-CRP and BMI, mainly for the adolescents with obesity. Similar findings, where high CRF was associated with a lower cardiometabolic risk score solely in children with obesity, have been presented by Nyström et al.8. This indicates that CRF may be important for decreasing cardiometabolic risk, especially in children with obesity.
In contrast to other studies41,42 we found no associations between VO2max (mL/kg/min) and SBP SDS, ALT, fasting insulin, HOMA-IR, fasting glucose, HbA1c, triglycerides, and LDL-, or total cholesterol. This may partly be explained by the relatively small study population. Further, the inclusion of solely children with obesity may have resulted in a narrower distribution of cardiometabolic risk outcomes compared with including children of different weight status. HDL cholesterol was inversely associated with CRF, but only when adjusted for BMI SDS. Further, when CRF was categorized no differences in HDL distribution could be detected between the different CRF levels. Additional studies with larger study populations should be conducted to either confirm or reject our findings.
Similarly to hs-CRP and CRF, the literature is mixed regarding if CRF is independently associated with other measures of cardiometabolic risk in children10,11,12,14. The diverse findings in the current literature may be explained by how adiposity and CRF is measured43. The widely used measurement of BMI and VO2max relative to body weight (assessed by indirect exercise tests), have been criticized for being imprecise measures of adiposity and CRF44. Fat free mass (FFM) is independent of adiposity and highly influential on VO2max in children and therefore a better indicator of physiological ability to maximally consume oxygen44,45. However, findings also differed when VO2max relative to FFM was used in adolescents and young adults of different weight status, from being significantly associated with46 or not associated with cardiometabolic risk factors43. However, when solely children with obesity were studied, VO2max relative to FFM was positively associated with insulin sensitivity9. Precise measures of adiposity, FFM (e.g., by dual-energy X-ray absorptiometry) and CRF (i.e., direct VO2max tests including analysis of gas exchange) are expensive and therefore not applicable in population-based or clinical settings. Moreover, direct VO2max assessment is highly dependent on motivation to get a valid test result and therefore difficult to use in untrained children. Nevertheless, assessing CRF among children in obesity treatment serves many purposes—from assessing functional ability to evaluating effects from exercise prescription. Moreover, since CRF is an important marker for health in children, clinically available assessments of CRF and adiposity are essential. However, since our findings were based on VO2max relative to body weight and adjusted for BMI SDS, the inverse relationship between hs-CRP and CRF may not be fully independent of adiposity.
No significant group differences were found between absolute VO2max and BMI SDS, BP, or any of the analyzed biochemical markers. Since absolute VO2max in children with obesity are similar, or higher than values from a general population17,45, these findings were not surprising. Although absolute VO2max should be reported to understand changes in CRF, VO2max relative to body weight or FFM serves a better purpose when comparing differences between individuals45,47.
Subjects included in this study differed significantly from excluded subgroups. Individuals without available blood samples or BP were younger and had a lower BMI SDS. This suggests that the clinical evaluation resulted in that blood samples for the identification of co-morbidities were not indicated. Because of the COVID-19 pandemic, appointments to the physiotherapist and pediatrician were further apart than intended, which may be a reason to why 77 children had BP and blood samples taken more 45 days from the performed cycle test. The studied population had lower relative VO2max (mL/kg/min) than the excluded subgroups; hence, the detected associations between CRF and cardiometabolic risk may have been stronger if patients at the obesity clinic with higher CRF levels could have been included.
The Åstrand-Rhyming cycle ergometer test was not developed for children19 and the test has not been validated for children with obesity. In children with normal weight the Åstrand-Rhyming test has similar validity compared with other indirect tests for CRF48. As previously stated in this discussion, indirect tests have several advantages and for children with obesity cycle tests enable assessment of CRF in those experiencing pain when walking or running. In our study, participants with a HR too high to estimate VO2max were significantly younger than the other subjects, and 76% of these children were younger than 12 years old. This indicates that the Åstrand-Rhyming test is not optimal for the youngest children, which is in accordance with previous findings18. Nevertheless, using the test in younger children may still be of clinical importance to assess level of exertion, changes in HR and ability to maintain speed. For children 12 years and older, a HR too high to estimate VO2max may indicate that CRF levels are extremely low.
