Abstract
This double-blind placebo-controlled cross-over study utilized comprehensive monitoring of blood bicarbonate (HCO3¯) kinetics and evaluation of gastrointestinal (GI) upset to determine their impact on an ergogenic potential of sodium bicarbonate (SB) co-ingested with carbohydrate (CHO). Nineteen CrossFit athletes performed 6 bouts of 15 s Wingate Anaerobic Test (WAnT) 90 min post-ingestion of 0.4 g·kg−1 body mass (BM) of SB (SB + CHO treatment) or PLA (PLA + CHO treatment) with 15 g CHO. Blood HCO3¯ concentration was evaluated at baseline, 30-, 60-, 75- and 90 min post-ingestion, in between WAnT bouts, and 3 and 45 min post-exercise, while GI upset at 120 min after protocol started. Control (no supplementation; CTRL) procedures were also performed. An effective elevation of extra-cellular buffering capacity was observed 60–90 min post-ingestion of SB + CHO. At mean peak blood HCO3¯, or at start of exercise an increase > 6 mmol·L−1 in HCO3¯ was noted in 84% and 52.6% participants, respectively. SB + CHO did not prevent performance decrements in WAnT bouts. There were no significant relationships between changes in blood HCO3¯ and WAnTs’ performance. Total GI was significantly higher in SB + CHO compared to CTRL, and stomach problems in SB + CHO compared to CTRL and PLA + CHO. There were inverse associations between peak- (p = 0.031; r = − 0.495), average- (p = 0.002; r = − 0.674) and minimum power (p = 0.008; r = − 0.585) and total GI upset, as well as average power and severe GI distress (p = 0.042; r = − 0.471) at SB + CHO. The implemented dose of SB + CHO was effective in improving buffering capacity, but did not prevent decrements in WAnTs’ performance. GI side effects were crucial in affecting the ergogenic potential of SB and thus must be insightfully monitored in future studies.
Similar content being viewed by others
Introduction
High-intensity functional training (HIFT) is derived from (1) well-studied high-intensity interval training (HIIT), in which relatively short bouts of high-intensity exercise separated by a recovery using low-intensity or inactivity are used as an alternative to traditional aerobic training to promote metabolic conditioning1,2, and (2) strength training paradigms3. HIFT training stresses both aerobic and anaerobic energy pathways, and develops power, strength, flexibility, speed, endurance, agility and coordination1. HIFT is executed at a high intensity and emphasizes functional, multi-joint movements via endurance, strength, power and speed stimulating exercises2,4.
The majority of studies focusing on HIFT methodology have used a cross-training template4,5. Cross-training’s specific modes of exercise (such as CrossFit’s programs) include power/Olympic lifting (i.e., squats, cleans, deadlifts, bench press, and push-presses), gymnastics (i.e., pull-ups, lunges, knees to elbows, handstand push-ups, push-ups, and sit-ups), and aerobic exercise/metabolic conditioning (i.e., swimming, running, and rowing). These high intensity exercise movements are often performed for specific lengths of time with little or no rest6.
Despite the growing popularity of HIFT and CrossFit training, limited interventional data exist on dietary and supplementation strategies to enhance performance in CrossFit7,8. So far, there are only two studies investigating the effectiveness of sodium bicarbonate (SB)—which in fact is one of the most widely studied ergogenic aids—on CrossFit-like performance9,10. According to the latest International Society of Sport Nutrition (ISSN) position stand11, supplementation with SB (doses from 0.2 to 0.5 g·kg−1 body mass < BM >) improves performance in muscular activities, various combat sports (e.g., boxing, judo, karate, taekwondo, wrestling), and in high-intensity cycling, running, swimming and rowing. The ergogenic effects of SB are mostly established for high-intensity exercise tasks that last between 30 s and 12 min11. With the latter in mind, SB may exert great ergogenic potential in HIFT such as CrossFit. During short-term high-intensity exercise, the intramuscular ATP demands exceed the maximum rate of ATP resynthesis by mitochondria. In these circumstances, ATP production heavily relies on anaerobic systems—phosphorylcreatine (ATP-PCr) hydrolysis or glycolysis. Intense exercises that are more reliant on glycolysis (i.e., those lasting from ~ 30 s to ~ 5 min) results in greater H+ generation11. Accumulation of H+ (leading to muscle acidosis and a decrease in muscle pH) has been shown to be an important contributor to fatigue due to its inhibitory effects on key glycolytic enzymes11, depressing Ca2+ sensitivity, as well as direct effects on cross-bridge cycling11. SB ingestion elicits ergogenic effect by increasing the concentration of blood bicarbonate, a buffer that contribute to maintain extra- and (indirectly) intracellular pH11. In the stomach, SB rapidly reacts with hydrochloric acid to form NaCl, CO2, and H2O; excess bicarbonate that does not neutralize gastric acid rapidly empties into the small intestine and is absorbed12. The reaction of NaHCO3 and HCl in gastric lumen increases the pH of the gastric lumen, which stimulates gastric parietal cells to secrete more HCl into the gastric lumen, thus resulting in the extrusion of HCO3¯ into the perigastric capillaries and eventually into the systemic circulation (one HCO3¯ ion on each H+ ion used in the reaction in the gastric lumen). The surplus HCO3¯ is eliminated via the kidney13 and carbon dioxide formed during this process is eliminated via the lungs14.
Numerous different protocols of SB supplementation have been investigated, however, so far only two protocols have been implemented in CrossFit-trained athletes9,10. One9 was a 10 day progressive-dose regime implemented in male and female CrossFit practitioners. The protocol resulted in overall improvement in discipline-specific performance (as measured during Fight Gone Bed test) by ~ 6.1% and enhancement in selected indices of aerobic capacity (as measured during incremental cycling). The chronic progressive supplementation regime prevented the occurrence of gastrointestinal (GI) side effects. The second dosing protocol10, was acute supplementation of 0.3 g·kg−1BM of SB ingested in three doses with 10 min intervals between doses, with the last dose ingested 60 min before exercise. This protocol neither improved discipline specific performance (as measured during ‘Cindy’ workout), nor altered hemodynamic and perceptual parameters. Moreover, no GI side effects were observed.
Although the performance benefits with SB are well documented, data linking the kinetics of blood HCO3¯, GI occurrence and anaerobic performance after ingestion of high doses of SB in conjunction with carbohydrate (CHO) in HIFT-trained athletes is lacking. Thus, it was hypothesized that the ingestion of 0.4 g·kg−1BM of SB + 15 g CHO ninety min before repeated-bouts of highly intensive exercise would result in the elevation of blood HCO3¯ above the almost certain ergogenic threshold (> 6 mmol·L−1)15,16 and minimize decrements of performance in consecutive bouts of the WAnT in CrossFit-trained athletes. It was also assumed that the conjunction of SB with small dose of CHO would minimize GI side effects.
