Abstract
Circulating tumor cells (CTCs) play a key role in tumor metastasis. CTCs have altered gene expression and can survive in the bloodstream. Finding the key genes whose expression are altered in CTCs could help explain the mechanism of tumor metastasis. We searched for genes differentially expressed in CTCs by analyzing four CTCs and primary tumor gene expression datasets in the GEO database. Key genes of clear cell renal cell carcinoma (ccRCC) CTCs were identified. The correlation between key genes and the immune microenvironment of ccRCC was explored. Finally, the CTCs cell model of ccRCC was constructed by in vivo screening method, and the expression of key genes was detected at the cell and tissue levels. A total of 771 DEGs were obtained. Gene enrichment analysis showed that DEGs of CTCs were mainly involved in the regulation of the tumor immune process and tumor cell apoptosis. Finally, we found 2 key genes, MMP9 and TYROBP in ccRCC CTCs. The high expression of these 2 genes predicted a poor prognosis of ccRCC, and the expression levels of these 2 genes were significantly increased in CTCs and ccRCC tissues. Our study suggested that genetic alterations in CTCs contribute to the ability of CTCs to survive in the blood by adapting to the tumor microenvironment. MMP9 and TYROBP are potential therapeutic and prognostic targets for ccRCC.
Similar content being viewed by others
Introduction
Renal cell carcinoma (RCC) is a malignant tumor with a high incidence in the human body, which seriously threatens people’s life and health1. Clear cell renal cell carcinoma (ccRCC) is the main histological subtype of RCC2. About one-third of patients are found to have metastasis at the time of presentation, recurrence and metastasis are the main causes of poor prognosis3. The development of early diagnosis and treatment of tumors has greatly reduced the mortality of malignant tumors, but there is still a lack of effective treatment for recurrent and metastatic ccRCC.
Circulating tumor cells (CTCs) are separated from the primary tumor site or metastatic tumor site4. CTCs enter the blood circulation through the vascular endothelium and survive in the blood, which was first observed by Ashworth in the blood of tumor patients5. Hematogenous metastasis is a major metastatic mode of ccRCC, and CTCs, as the seeds of tumor metastasis, play a key role in the process of ccRCC metastasis6. Only a small part of tumor cells that enter the blood circulation survive and metastasize in the blood circulation7. As CTCs are scarce in the blood, it is difficult to capture and analyze CTCs. In recent years, the development of various detection technologies has made it possible to analyze CTCs in vitro, and liquid biopsy technology has been developed to perform tumor screening, treatment response monitoring, diagnosis, and prognosis assessment through biological analysis of CTCs8. A large number of studies have focused on the survival and metastasis of CTCs in the blood, providing new ideas for drug treatment of tumor recurrence and metastasis.
In this study, we used the GEO database to analyze the gene expression differences between CTCs and primary tumor cells and to analyze the functions of these differentially expressed genes (DEGs) and the mechanisms that regulate the development of ccRCC. Finally, the CTCs model of ccRCC was constructed by in vivo screening method, and the expression of key genes was detected at the cellular and tissue levels, which provides potential targets for the treatment and prognosis of ccRCC.
Methods
Data collection and processing
We obtained four databases GSE18670, GSE50991, GSE82198 and GSE82198 from GEO database9,10. We list the details of the data set in Table 1. The GEO2R tool was used to process the data to obtain differentially expressed genes (DEGs) between CTCs and primary tumors. |logFC|> 1 and adjust p < 0.05 were used as the threshold to select DEGs for subsequent analysis. After obtaining DEGs in all four data sets, the Venn diagram method was used to include DEGs in more than three datasets for further analysis. The DAVID tool was used to perform GO and KEGG enrichment analysis on the 771 DEGs included11.
Screening and analysis of key genes
The String database was used to analyze the interactions between DEGs. Cytoscape tool was used to screen and analyze key genes. We first analyzed the entire PPI network using the Cytohubba plug-in and obtained the top 10 hub genes using the MNC algorithm. Then the MCODE plug-in was used to analyze the subnetwork, and the node genes in the subnetwork with the highest score were obtained. The intersection of 10 hub genes and node genes was obtained for subsequent analysis.
Expression and survival analysis of key genes in ccRCC
We acquired the TCGA-KIRC dataset to analyze the expression differences of key genes between ccRCC tumor tissues and normal tissues. The Kaplan–Meier Plotter tool was used to analyze the association between key genes and OS in ccRCC12.