Several limitations to this study have previously been stated in the discussion. Additionally, this study is limited using clinical data resulting in a time difference between assessed BP together with blood samples and CRF. However, weight changes during the BP and blood sample collection, if any, is believed to be modest. Further, our results are in line with previous findings that CRF attenuate the effects of obesity on cardiometabolic risk in children8,9,13,40. In accordance with many studies reporting fitness outcomes in children11,49,50, this study is limited by not having available data on pubertal stage—a variable related to CRF in children44.We strived to eliminate other confounders for CRF and cardiometabolic risk factors by excluding children with diseases or medications affecting HR, BP, or biochemical markers. The inclusion of solely children with obesity, of different migrant background, was a strength of this study. That our findings are presented in relation to reference values for CRF in children with obesity, enable interpretation of CRF assessed in a clinical setting. This will allow further investigation regarding if also minor improvements of CRF in children with obesity could decrease hs-CRP.
Clinical implications
Our results indicate that there is an association between lower CRF and increased hs-CRP in children with obesity. Low-grade inflammation in young children seems to be an important factor behind the association between pediatric obesity and autoimmune disease and cancers later in life32,51. Inflammation is also involved in the development of atherosclerosis and type 2 diabetes31,33,51. Thus, it is of major importance to reduce inflammation in young children with obesity. Exercise training in adults can reduce inflammation34 and it is likely, but not yet demonstrated, that improvements of CRF according to the reference values in children18, would reduce inflammatory markers. Previous findings indicate that high-intensity interval training may improve CRF more than moderate-intensity continuous training, in children with obesity49. However, for children with obesity, increased physical activity can be challenging—especially high-intensity exercise. Further, it is likely a high individual variation to which extent physical activity will improve CRF. Therefore, CRF and markers for low-grade inflammation should be assessed regularly in children with obesity. The reference values of CRF in this population18 could be helpful for initial clinical evaluation to obtain an understanding of the patient’s CRF health status.
Conclusions
When using recently developed reference values for CRF, which enable improved grading of CRF for children with obesity, we found that children with lower CRF had more pronounced signs of inflammation in terms of increased hs-CRP, regardless of degree of obesity. As low-grade inflammation early in life is of major importance for later development of obesity related comorbidities, regular assessment of CRF and exercise interventions to increase CRF should be encouraged. Future research, including exercise interventions in children with obesity, are needed to investigate to which extent low-grade inflammation decreases when CRF is improved.
Data availability
The datasets are available from the corresponding author on reasonable request.
Abbreviations
- CRF:
-
Cardiorespiratory fitness
- BP:
-
Blood pressure
- hs-CRP:
-
High-sensitivity C-reactive protein
- HDL:
-
High-density lipoprotein
- LDL:
-
Low-density lipoprotein
- BMI:
-
Body mass index
- SDS:
-
Standard deviation score
- VO2max :
-
Maximal oxygen uptake
- BORIS:
-
Swedish Childhood Obesity Treatment Register
- IOTF:
-
International Obesity Task Force
- HR:
-
Heart rate; bpm, beats per minute
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- ALT:
-
Alanine aminotransferase
- HbA1c:
-
Glycated hemoglobin A1c
- HOMA-IR:
-
Homeostasis model assessment of insulin resistance
- LGI:
-
Low-grade inflammation
- IFG:
-
Impaired fasting glucose
References
Lindberg, L., Danielsson, P., Persson, M., Marcus, C. & Hagman, E. Association of childhood obesity with risk of early all-cause and cause-specific mortality: A Swedish prospective cohort study. PLoS Med. 17, e1003078. https://doi.org/10.1371/journal.pmed.1003078 (2020).
Twig, G. et al. Body-mass index in 2.3 million adolescents and cardiovascular death in adulthood. N. Engl. J. Med. 374, 2430–2440. https://doi.org/10.1056/NEJMoa1503840 (2016).
Skinner, A. C., Perrin, E. M., Moss, L. A. & Skelton, J. A. Cardiometabolic risks and severity of obesity in children and young adults. N. Engl. J. Med. 373, 1307–1317. https://doi.org/10.1056/NEJMoa1502821 (2015).