Results
All the performed and presented results’ analyses of both primary and secondary outcomes covered 19 participants and the analyses were performed according to originally assigned groups.
Blood sample analysis
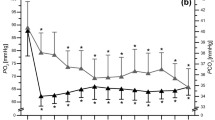
The intake of 0.4 g·kg−1BM of SB + 15 g CHO (SB + CHO) resulted in a mean peak of blood HCO3¯ (33.0 ± 2.5 mmol·L−1) 75 min post-ingestion (Fig. 1A), which was 15 min before the test exercises started (− 15′EX). As many as 79% of participants reached peak HCO3¯at this time point, while for the remaining participants, the pre-exercise peak HCO3¯ occurred between 60 < 5% > and 90 < 16% > min after ingestion, which was − 30′EX and 0′EX. The HCO3¯at CTRL and PLA + CHO at 75 min post-ingestion (− 15′EX) were 26.1 mmol·L−1 and 24.2 mmol·L−1 (substantially lower compared to SB + CHO and significantly different between PLA + CHO vs CTRL), and were not different from the corresponding pre-ingestion values (− 90′EX; CTRL: 26.0 mmol·L−1 and PLA + CHO: 25.4 mmol·L−1). HCO3¯ was clearly and effectively elevated after SB + CHO ingestion. Except the baseline measurement (− 90′EX), mean HCO3¯ at each measuring point at PLA + CHO and at CTRL were substantially lower compared to SB + CHO. Concentration of HCO3¯ at − 15′EX, − 0′EX and from WAnT_2POST to WAnT_6POST were significantly lower at PLA + CHO compared to CTRL.
Mean and individual (light-colour lines) blood bicarbonate (A), pH (B), lactate (C) values. Data are expressed as means (square), 95% CI (box), 95% CI + SD (whisker), raw data (light-colour lines). a,b,cDifferent lowercased letters refers to significant differences between study conditions (CTRL, SB + CHO, PLA + CHO); A–Gdifferent uppercased letters refers to significant differences between measuring time points (from − 90′EX to + 45′EX) within SB + CHO condition.
The mean peak change (increase) in HCO3¯ from baseline was + 7.8 ± 1.9 mmol·L−1 and was observed 75 min after ingestion of SB + CHO (− 15′EX; and 2 min before warm-up) (Fig. 2). Approximately 16% of participants’ peak increase in HCO3¯ was within the range 4–6 mmol·L−1 and 84% of participants had increases of > 6 mmol·L−1. The mean increase of HCO3¯ at 0′EX (90 min post-ingestion) was + 6.1 ± 1.9 mmol·L−1. In 9 participants (47.4%) the increase in HCO3¯ at this time point fell within range 4–6 mmol·L−1 and in the remaining 10 participants (52.6%) it was > 6 mmol·L−1. The increase in HCO3¯ at − 15′EX was substantially higher compared to remaining time points, and at − 30′EX and − 15′EX substantially higher compared to − 60′EX.
∆ of mean and individual (light-colour line) bicarbonate changes between baseline (pre-ingestion) and 30, 60, 75 and 90 min after SB + CHO or PLA + CHO intake or the first blood drawn (CTRL). Data are expressed as means (square), 95% CI (box), 95%CI + SD (whisker), raw data (light-colour lines). A–CDifferent uppercased letters refers to significant differences between measuring time points (from − 90′EX to + 45′EX) within SB + CHO condition.
There were no differences in baseline (pre-ingestion) pH values between study conditions (Fig. 1B). However, at each remaining time-point, the mean pH was substantially higher in SB + CHO compared to PLA + CHO and CTRL. Furthermore, at the points from − 30′EX to 0′EX and from WAnT_2POST to WAnT_6POST, pH was significantly higher in CTRL compared to PLA + CHO.
After SB + CHO ingestion the mean peak of blood pH (7.503 ± 0.039) was observed after the first bout of WAnT (WAnT_1POST; Fig. 1B), with ~ 42% of participants peaking at this point. The mean increase in pH at SB + CHO at WAnT_1POST from baseline was + 0.091 ± 0.037 and the absolute differences in pH at this time point between SB + CHO vs CTRL and SB + CHO vs PLA + CHO were + 0.100 ± 0.045 and + 0.121 ± 0.059. Furthermore, the peak pH increase (based on individual blood pH peaks reached at the individual time points) was + 0.106 ± 0.027.
There were no differences in lactate concentration between study conditions from − 90′EX to − 15′EX and at WAnT_1POST (Fig. 1C). At each remaining time point, lactate was higher after SB + CHO ingestion compared to lactate in PLA + CHO and CTRL (excluding WAnT_5POST). However, there were differences between CTRL and PLA + CHO at 0′EX, WAnT_5POST, WAnT_6POST and + 45′EX (recovery phase), with blood lactate higher in CTRL compared to PLA + CHO.
It should also be highlighted that at all time points after ingestion of SB + CHO compared to CTRL and PLA + CHO, the concentration of H+ was significantly lower, while base excess was significantly higher (Supplementary table S1). The results of remaining blood acid–base balance indices are given in Supplementary table S1.
The WAnT bouts performance
Within each of the study conditions (CTRL, SB + CHO, PLA + CHO), performance during the WAnTs decreased gradually in consecutive bouts (Fig. 3A–C). The PP in WAnT_3 to WAnT_6 was significantly lower compared to WAnT_1 and WAnT_2 within each of study conditions (Fig. 3A). Regarding AP (Fig. 3B), there was a decline from WAnT_1 to WAnT_4 in CTRL, and from WAnT_1 to WAnT_3 in SB + CHO and PLA + CHO, and remained stable (but significantly lower from previous bouts) between WAnT_5 and WAnT_6 in CTRL, and between WAnT_4 and WAnT_6 for SB + CHO and PLA + CHO. Moreover, TtoPP (Fig. 3C) significantly increased between WAnT_1 and WAnT_6 within the SB + CHO and PLA + CHO conditions. It was also significantly higher at WAnT_5 compared to WAnT_2 in CTRL.
Mean peak power (A), average power (B), time to peak power (C) during consecutive bouts of 15-s WAnTs. Data are expressed as means (square), 95% CI (box), 95% CI + SD (whisker), raw data. a–eDifferent lowercased letters refers to significant differences between the WAnT bouts (WAnT_1-WAnT_6); RM ANOVA models: †bouts x treatment, ǂbouts x treatment x treatment order and §bouts x treatment x gender).