Functional analysis and immune infiltration analysis of prognostic genes
The GEPIA database was used to obtain a list of genes co-expressed with prognostic genes in ccRCC13. We selected the top 50 genes that correlate with each prognostic gene for GO gene enrichment analysis. The Timer database was used to analyze the relationship between prognostic genes and the level of tumor-associated immune cell infiltration in ccRCC14. Finally, we used the TISIDB database to analyze the association of these 2 prognostic genes with immune markers and disease immune subtypes in ccRCC15.
Cells and reagents
Renal cell carcinoma cell lines 786O, OSRC2, 769P, ACHN, CAKI2, and normal cell HK2 were purchased from ATCC. HK2 cells were cultured in DMEM/F12 medium (Gibco, USA) containing 10% fetal bovine serum (Excell, China), renal cell carcinoma cells 786O, OSRC2, 769P, and ACHN were cultured in RPMI 1640 medium (Gibco, USA) containing 10% fetal bovine serum, and CAKI2 cells were cultured in McCoy’s 5A medium (Procell, China) containing 10% fetal bovine serum. All cell lines were incubated at 37 ° with 5% CO2.
Primary antibody against pan Keratin was bought from Cell Signaling Technology (Danvers, USA), Fluorescein (FITC)–conjugated Affinipure Goat Anti-Mouse IgG(H + L) was bought from Proteintech Group Inc. (USA).
Animal experiments and clinical samples
Animal experiments were performed with the approval of Animal Care and Use Committee of the Second Affiliated Hospital of Xi’an Jiaotong University. Athymic BALB/c nude male mice (6 weeks old) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). 786O cells were resuspended in PBS to 5*10^6/ml, and 100ul of the cell suspension was injected into the tail vein of nude mice as described in previous literature. Six weeks after cell inoculation, the tumorc cells injected mice were sacrificed by cervical dislocation following anesthesia, blood was collected by cardiac puncture. Lymphocyte isolation solution (Solarbio, China) was used to remove red blood cells and plasma from the blood, and the remaining cells were resuspended in RPMI 1640 medium and seeded on cell culture plates. The solution was changed every 24 h until cell clones appeared, which was the CTC cell line.
We collected 15 pairs of ccRCC tumor tissue and normal control tissue samples at the department of urology, the second affiliated hospital of Xi’an Jiaotong University. All patients submitted informed consent for tissue use. Our studies involving human participants were in accordance with the ethical standards of the relevant guidelines and regulations. The study protocol was approved by the Ethics Committees of the second affiliated hospital of Xi'an Jiaotong University.
Immunofluorescence
The adherent cells were washed with PBS, then fixed with 4% paraformaldehyde for 15 min and washed with PBS. Cells were treated with 0.5% Triton x-100 for 20 min at room temperature. After blocking with goat serum, the primary antibody was incubated overnight. Then the cells were incubated with secondary antibody for 1 h, and the nuclei were stained with DAPI. Finally, a fluorescence microscope was used to observe the results.
Gene expression analysis by qPCR and weatern blot
Total RNA was extracted from cells using the Trizol method and subsequently reverse transcribed to cDNA using the reverse transcription kit (abm, Canada). The mRNA expression level was detected by the qPCR kit (DiNing, China). The primer sequences of all genes were shown in Table 2. All reagents are used in strict accordance with the actual instructions. Relative genes expression analysis was performed using the 2 –△△CT, with GAPDH used as an internal control.
Protein samples of cells and tissues were separated by 10% SDS-PAGE and then transferred to membranes. After blocking with 5% skim milk, the corresponding primary antibody was added and incubated overnight. After 1 h incubation with secondary antibody, BCL luminescent solution was used for detection.
Statistical analysis
Statistical analyses were performed using GraphPad (8.0.1) and R (4.1.1) software, and two tailed student’s t test was used to assess statistical differences between two groups. p < 0.05 was considered statistically significant.
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committees of the second affiliated hospital of Xi’an Jiaotong University. Animal experiments were performed with the approval of Animal Care and Use Committee of the Second Affiliated Hospital of Xi’an Jiaotong University. All animal experiments were performed in accordance with ARRIVE guidelines. All patients submitted informed consent for tissue use. All procedures performed in studies involving human participants were in accordance with the ethical standards of the 1964 Helsinki declaration, and its later amendments or comparable ethical standards.