Hagman, E., Danielsson, P., Elimam, A. & Marcus, C. The effect of weight loss and weight gain on blood pressure in children and adolescents with obesity. Int. J. Obes. 2005(43), 1988–1994. https://doi.org/10.1038/s41366-019-0384-2 (2019).
Lund, M. A. V. et al. Low-grade inflammation independently associates with cardiometabolic risk in children with overweight/obesity. Nutr. Metab. Cardiovasc. Dis. 30, 1544–1553. https://doi.org/10.1016/j.numecd.2020.04.024 (2020).
Ortega, F. B., Ruiz, J. R., Castillo, M. J. & Sjostrom, M. Physical fitness in childhood and adolescence: A powerful marker of health. Int. J. Obes. 2005(32), 1–11. https://doi.org/10.1038/sj.ijo.0803774 (2008).
Anderssen, S. A. et al. Low cardiorespiratory fitness is a strong predictor for clustering of cardiovascular disease risk factors in children independent of country, age and sex. Eur. J. Cardiovasc. Prev. Rehabil. 14, 526–531. https://doi.org/10.1097/HJR.0b013e328011efc1 (2007).
Nystrom, C. D. et al. Does cardiorespiratory fitness attenuate the adverse effects of severe/morbid obesity on cardiometabolic risk and insulin resistance in children? A Pooled Analysis. Diabetes care 40, 1580–1587. https://doi.org/10.2337/dc17-1334 (2017).
Morinder, G., Larsson, U. E., Norgren, S. & Marcus, C. Insulin sensitivity, VO2max and body composition in severely obese Swedish children and adolescents. Acta Paediatr. 98, 132–138. https://doi.org/10.1111/j.1651-2227.2008.01030.x (2009).
Martinez-Gomez, D. et al. Associations of physical activity, cardiorespiratory fitness and fatness with low-grade inflammation in adolescents: The AFINOS Study. Int. J. Obes. 2005(34), 1501–1507. https://doi.org/10.1038/ijo.2010.114 (2010).
Perez-Bey, A. et al. The influence of cardiorespiratory fitness on clustered cardiovascular disease risk factors and the mediator role of body mass index in youth: The UP&DOWN Study. Pediatr. Diabetes 20, 32–40. https://doi.org/10.1111/pedi.12800 (2019).
Wang, P. G. et al. Relationship of body fat and cardiorespiratory fitness with cardiovascular risk in Chinese children. PLoS One 6, e27896. https://doi.org/10.1371/journal.pone.0027896 (2011).
Shang, X. et al. Independent and interactive associations of fitness and fatness with changes in cardiometabolic risk in children: A longitudinal analysis. Front. Endocrinol. 11, 342. https://doi.org/10.3389/fendo.2020.00342 (2020).
Zaqout, M. et al. Influence of physical fitness on cardio-metabolic risk factors in European children. The IDEFICS study. Int. J. Obes. 2005(40), 1119–1125. https://doi.org/10.1038/ijo.2016.22 (2016).
American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription (Wolters Kluwer, 2018).
Ruiz, J. R. et al. Cardiorespiratory fitness cut points to avoid cardiovascular disease risk in children and adolescents; What level of fitness should raise a red flag? A systematic review and meta-analysis. Br. J. Sports Med. 50, 1451–1458. https://doi.org/10.1136/bjsports-2015-095903 (2016).
Berndtsson, G., Mattsson, E., Marcus, C. & Larsson, U. E. Age and gender differences in VO2max in Swedish obese children and adolescents. Acta Paediatr. 96, 567–571. https://doi.org/10.1111/j.1651-2227.2007.00139.x (2007).
Johansson, L., Brissman, M., Morinder, G., Westerståhl, M. & Marcus, C. Reference values and secular trends for cardiorespiratory fitness in children and adolescents with obesity. Acta Paediatr. 109, 1665–1671. https://doi.org/10.1111/apa.15163 (2020).
Astrand, I. Aerobic work capacity in men and women with special reference to age. Acta Physiol. Scand. Suppl. 49, 1–92 (1960).
Hagman, E., Danielsson, P., Lindberg, L. & Marcus, C. Paediatric obesity treatment during 14 years in Sweden: Lessons from the Swedish Childhood Obesity Treatment Register-BORIS. Pediatr. Obes. https://doi.org/10.1111/ijpo.12626 (2020).