There was no effect of treatment order on exercise performance (PP, AP, TtoPP) in individual bouts (WAnT_1-WAnT_6) of WAnT.
There was a significant treatment x WAnT bout main effect for PP. However, the main effects were no longer significant when treatment order (WAnT bouts x treatment x treatment order) or gender (WAnT bouts x treatment x gender) were considered in the models (Fig. 3A). None of the examined main effects were significant with respect to AP (Fig. 3B) or TtoPP (Fig. 3C) in any of the studied treatment conditions.
The mean PP of the 6 bouts of WAnT was significantly higher in SB + CHO and PLA + CHO compared to CTRL, and the main effect remained significant after including treatment order (Table 1), but not gender, in the model. Mean PD was significantly higher in SB + CHO and PLA + CHO compared to CTRL, but was no longer significant after considering treatment order or gender in the models. However, mean TtoPP was significantly lower in SB + CHO compared to CTRL (but not PLA + CHO), and the main effect remained significant when considering treatment order in the model.
Elevation of blood bicarbonate and anaerobic capacity
There were no significant linear relationships between changes in blood HCO3¯ from baseline to peak HCO3¯ concentration or changes from baseline and blood HCO3¯ at 0′EX, and PP, AP, MP, PD and TtoPP in consecutive individual bouts of WAnT (Supplementary Table S2).
Gastrointestinal side effects
Reporting of stomach problems was substantially higher in SB + CHO compared to CTRL and PLA + CHO (Table 2). Apart from that, there were no substantial differences in the intensification of any other of GI side effects between SB + CHO and PLA + CHO (Table 2). Symptoms such as flatulence, urge to defecate, belching or diarrhea, as well as total GI symptoms and less severe GI symptoms were higher after SB + CHO ingestion compared to CTRL.
Gastrointestinal side effects and anaerobic performance
There was an inverse relationship between mean PP, AP, MP and total GI symptoms in SB + CHO, and an inverse relation between MP and total GI symptoms in PLA + CHO (Table 3). The score of severe GI symptoms was inversely related to AP in SB + CHO and the score of less severe GI symptoms was positively correlated with TtoPP in PLA + CHO.
Discussion
In this study, mean peak blood bicarbonate (HCO3¯) occurred after 75 min following ingestion of SB + CHO, with 79% of participants peaking at this time point. There were clear decrements in performance during subsequent WAnTs, regardless of study condition. There was a significant main effect between conditions for PP, however it lost significance after including treatment order or gender in the models. Mean PP across the six WAnTs was higher with SB + CHO and PLA + CHO compared to CTRL, but there was no difference between SB + CHO and PLA + CHO. There were no associations between the level of elevation of blood HCO3¯ and WAnT performance. Severity of GI side effects were not significantly different between SB + CHO and PLA + CHO, but substantially higher compared to CTRL. However, there was an inverse interaction between GI side effects and performance indices (PP, AP, MP, PD) with SB + CHO, meaning GI worsened performance in WAnT.
In the current study after ingestion of 0.4 g·kg−1BM SB combined with 15 g CHO an effective and desirable plateau in an increased HCO3¯ concentration was achieved and maintained from − 30′EX (60 min post-ingestion) until WAnT_1POST, fluctuating between 31.4 and 33.0·mmol L−1. There was a non-significant reduction in blood HCO3¯ between 75 and 90 min after SB + CHO (− 1.6 ± 1.7 mmol·L−1) and PLA + CHO (− 1.6 ± 1.3 mmol·L−1) ingestion (or after baseline blood draw < − 1.9 ± 1.6 mmol·L−1 >) due to a standard 10 min warm-up (which took place between minutes 77 and 87). The reduction seems to be a physiological response to exercise, however, rarely taken into account with respect to its impact on buffering potential of ingested SB. The maintenance of increased level of blood HCO3¯ after the first bout of WAnT may arise from the fact that the metabolic perturbations elicited by maximal all-out exercises lasting less than 30 s are distinct from those occurring during intense exercise lasting from ~ 30 s to 5 min17.
Jones et al.15 observed that 75 min after ingestion of 0.3, 0.2 or 0.1 g·kg−1BM of SB, mean blood HCO3¯ was 31.7 ± 1.9, 30.1 ± 1.0, and 27.9 ± 1.3 mmol·L−1, while the mean blood HCO3¯ peaks occurred at 120 (~ 32.4 mmol·L−1), 90–105 (~ 30.5 mmol·L−1) and 105 (~ 28.1 mmol·L−1) min post-ingestion. In the study by Gough et al.18 the mean time to peak blood HCO3¯ after ingestion of 0.3 and 0.2 g·kg−1BM of SB ranged from 87 to 89 and 77 to 83 min. In general, it could be stated that the higher the dose of SB, the higher the peak HCO3¯ and the longer the time to reach this peak. Both the aforementioned studies15,18 investigated resting kinetics of blood HCO3¯ after SB intake. However, little is known about the kinetics of blood HCO3¯ when doses higher than 0.3 g·kg−1BM are ingested or protocols in which SB ingestion was combined with the intake of small amounts of CHO. Protocols which tested acute single SB doses equal to 0.4 g·kg−1BM are relatively scarce19,20,21,22,23,24,25,26,27, and unlike our protocol, most of them provided the acute dose of 0.4 g·kg−1BM split into smaller doses before exercise (e.g., 2 × 0.2 g·kg−1BM ingested 90 and 20 min23,25 or 90 and 60 min24 pre-exercise23,24,25) or over 1 h period from starting until the end of SB ingestion19), and no information on blood HCO3¯ kinetics is given. It seems reasonable to verify, if higher doses of ingested SB would also result in a longer time of sustaining a desirable and effective blood HCO3¯ elevation. In these circumstances, peak blood HCO3¯ might not be perceived as a single time point, but as a time frame or ‘peak bicarbonate window’. Indeed, in this study, HCO3¯ was elevated and remained stable for a 30 min period. It has been previously shown that in a rested condition ingestion of 0.3 g·kg−1BM of SB resulted in HCO3¯ increase > 6 mmol∙L−1 between 90 and 225 min28. Regarding practical applications, the individualized ingestion timing might be more important when implementing lower doses of SB (e.g., ≤ 0.2 g·kg−1BM). It is also worth mentioning, that when the HCO3¯ load exceeds the amount of acid in the stomach, HCO3¯ enters the intestine and reaches the jejunum, where it can be absorbed11. Thus, this phenomenon may partially explain the longer time to obtain peak HCO3¯ after consumption of high dose of SB compared to lower doses.