Results
Identification of DEGs in CTCs
We included 4 ccRCC GEO profile datasets in the study. The details of GSE18670, GSE50991, GSE82198, and GSE106363 are shown in the table (Table 1). The volcano map shows the DEGs in each dataset (Fig. 1A). We used the Venn diagram method to obtain the intersection of up-or down-regulated DEGs in the four data sets (Fig. 1B,C). We selected genes that were differentially expressed in more than three data sets. Finally, 344 up-regulated genes and 436 down-regulated genes were included. Next, we aimed to explore the biological processes associated with these DEGs. The DAVID database was used to annotate these robust DEGs, and GO and KEGG analyses were performed for these DEGs. In the BP part of GO analysis, DEGs are mainly enriched in the ‘Positive regulation of transcription from RNA polymerase II promoter’ and ‘Apoptotic process’ (Fig. 2A). The results of CC showed that DEGs were mainly concentrated in the ‘Cytosol’ and ‘Cytoplasm’(Fig. 2B). The main molecular function performed by DEGs was ‘Protein binding’(Fig. 2C). Through KEGG analysis, we learned that DEGs were mainly involved in ‘Platelet activation’, ‘B cell receptor signaling pathway’, ‘Lipid and atherosclerosis’ as well as ‘Biosynthesis of antibiotics’ (Fig. 2D). According to the results of these enrichment analyses, we found that the biological functions of these DEGs involved in apoptosis regulation and immune cell interaction were closely related to the onset and recurrence of the tumors.
Differentially expressed genes in CTCs. (A) Differentially expressed genes in different datasets. (B) Venn diagram of up-regulated DEGs in CTCs. (C) Venn diagram of down-regulated DEGs in CTCs. The red and blue dots represent up-regulated and down-regulated genes, respectively. The threshold for fold change > 1.0 and a corrected p-value of < 0.05. Black points represent genes with no significant difference. GEO gene expression omnibus, DEGs differentially expressed genes.
Hub genes selection and analysis
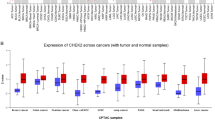
Using the Cytoscape software, a PPI network of DEGs was constructed. The MCODE plug-in was used to analyze the subnetwork, and selected the node genes in the subnetwork with the highest score (Fig. 3A). CytoHubba plug-in of Cytoscape software was used to identify hub genes of ccRCC according to the MNC algorithms (Fig. 3B). The overlapped hub genes in the results of the two methods included 4 genes (TYROBP, FGR, HCK, and MMP9) (Fig. 3C). The expression levels and prognostic value of these 4 hub genes were studied in ccRCC. The expression levels of these 4 genes in ccRCC tissues were higher than those in normal tissues (Fig. 4). Furthermore, high expression of TYROBP, and MMP9 suggested shorter OS in ccRCC patients, while the expression levels of the remaining 2 genes were not associated with OS in ccRCC patients (Fig. 5).
Gene enrichment analysis
To deeply understand the molecular mechanism of these 2 genes involved in the regulation of ccRCC disease progression, we used the GEPIA database to obtain the top 50 genes with the highest correlation with the expression of these two genes in ccRCC for functional analysis. The results showed that these genes were mainly involved in ‘Immune response’, ‘Innate immune response’, ‘Positive regulation of T cell proliferation’, ‘staphylococcus aureus infection’, and ‘systemic lupus erythematosus’ (Fig. 6). These biological processes are mostly related to tumor immune infiltration, suggesting that the genetic changes of CTCs in ccRCC affect tumor progression by regulating the tumor immune microenvironment.
Relationship between prognostic DEGs and immune characteristics
In recent years, immune checkpoint inhibitors are considered to be effective against a wide range of tumors, the research on tumor immune checkpoints has provided many important targets for tumor immunotherapy. According to the TIMER database, we found that these two genes are strongly associated with the infiltration levels of various immune cells in ccRCC. In particular, the expression of TYROBP and MMP9 showed a significant positive correlation with the infiltration level of various immune cells. (Fig. 7A,B).
The results of the TISIDB database showed that these 2 genes were closely related to immune-related markers of ccRCC (Fig. 8A,B). The immune stimulators that showed the strongest positive correlation with MMP9 and TYROBP genes were CD276 and CD86 respectively (SFig. 1A). TNFSF13 and IL6R had the strongest negative correlation with these 2 genes (SFig. 1B). Among the immune inhibitors, TGFB1 and LGALS9 showed the strongest positive correlation with these 2 genes (SFig. 1C), while KDR showed the strongest negative correlation (SFig. 1D). In addition, the analysis results of immune subtypes showed that the expression of these 2 genes was significantly different among different immune subtypes of ccRCC (Fig. 8C), which indicated that these genes were widely involved in the regulation of immune microenvironment of ccRCC.