Cole, T. J. & Lobstein, T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr. Obes. 7, 284–294. https://doi.org/10.1111/j.2047-6310.2012.00064.x (2012).
Astrand, P.O. & Rodahl, K. Textbook of work physiology, 2nd ed (McGraw-Hill, 1977).
Andersson, G., Forsberg, A. & Malmgren, S. Konditionstest på cykel : [testledarutbildning]. Rev. [utg.] edn, (SISU idrottsböcker, 2005).
National high blood pressure education program working group on high blood pressure in children and adolescents. Fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114, 555–576 (2004).
Vos, M. B. et al. NASPGHAN clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children: Recommendations from the expert committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J. Pediatr. Gastroenterol. Nutr. 64, 319–334. https://doi.org/10.1097/mpg.0000000000001482 (2017).
De Jesus, J. M. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. Pediatrics 128(Suppl 5), S213-256. https://doi.org/10.1542/peds.2009-2107C (2011).
Sørensen, C. J. et al. Combined oral contraception and obesity are strong predictors of low-grade inflammation in healthy individuals: results from the Danish Blood Donor Study (DBDS). PLoS One 9, e88196. https://doi.org/10.1371/journal.pone.0088196 (2014).
Imhof, A. et al. Distributions of C-reactive protein measured by high-sensitivity assays in apparently healthy men and women from different populations in Europe. Clin. Chem. 49, 669–672. https://doi.org/10.1373/49.4.669 (2003).
World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: Report of a WHO/IDF consultation (2006).
Skinner, A. C., Steiner, M. J., Henderson, F. W. & Perrin, E. M. Multiple markers of inflammation and weight status: Cross-sectional analyses throughout childhood. Pediatrics 125, e801-809. https://doi.org/10.1542/peds.2009-2182 (2010).
Geovanini, G. R. & Libby, P. Atherosclerosis and inflammation: Overview and updates. Clin. Sci. (Lond.) 132, 1243–1252. https://doi.org/10.1042/cs20180306 (2018).
Umano, G. R. et al. Pediatric obesity and the immune system. Front. Pediatr. 7, 487. https://doi.org/10.3389/fped.2019.00487 (2019).
Donath, M. Y. & Shoelson, S. E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 11, 98–107. https://doi.org/10.1038/nri2925 (2011).
Fedewa, M. V., Hathaway, E. D. & Ward-Ritacco, C. L. Effect of exercise training on C reactive protein: A systematic review and meta-analysis of randomised and non-randomised controlled trials. Br. J. Sports Med. 51, 670–676. https://doi.org/10.1136/bjsports-2016-095999 (2017).
You, T., Arsenis, N. C., Disanzo, B. L. & Lamonte, M. J. Effects of exercise training on chronic inflammation in obesity: Current evidence and potential mechanisms. Sports Med. 43, 243–256. https://doi.org/10.1007/s40279-013-0023-3 (2013).
González-Gil, E. M. et al. Improving cardiorespiratory fitness protects against inflammation in children: The IDEFICS study. Pediatr. Res. 91, 681–689. https://doi.org/10.1038/s41390-021-01471-0 (2022).
Steene-Johannessen, J., Kolle, E., Andersen, L. B. & Anderssen, S. A. Adiposity, aerobic fitness, muscle fitness, and markers of inflammation in children. Med. Sci. Sports Exerc. 45, 714–721. https://doi.org/10.1249/MSS.0b013e318279707a (2013).
Garcia-Hermoso, A. et al. Adiposity as a full mediator of the influence of cardiorespiratory fitness and inflammation in schoolchildren: The FUPRECOL Study. Nutr. Metab. Cardiovasc. Dis. 27, 525–533. https://doi.org/10.1016/j.numecd.2017.04.005 (2017).
Llorente-Cantarero, F. J. et al. Non-traditional markers of metabolic risk in prepubertal children with different levels of cardiorespiratory fitness. Public Health Nutr. 15, 1827–1834. https://doi.org/10.1017/s1368980011003533 (2012).