In the current study, SB was co-ingested with 15 g of CHO, which may have impacted the kinetics of blood HCO3¯. The addition of CHO was implemented based on previous literature, as a means to alleviate possible GI symptoms, and improving SB absorption. Carr et al.29, compared 8 different protocols of SB ingestion (0.3 g·kg−1BM), which differed in the form of supplement (solution or capsule), ingestion period (30 or 60 min), volume of fluid (7 or 14 mL·kg−1BM) and SB intake with a meal (containing 1.5 g CHO·kg−1BM). The most effective protocol for eliciting increases in blood HCO3¯ was SB supplementation in the form of capsules in conjunction with a CHO-rich meal, and when participants ingested the supplement within 30 min from commencement to completion of ingestion. Peak blood HCO3¯ (~ 30.9 mmol·L−1) was reached 150 min after ingestion, and the HCO3¯ elevation compared to baseline was ~ + 6.6 mmol·L−1. The analogous model which, however, did not include co-ingestion of the meal, resulted in achieving earlier (~ 120 min) peak blood HCO3¯ after SB intake. Thus, the co-ingestion of SB with CHO may delay reaching the peak of blood HCO3¯, and as a result separates the moment of peak HCO3¯ (which presumably refers to highest ergogenic effect) and the moment of peak GI symptoms (which results in performance decrements). However, the absolute dose of CHO provided in the current study was equal for each participant (15 g) and when expressed in relation to BM, was substantially lower than in the study by Carr et al.29. Thus, CHO impact on bicarbonate kinetics after ingestion of SB in the current study might be less pronounced compared to the study by Carr et al.29, and additionally affected by the warm-up which preceded the test exercise. Furthermore, in the current study, the GI side effects were evaluated only at a single time point, which was 120 min after SB + CHO or PLA + CHO intake. In the study by Gough et al.18, the time to peak GI upset was reached 36 and 29 min after ingestion of 0.3 and 0.2 g·kg−1BM. However, Carr et al.29, observed the greatest incidence of GI side effects 90 min after ingestion of 0.3 g·kg−1BM SB in a solution form. On the one hand, the great discrepancy with regard to peak GI upset after ingestion of the same dose of SB suggests the need for more precise investigation of that aspect in the future studies. On the other hand, due to its subjective nature GI symptoms are difficult to measure accurately.
It is also worth considering that not the absolute value of peak blood HCO3¯, but the actual increase (change) in blood bicarbonate from baseline to peak HCO3¯, or to the moment exercise is initiated, might be the crucial factor for extra-cellular buffering support and the ergogenic efficiency of SB. According to a recent meta-analysis16, greater effects in exercise were obtained when blood bicarbonate increases were medium (4–6 mmol·L−1) and large (> 6 mmol·L−1) compared to small (≤ 4 mmol·L−1). In the current study, the mean increase in blood bicarbonate from baseline to blood HCO3¯ peak after SB + CHO ingestion was 7.8 ± 1.9 mmol·L−1; 16% of participants reached an increase in blood HCO3¯ of 4–6 mmol·L−1, which according to the previous literature corresponds to a zone of a potential ergogenic effect, and 84% of participants reached the increase of > 6 mmol·L−1, which corresponds to an almost certain ergogenic effect. However, what seems to be more important, is the mean increase of blood bicarbonate at 0′EX which was 6.1 ± 1.9 mmol·L−1. In 9 participants (47.4%) the blood bicarbonate increase fell within the range of 4–6 mmol·L−1 at 0′EX (90 min post-ingestion) and in the remaining 10 participants (52.6%) it was > 6 mmol·L−1. However, in the current study, in accordance with previous findings30 no significant relationships between HCO3¯ increase and WAnTs performance were observed. The lack of simultaneous data on blood HCO3¯ time to peak and/or peak concentration increase and time to peak GI symptoms upset, could be perceive as the limitation of the current study.
Based on the results of the current study and assuming that the greatest ergogenic potential of SB is noted when blood HCO3¯ reach maximum and the elevation is > 6 mmol·L−1, the implemented dosage of SB + CHO should have been ingested at least 75 min before the exercises started, or individualized ingestion times should be established based on individual blood HCO3¯ peak increases. However, it should also be emphasized, that the slight depletion in HCO3¯ concentration is unavoidable, as a consequence of the warm-up, which in turn is indispensable and essential to perform before staring competition or main training31. Furthermore, the exclusion of occurrence of GI side effects (impairing SB-induced exercise performance benefits) presumably requires extending the time between SB intake and exercise.
The ingestion of 0.4 g·kg−1BM of SB + 15 g CHO ninety min before exercise did not prevent decrements in performance during consecutive bouts of 15-s WAnTs. To our knowledge, this the first study to implement such a demanding exercise protocol, comprising of 6 bouts of the WAnT with 30-s rest between bouts. In previous studies on SB supplementation, single32, two33,34,35, three36,37,38 or four39,40,41 bouts of 30-s WAnT were implemented. Similarly to our study, Zabala et al.37 found no impact of 0.3 g·kg−1BM of SB 90 min before exercise on performance during 3 bouts of 30-s WAnT separated by 30-min or 15-min rest periods in elite cyclists. Only one study utilizing 4 bouts of the WAnT in judoists following acute SB supplementation (0.3 g·kg−1BM of SB ingested 120 min before exercise) showed significantly higher AP (3rd and 4th bouts) and PP (4th bout) of upper limb WAnTs39. This SB supplementation timing could be entirely sufficient to avoid any GI disorders while effectively increasing the buffering capacity at the same time. This idea may be supported by the study by de Oliveira and colleagues40, where 5-day supplementation with 0.5 g·kg−1BM of SB (split into four equal doses per day) resulted in the overall improvement (as measured by total mechanical work) in four bouts of upper limb WAnTs. The last dose of SB was ingested 4 h before the performance assessment. Similarly, Tobias et al.41 found that 7 days supplementation of 0.5 g·kg−1BM of SB (no data of the last dose intake time before exercise) increased total work done in 4 bouts of 30-s upper-body WAnTs in combat sport athletes.
The undeniable and urgent need for controlling and quantifying the impact of GI side effects on performance after SB ingestion has been raised in two recent extensive papers on SB efficacy11,16. In general, the severity of GI side effects is dose-dependent, with higher doses resulting in more severe GI upset11,21, while studies indicate a variability in time to peak of GI upset18,29. Siegler et al.42 noted significant increases in the incidence of GI symptoms after ingestion of 0.3 g·kg−1BM of SB at 30, 60, 90, 120 and 150 min, but not 180 min post-ingestion compared to baseline. Simultaneously, the individual susceptibility to GI side effects and their severity should be taken into thoughtful consideration. In the current study, based on the IQR of total GI symptoms score (Table 2), large variability in occurrence and severity of GI upset between study participants was observed. Furthermore, there were some participants whose total GI symptoms score was higher (n = 2) or equal (n = 1) in CTRL compared to SB + CHO. This suggests that there may be some individuals who exhibit high levels of anxiety, which naturally predisposes them to GI distress in laboratory or field-based conditions, regardless of supplementation or familiarization procedures. Saunders and colleagues30 found a significant overall performance improvement after SB ingestion only when excluding participants (4 individuals out of 21 participants) who experienced GI discomfort. In the current study, based on regression analysis, we found a significant adverse impact of GI symptoms severity on WAnT performance. Thus, the impact of GI distress must be taken into consideration when analyzing the ergogenic potential of SB, and particularly when protocol assuming implementation of (1) single (2) acute (3) high (≥ 0.4 g·kg−1BM) doses of SB. Highly-motivated elite athletes may overcome GI upset and still benefit from high doses of SB supplementation, though this should be experimentally confirmed.
One of the suggested strategies to minimize GI side effects after SB ingestion, is to ingest SB alongside a high-carbohydrate meal11,29. In the current study we combined SB and PLA ingestion with a small CHO (15 g) dose, in an attempt to maximize tolerance to the relatively high SB dose. Though the implemented strategy did not avoid GI symptoms, adopted protocol did not intend to provide greater amounts of CHO, to avoid achieving ergogenic effects arising from consumption of CHO. The source of CHO was an orange juice (150 mL) added to the tested preparations just before intake. It must to be mentioned, that the organic acids present in the juice (e.g., citric, ascorbic or malic) were available to react with H2O or NaHCO3. These weak organic acids partially dissociate in water. They react with sodium bicarbonate, meaning sodium salts, water and carbon dioxide are produced. Thus, one the hand, the addition of orange juice might slightly decrease the overall buffering potential of the implemented dose of SB. On the other hand, it improved substantially the taste of the preparations, and eventually might lead to greater palatability of supplementation. Moreover, the addition of the juice did not prevent from obtaining effective increase in blood bicarbonate.
One of the limitations of the current study is evaluation of the incidence and severity of GI symptoms at one solitary time point. Knowing the results of the study, and the fact that the severity of GI symptoms negatively impacted the WAnTs, it would be reasonable to evaluate GI symptoms at additional time points e.g., pre-supplementation and during exercise. This approach might help to identify the time of peak GI symptoms, and in the future studies to establish time of SB ingestion according to individual bicarbonate kinetics and GI symptoms occurrence. Another limitation of the study is that mean peak increase in blood HCO3¯ (7.8 ± 1.9 mmol·L−1) was achieved 15 min before the exercise test started. At the time point, right after 10 min standardized warm-up and before the exercise started (0′EX), the mean increase of blood HCO3¯ was just slightly above the threshold of almost certain ergogenic effect (~ 6.1 mmol·L−1). The strength of our study is a simultaneous comparison of blood bicarbonate kinetics and anaerobic performance. Most of the previous studies, which considered indices related to physical performance and high doses of SB (≥ 0.4 g·kg−1BM) controlled blood HCO3¯, lactate or pH (or other indices related to acid–base blood balance) solely at baseline, before and after exercise. In our study we insightfully controlled blood indices at 12 distinct time points. Thus, the current study must be perceived as highly innovative, and its undeniable strength is the ability to identify the peak blood HCO3¯, as well as peak increase (changes) in blood HCO3¯ and an increase (changes) in blood HCO3¯ directly before exercise was initiated, and to demonstrate there was a difference between these. Additionally, these blood collections clearly indicated the effect of warm-up causing a slight blood HCO3¯ depletion. The phenomenon is unavoidable, and has been raised up in one previous study43, in which similarly to current results, a warm-up (regardless its intensity) led to decrease in blood bicarbonate, but did not affect the ergogenic potential of ingested SB. That seems to be valuable in the perspective of the future studies, to control if the time of starting the exercise and time of reaching peak increase in blood HCO3¯ overlap and/or are located in the assumed and desirable time gap (e.g. in case of longer-lasting exercises). It is possible that in some previous studies the time for the exercise tests to initiate following supplementation was not optimal from the point of view of peak HCO3¯ increases in blood, which may affect conclusions. To the best of our knowledge, this is the first study to investigate the kinetics of blood bicarbonate (and other acid–base blood indices) after ingestion of a single acute dose of 0.4 g·kg−1BM of SB. Finally, the undeniable strength of our study is also proving that to have a better insight into factors determining the buffering and ergogenic potential of SB, it is crucial to monitor blood HCO3¯ not only at baseline (pre-ingestion) and right before the exercise start, but also in between the ingestion and start of the exercise.
Conclusions
In this study in CrossFit trained athletes, the acute ingestion of single dose of 0.4 g·kg−1BM of sodium bicarbonate combined with a small amount of carbohydrate resulted in peak blood bicarbonate about 75 min after ingestion and significant increase in extra-cellular buffering capacity of plasma persisting from 60 until 90 min after intake. There was a significant negative impact of GI symptoms distress on mean indices of anaerobic performance of WAnT bouts. This may partially explain lack of improvement and/or lack of preserving from the decrements in performance in the six following bouts of the WAnT after SB ingestion. Thus, monitoring the time course of GI symptoms occurrence and severity post-SB intake seems to be crucial factors to control when implementing high, single, and acute doses of SB. Future studies should aim to investigate individualization of time of ingesting SB, combining the simultaneous elevation of blood bicarbonate referring to the zone of almost certain ergogenic action and lowest possible level of GI upset.
Material and methods
Participants
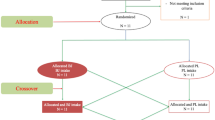
Twenty male and female CrossFit-trained practitioners were initially enrolled in this study and randomly allocated to the double-blinded treatment sequence (SB + CHO → PLA + CHO or PLA + CHO → SB + CHO) in a balanced design. There was one drop-out from the study, due to personal reasons (PLA + CHO → SB + CHO sequence). A total of 19 participants aged 37 ± 8 years and of mean body mass 75.1 ± 13.1 kg, completed the whole study protocol and were included in the analyses (Table 4).
The inclusion criteria were: good general health, a valid and up-to-date medical certificate that confirmed the athlete’s ability to practice sports, at least 4 years of training experience and participation in a minimum of four CrossFit workout sessions a week. The exclusion criteria were: current injury, health-related contraindication, declared general feeling of being unwell and unwillingness to follow the study protocol. The entire protocol for each participant lasted 4 weeks. All the study procedures were performed in the sport club in which participants practice CrossFit on regular basis (Lubin, Lubuskie Province, Poland).