Agostinis-Sobrinho, C. et al. Higher cardiorespiratory fitness levels may attenuate the detrimental association between weight status, metabolic phenotype and C-reactive protein in adolescents-a multi-cohort study. Nutrients https://doi.org/10.3390/nu12051461 (2020).
Pozuelo-Carrascosa, D. P. et al. Obesity as a mediator between cardiorespiratory fitness and blood pressure in preschoolers. J. Pediatr. 182, 114-119.e112. https://doi.org/10.1016/j.jpeds.2016.11.005 (2017).
Jones, P. R. et al. Cross-sectional and prospective associations between aerobic fitness and lipoprotein particle profile in a cohort of Norwegian schoolchildren. Atherosclerosis 321, 21–29. https://doi.org/10.1016/j.atherosclerosis.2021.02.002 (2021).
Hinriksdóttir, G., Tryggvadóttir, Á., Ólafsdóttir, A. S. & Arngrímsson, S. Fatness but not fitness relative to the fat-free mass is related to C-reactive protein in 18 year-old adolescents. PLoS One 10, e0130597. https://doi.org/10.1371/journal.pone.0130597 (2015).
Armstrong, N. & Welsman, J. Traditional and new perspectives on youth cardiorespiratory fitness. Med. Sci. Sports Exerc. 52, 2563 (2020).
Goran, M., Fields, D. A., Hunter, G. R., Herd, S. L. & Weinsier, R. L. Total body fat does not influence maximal aerobic capacity. Int. J. Obes. Relat. Metab. Disord. 24, 841–848 (2000).
Sævarsson, E. S., Magnússon, K. T., Sveinsson, T., Jóhannsson, E. & Arngrímsson, S. The association of cardiorespiratory fitness to health independent of adiposity depends upon its expression. Ann. Hum. Biol. 43, 229–234. https://doi.org/10.3109/03014460.2015.1042522 (2016).
Martin-Rincon, M. & Calbet, J. A. L. Progress update and challenges on VO2max testing and interpretation. Front. Physiol. 11, 1070. https://doi.org/10.3389/fphys.2020.01070 (2020).
Ekblom, O. Testing the validity of three submaximal ergometer tests for estimating maximal aerobic capacity in children. Acta Paediatr. 103, 559–563. https://doi.org/10.1111/apa.12582 (2014).
Cao, M., Tang, Y., Li, S. & Zou, Y. Effects of high-intensity interval training and moderate-intensity continuous training on cardiometabolic risk factors in overweight and obesity children and adolescents: A meta-analysis of randomized controlled trials. Int. J. Environ. Res. Public Health https://doi.org/10.3390/ijerph182211905 (2021).
Tomkinson, G. R. et al. European normative values for physical fitness in children and adolescents aged 9–17 years: Results from 2 779 165 Eurofit performances representing 30 countries. Br. J. Sports Med. 52, 1445–14563. https://doi.org/10.1136/bjsports-2017-098253 (2018).
Marcus, C., Danielsson, P. & Hagman, E. Pediatric obesity – Long-term consequences and effect of weight loss. J. Intern. Med. 292, 870 (2022).
Acknowledgements
We would like to thank all staff at Martina Children’s Hospital involved in data collection and registration of data in BORIS. We would further like to thank Emilia Hagman for data exportation from BORIS and Markus Brissman for valuable input.
Funding
Open access funding provided by Karolinska Institutet. This research was supported by grants provided by the Stockholm Freemasons’ Foundation of Children’s Welfare, HRH Crown Princess Lovisa’s Foundation for Pediatric Care, Mjölkdroppen Foundation, and by research, education, and intervention at Karolinska University Hospital.
Author information
Authors and Affiliations
Contributions
L.J. planned the study design together with C.M., M.H., and P.D. L.J. collected parts of the data, compiled data, and conducted analyses with support from R.R.P. L.J., R.R.P., and C.M. interpreted results. L.J. drafted the manuscript and coordinated contributions from all other authors. All the authors made critical comments and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Johansson, L., Putri, R.R., Danielsson, P. et al. Associations between cardiorespiratory fitness and cardiometabolic risk factors in children and adolescents with obesity. Sci Rep 13, 7289 (2023). https://doi.org/10.1038/s41598-023-34374-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-34374-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.