The study protocol was reviewed and approved by the local institutional review board (Bioethics Committee at Poznan University of Medical Sciences, reference number: 1000/18 of 11 October 2018), and registered at ClinicalTrials.Gov (NCT03810404, 18/01/2019). Written informed consent was obtained from all participants before their participation in the study began. All procedures were conducted in accordance with the ethical standards of the 1975 Declaration of Helsinki. The study complies with the CONSORT statement for randomized trials, as shown in Fig. 4.
G*Power software (version 3.1.9.4, Universität Düsseldorf, Germany) was used to calculate the sample size required to obtain a power of approximately 80% (α = 0.05) and large effect size partial eta squared 0.1444 in analysis of variance (ANOVA) with repeated measurements (RM) within factors. Analysis indicated that a sample size of 12 would be suitable for detecting a difference between three measurements. To account for possible dropouts, 20 participants were initially enrolled into the study.
Experimental protocol
In the current study, the efficacy of SB supplementation was evaluated in a randomized, cross-over, placebo-controlled double-blind trial. The protocol of the study consisted of four study visits (T1–T4) (Fig. 4), of which the T1 was arranged to obtain the anthropometric measurements of the participants and to get the participants familiarized with the study procedures, including the exercise protocol—several consecutive bouts of the 15 s WAnTs; the T2 was the control (CTRL) visit—no supplementation was provided, but all the testing procedures were performed; during the T3 and T4 visits the participants randomly ingested SB + CHO or placebo (PLA) + CHO. The T2–T4 visits were separated by 7-days washout period. Participants were obligated to eat a standardized meal two hours before each study visit. Furthermore, the T2–T4 visits started with BM and body composition evaluation, then the baseline blood samples (− 90′EX) were drawn, after which the participants ingested SB + CHO or PLA + CHO (T3 and T4 visits). Subsequent blood samples were taken 30 (− 60′EX), 60 (− 30′EX), 75 (− 15′EX) and 90 (0′EX) min after supplement ingestion, immediately after each of six bouts of WAnT (WAnT_1POST, WAnT_2POST, WAnT_3POST, WAnT_4POST, WAnT_5POST, WAnT_6POST), and the last sample was drawn during recovery − 45 min after completion of the whole exercise protocol (+ 45′EX). In total, 12 blood samples were taken from each participants during T2–T4 visits. At 120 min post-ingestion (in T3–T4) or at 120 min post-baseline blood draw (T2 at CTRL), participants completed a GI side effect questionnaire. Blood bicarbonate kinetics and performance in subsequent bouts of WAnT were considered as primary outcomes, while gastrointestinal side effects as secondary outcomes.
Supplementation
The participants were provided with individually adjusted supplementation doses. The dose of supplemented SB was 0.4 g·kg−1BM of SB + 15 g CHO. SB provided in the form of powder containing 89.1% of sodium bicarbonate, 9.9% of potassium bicarbonate, and 1.0% of sodium citrate (Alkala N, Sanum-Kehlbeck GmbH & Co. KG, Germany). The share of SB in the powder was taken into account when establishing individual supplemented doses. PLA preparation was provided in a similar powder form and contained sodium chloride in an equimolar amount of sodium as SB. Both preparations were dissolved in approximately 750 mL of fluid (600 mL of water and 150 mL of orange juice). Juice was provided as a source of CHO, which was supposed to alleviate GI symptoms29 and improve the taste of the preparations. Participants were asked to drink the preparations within 5 min. Neither main investigators, nor the participants were aware about the ingested preparations. The random allocation sequence and matching were performed using stratified randomization via impartial biostatistics, who was not directly involved in performing test procedures.
Anthropometric measurements
Body mass and height were measured using a professional medical scale with a stadiometer (WPT 60/150 OW, RADWAG, Radom, Poland; an accuracy of 0.1 cm and 0.1 kg for height and BM). Body composition and total body water were assessed by means of bioelectric impedance, using Bodystat 1500 (Bodystat Inc., Douglas, UK).
Blood samples analysis
Capillary blood was collected from a fingertip of the nondominant hand using a disposable lancet-spike Medlance Red (HTL-STREFA, Łódź, Poland) with a 1.5 mm blade and 2.0 mm penetration depth. Approximately 65 μL of blood was collected to a heparinized capillary tube where HCO3−, H+, Na+, K+, Cl−, base excess, anion gap, glucose, and lactate concentrations and pH value were determined on blood gas analyzer (ABL90 FLEX, Radiometer, Brønshøj, Denmark). The blood gas analyzer was each time calibrated before use.
Power indices monitoring—series of modified Wingate Anaerobic Test (WAnT) bouts
Six bouts of the 15 s WAnT on a cycloergometer (Monark 894E, Varberg, Sweden) were performed with 30 s rests between consecutive bouts. The recommendations proposed by Bar-Or 45 were followed, as described in our previous studies33,34. The following indices were evaluated: peak power (PP), average power (AP), minimum power (MP), power drop (PD), and time to PP (TtoPP). The series of six bouts of 15 s WAnTs was preceded by a standardized 10 min warm-up (from 77 until 87 min after SB + CHO or PLA + CHO ingestion or baseline blood drawn < CTRL >), of which the first 5 min were cycling at approximately 50 W followed by 5 min of self-selected stretching.
Gastrointestinal side effects
GI side effects were evaluated via a standardized questionnaire46, which has been previously adapted and effectively utilized in our former studies9,33 and studies on SB supplementation by others18,29. The questionnaire consists of 19 items describing common GI symptoms and the numeric rating scale (NRS) was used to rate the intensity of these symptoms. NRS ranged between 0 and 10, where 0 reflected no GI distress at all and 10 being the most severe GI distress imaginable. The total GI score was calculated as a sum of all 19 GI symptoms. GI symptoms were considered severe when a score ≥ 5 was given, and less severe when < 5. The exception was vomiting, which was considered severe when scores were > 0. The severe GI symptoms included nausea, stomach ache, urge to vomit, vomiting and intestinal cramps. Less severe GI symptoms included eructation, flatulence, urge to defecate, heartburn and abdominal pressure (bloating). The sum of scores was then calculated for severe and less severe symptoms46.
Statistical analysis
All variables were checked for normal distribution using the Shapiro–Wilk test. Blood variables and the WAnTs are presented as mean ± 1 standard deviation (SD); 95% confidence interval (95% CI). Baseline comparison between subgroups according to supplementation sequence were performed using T-test for independent samples or Mann–Whitney U-test. Blood variables between study conditions (CTRL, SB + CHO, PLA + CHO) at each of twelve particular time points (from − 90′EX to + 45′EX), were tested with repeated-measures analysis of variance (RM ANOVA). Similarly, the results of the WAnTs (the differences between six consecutive bouts from WAnT_1 to WAnT_6) between the study conditions (CTRL, SB + CHO or PLA + CHO) were analyzed via RM ANOVA. For the WAnT (performed six times on each testing day at each of the three study conditions—CTRL, SB + CHO, PLA + CHO) the three different models of RM ANOVA were used, of which the first one was univariate model (†bouts x treatment) and the two remaining were double-multivariate models (ǂbouts x treatment x treatment order or §bouts x treatment x gender). Taking into account the robustness of F-test in terms of Type 1 error47, if the normality assumptions based on Shapiro–Wilk test was violated, the kurtosis and skewness variables were also evaluated. Eventually, all the variables met the assumptions to be analyzed by various models of ANOVA. A Huynh–Feldt adjustment was made when sphericity was violated (as indicated by Mauchly’s test). Post-hoc comparisons were performed by Bonferroni test. Effect sizes are expressed as partial eta square (η2p). The results of GI side effects are presented as median ± interquartile range (IQR); quartile range. The results were analyzed by Friedman’s ANOVA, followed by post-hoc for Friedman. However, the power of post-hoc for Friedman was not always strong enough to detect the substantial differences between the compared groups (i.e., nausea, dizziness, urge to urinate, bloating, cold shivering), even though the Friedman’s ANOVA indicated their existence. Effect sizes were calculated using Kendall’s concordance coefficient W (0—no agreement and 1—complete agreement). The relationship between GI symptoms (total GI symptoms score, severe GI symptoms and less severe GI symptoms) and WAnT performance (the mean results of six WAnT bouts), as well as the relationship between the increase in blood bicarbonate and performance in particular bouts of WAnT were analyzed using Spearman's rank correlation. Statistical significance was set at p < 0.05. The different letter inscriptions in the results presentation indicate substantial differences between measurements/study conditions. Data were analyzed using STATISTICA 13.3 (StatSoft Inc., Tulsa, OK, USA) software.
Data availability
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
References
Haddock, C. K., Poston, W. S. C., Heinrich, K. M., Jahnke, S. A. & Jitnarin, N. The benefits of high-intensity functional training fitness programs for military personnel. Mil. Med. 181, e1508–e1514 (2016).
Lu, Y., Wiltshire, H. D., Baker, J. S. & Wang, Q. The effects of running compared with functional high-intensity interval training on body composition and aerobic fitness in female university students. IJERPH 18, 11312 (2021).
Ben-Zeev, T. & Okun, E. High-intensity functional training: Molecular mechanisms and benefits. Neuromol. Med. 23, 335–338 (2021).
Feito, Y., Heinrich, K., Butcher, S. & Poston, W. High-intensity functional training (HIFT): Definition and research implications for improved fitness. Sports 6, 76 (2018).
Durkalec-Michalski, K. et al. Effect of a four-week vegan diet on performance, training efficiency and blood biochemical indices in CrossFit-trained participants. Nutrients 14, 894 (2022).
Weisenthal, B. M., Beck, C. A., Maloney, M. D., DeHaven, K. E. & Giordano, B. D. Injury rate and patterns among CrossFit athletes. Ortho. J. Sports Med. 2, 2325967114531177 (2014).
dos Santos Quaresma, M. V. L., Guazzelli Marques, C. & Nakamoto, F. P. Effects of diet interventions, dietary supplements, and performance-enhancing substances on the performance of CrossFit-trained individuals: A systematic review of clinical studies. Nutrition 82, 110994 (2021).
de Souza, R. A. S. et al. A systematic review of CrossFit workouts and dietary and supplementation interventions to guide nutritional strategies and future research in CrossFit. Int. J. Sport Nutr. Exerc. Metab. 31, 187–205 (2021).
Durkalec-Michalski, K. et al. The effect of chronic progressive-dose sodium bicarbonate ingestion on CrossFit-like performance: A double-blind, randomized cross-over trial. PLoS One 13, e0197480 (2018).
Toledo, L. P., Vieira, J. G. & Dias, M. R. Acute effect of sodium bicarbonate supplementation on the performance during CrossFit training. Motriz Rev. Educ. Fis. 26, e10200075 (2020).
Grgic, J. et al. International Society of Sports Nutrition position stand: Sodium bicarbonate and exercise performance. J. Int. Soc. Sports Nutr. 18, 61 (2021).
McEvoy, G. K. & American Society of Health-System Pharmacists. AHFS Drug information 2007. (American Society of Health-System Pharmacists, 2007).
American Medical Association, AMA Department of Drugs. AMA Drug Evaluations. (American Medical Association, 1980).
Stanton B.A., & Koeppen B.M. The kidney. in Thomson/Micromere. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006. (1998).
Jones, R. L. et al. Dose-response of sodium bicarbonate ingestion highlights individuality in time course of blood analyte responses. Int. J. Sport Nutr. Exerc. Metab. 26, 445–453 (2016).
de Oliveira, L. F. et al. Extracellular buffering supplements to improve exercise capacity and performance: A comprehensive systematic review and meta-analysis. Sports Med. 52, 505–526 (2022).
Gastin, P. B. Energy system interaction and relative contribution during maximal exercise. Sports Med. 31, 725–741 (2001).
Gough, L. A., Deb, S. K., Sparks, A. S. & McNaughton, L. R. The reproducibility of blood acid base responses in male collegiate athletes following individualised doses of sodium bicarbonate: A randomised controlled crossover study. Sports Med. 47, 2117–2127 (2017).
Goldfinch, J., Mc Naughton, L. & Davies, P. Induced metabolic alkalosis and its effects on 400-m racing time. Eur. J. Appl. Physiol. 57, 45–48 (1988).
Marriott, M., Krustrup, P. & Mohr, M. Ergogenic effects of caffeine and sodium bicarbonate supplementation on intermittent exercise performance preceded by intense arm cranking exercise. J. Int. Soc. Sports Nutr. 12, 13 (2015).
McNaughton, L. R. Bicarbonate ingestion: Effects of dosage on 60 s cycle ergometry. J. Sports Sci. 10, 415–423 (1992).
McNaughton, L. et al. Anaerobic work and power output during cycle ergometer exercise: Effects of bicarbonate loading. J. Sports Sci. 9, 151–160 (1991).
Afman, G. et al. Effect of carbohydrate or sodium bicarbonate ingestion on performance during a validated basketball simulation test. Int. J. Sport Nutr. Exerc. Metab. 24, 632–644 (2014).
Ansdell, P. & Dekerle, J. Sodium bicarbonate supplementation delays neuromuscular fatigue without changes in performance outcomes during a basketball match simulation protocol. J. Strength Cond. Res. 34, 1369–1375 (2020).
Bishop, D. & Claudius, B. Effects of induced metabolic alkalosis on prolonged intermittent-sprint performance. Med. Sci. Sports Exerc. 37, 759–767 (2005).
Krustrup, P., Ermidis, G. & Mohr, M. Sodium bicarbonate intake improves high-intensity intermittent exercise performance in trained young men. J. Int. Soc. Sports Nutr. 12, 25 (2015).
Verbitsky, O., Mizrahi, J., Levin, M. & Isakov, E. Effect of ingested sodium bicarbonate on muscle force, fatigue, and recovery. J. Appl. Physiol. 83, 333–337 (1997).
de Oliveira, L. F., Saunders, B., Yamaguchi, G., Swinton, P. & Giannini Artioli, G. Is individualization of sodium bicarbonate ingestion based on time to peak necessary? Med. Sci. Sports Exerc. 52, 1801–1808 (2020).
Carr, A. J., Slater, G. J., Gore, C. J., Dawson, B. & Burke, L. M. Effect of sodium bicarbonate on [HCO3−], pH, and gastrointestinal symptoms. Int. J. Sport Nutr. Exerc. Metab. 21, 189–194 (2011).
Saunders, B., Sale, C., Harris, R. C. & Sunderland, C. Sodium bicarbonate and high-intensity-cycling capacity: Variability in responses. Int. J. Sports Physiol. Perform. 9, 627–632 (2014).
McGowan, C. J., Pyne, D. B., Thompson, K. G. & Rattray, B. Warm-up strategies for sport and exercise: Mechanisms and applications. Sports Med. 45, 1523–1546 (2015).
Douroudos, I. I. et al. Dose-related effects of prolonged nahco3 ingestion during high-intensity exercise. Med. Sci. Sports Exerc. 38, 1746–1753 (2006).
Durkalec-Michalski, K. et al. The effect of a new sodium bicarbonate loading regimen on anaerobic capacity and wrestling performance. Nutrients 10, 697 (2018).
Durkalec-Michalski, K., Zawieja, E. E., Zawieja, B. E., Michałowska, P. & Podgórski, T. The gender dependent influence of sodium bicarbonate supplementation on anaerobic power and specific performance in female and male wrestlers. Sci. Rep. 10, 1878 (2020).
Mündel, T. Sodium bicarbonate ingestion improves repeated high-intensity cycling performance in the heat. Temperature. 5, 343–347 (2018).
Parry-Billings, M. & MacLaren, D. P. M. The effect of sodium bicarbonate and sodium citrate ingestion on anaerobic power during intermittent exercise. Eur. J. Appl. Physiol. 55, 524–529 (1986).
Zabala, M. et al. Effects of sodium bicarbonate ingestion on performance and perceptual responses in a laboratory-simulated BMX cycling qualification series. J. Strength Cond. Res. 22, 1645–1653 (2008).
Zabala, M. et al. Bicarbonate ingestion has no ergogenic effect on consecutive all out sprint tests in BMX elite cyclists. Eur. J. Appl. Physiol. 111, 3127–3134 (2011).
Artioli, G. G. et al. Does sodium-bicarbonate ingestion improve simulated judo performance?. Int. J. Sport Nutr. Exerc. Metab. 17, 206–217 (2007).
de Oliveira, L. F. et al. Chronic lactate supplementation does not improve blood buffering capacity and repeated high-intensity exercise. Scand. J. Med. Sci. Sports. 27, 1231–1239 (2017).
Tobias, G. et al. Additive effects of beta-alanine and sodium bicarbonate on upper-body intermittent performance. Amino Acids 45, 309–317 (2013).
Siegler, J. C., Marshall, P. W. M., Bray, J. & Towlson, C. Sodium bicarbonate supplementation and ingestion timing: Does it matter?. J. Strength Cond. Res. 26, 1953–1958 (2012).
Jones, R. L. et al. Warm-up intensity does not affect the ergogenic effect of sodium bicarbonate in adult men. Int. J. Sport Nutr. Exerc. Metab. 31, 482–489 (2021).
University of Cambrige. MRC CBU Wiki. Rules of thumb on magnitudes of effect sizes. https://imaging.mrc-cbu.cam.ac.uk/statswiki/FAQ/effectSize.
Bar-Or, O. The Wingate Anaerobic Test: An update on methodology, reliability and validity. Sports Med. 4, 381–394 (1987).
Jeukendrup, A. E. et al. Relationship between gastro-intestinal complaints and endotoxaemia, cytokine release and the acute-phase reaction during and after a long-distance triathlon in highly trained men. Clin. Sci. (Lond) 98, 47–55 (2000).
Blanca, M. J., Alarcón, R. & Arnau, J. Non-normal data: Is ANOVA still a valid option?. Psicothema 29, 552–557 (2017).
Acknowledgements
We thank the athletes for their participation and their coaches for help in conducting this research. We thank the company Sanum Polska sp. z o. o. for providing the alkalizing powder for the study.
Funding
This study was partially funded by the National Science Centre, Poland. K.D.-M has received research Grant from the Polish National Science Centre (Grant number 2018/02/X/NZ7/03217). Furthermore, K.D.-M. has participated in the Exchange Programme for Scientists as part of bilateral cooperation financed by The Polish National Agency for Academic Exchange (NAWA: BPN/BIL/2021/1/00108/U/00001). B.S. (2016/50438-0 and 2021/06836-0) acknowledges a personal research Grant from Fundação de Amparo à Pesquisa do Estado de São Paulo. B.S. also receives a Grant from Faculdade de Medicina da Universidade de São Paulo (2020.1.362.5.2).
Author information
Authors and Affiliations
Contributions
K.D.-M.: conception, design of the work, and funding acquisition. K.D.-M., J.K., D.C., and T.P.: investigation and resources. K.D.-M., and P.M.N.: analysis, and interpretation of data for the work. K.D.-M., P.M.N., J.K., B.S., I.Ł., D.C., M.S., and T.P.: drafting of the work and revising it critically for important intellectual content. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Corresponding author
Ethics declarations
Competing interests
I.Ł. is a shareholder of an alkali products distributor and has received honoraria from the Sanum Polska sp. z o. o. However, the content of this study was not constrained by this fact in absolutely no extend. K.D.-M., P.M.N., J.K., B.S., D.C., M.S., and T.P. report no conflicts of interest. The results of the present study do not constitute endorsement by the Scientific Reports. The authors declare that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Durkalec-Michalski, K., Nowaczyk, P.M., Kamińska, J. et al. The interplay between bicarbonate kinetics and gastrointestinal upset on ergogenic potential after sodium bicarbonate intake: a randomized double-blind placebo-controlled trial. Sci Rep 13, 7081 (2023). https://doi.org/10.1038/s41598-023-34343-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-34343-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.