Immunocorrelation analysis of key genes. (A) Correlations between key genes and immune stimulators. (B) Correlations between key genes and immune inhibitors. (C) Association between key genes and immune subtypes of ccRCC. Red represents a positive relationship and blue represents a negative relationship.
CTCs isolation and validation
In this study, we used in vivo selection method to construct CTC cells according to previous reports16,17 (Fig. 9A). To verify that our method resulted in ccRCC CTC cells, we stained the obtained cells with antibodies to CK, a protein widely expressed in CTC cells. The results of immunofluorescence showed that the cells we isolated were positive for CK, indicating that these cells were CTCs (Fig. 9B).
The expression of key genes
Subsequently, we used the constructed CTCs cell model of ccRCC to detect the expression of these two genes. The results showed that the expression levels of MMP9 and TYROBP in CTCs were significantly increased compared with the 786O cell line (Fig. 10A). We also examined the mRNA expression of these 2 genes in renal cancer tissues, and the results showed that the mRNA expression levels of MMP9 and TYROBP in ccRCC tissues were significantly increased (Fig. 10B). The expression levels of these 2 genes in renal cancer cell lines 786O, OSRC2, and CAKI2 were significantly higher than that in normal control HK2 cell lines (Fig. 10C). Previous results of immunocorrelation analysis showed that MMP9 and TYROBP were most closely correlated with the level of immunoinfiltration in ccRCC, and western blot experiment was used to detect the expression of these two molecules. The results showed that these two molecules were significantly upregulated in ccRCC tissues compared with adjacent normal tissues (Fig. 10D). The protein expression levels of these 2 genes in CTCs were significantly increased compared with the 786O cell line (Fig. 10E).
The expression of key genes. (A) The expression levels of MMP9 and TYROBP in CTCs. (B) The mRNA expression levels of MMP9 and TYROBP in renal cancer tissues. (C) The mRNA expression levels of MMP9 and TYROBP in renal cancer cell lines. (D) Western blot analyses of MMP9 and TYROBP expression in 4 representative tumors and paired adjacent tissues. (E) Western blot analyses of MMP9 and TYROBP expression in CTCs. *p < 0.05; **p < 0.01; ***p < 0.001.
Discussion
Cancer is a complex disease involving multiple genes. It is widely believed that a tumor is not composed of a single type of cell population, but consists of subsets of cells with different genes and phenotypes18. Tumor heterogeneity can result from genetic or non-genetic changes, such as changes in the expression of tumor molecules or different external stimuli19. After the tumor cells escape from the extracellular matrix and enter the blood circulation, most of the tumor cells die due to the shear force of the intravascular fluid, the anoikis, and the killing effect of immune cells20,21. Only a few of the tumor cells survive. These surviving CTCs are a group of genetically and phenotypically highly heterogeneous cells, often with specific biological characteristics to adapt to the new environment22. In this study, we analyzed the key genes with altered expression in ccRCC CTCs, explored the association between these genes and the progression of ccRCC, and finally determined that MMP9 and TYROBP played a key role in the development of ccRCC CTCs.
After entering the peripheral blood circulation, single tumor cells will be attacked by the body’s immune cells and undergo apoptosis. Immune cells are the main reason that restricts the survival of CTCs in blood circulation23. A large number of CTCs die due to the killing effect of immune cells, and only a small part of CTCs have the ability to escape the killing of immune cells and survive in the blood circulation24. These surviving CTCs evade immune killing by regulating their gene expression, downregulating the ligands that react with NK cells, and taking refuge from other cellular components25.
In our study, we also found that DEGs in CTCs were widely involved in various immune processes in the body. The results of gene enrichment analysis of DEGs showed that these DEGs affected 'Platelet activation' and 'B cell receptor signaling pathway’. Genes associated with MMP9 and TYROBP expression in ccRCC were involved in the regulation of ‘Immune response’ and ‘Innate Immune response’.
Immunotherapy is an effective treatment for advanced tumors26. Recently, tumor-associated immune cells have been widely concerned because of their important role in tumor progression and treatment27. The specific mechanism of tumor-associated immune cells in tumors is often extremely complex. Further exploration of immune infiltration of tumors is helpful to deepen our understanding of the progression of tumor diseases.
Our study found that among the two key genes of CTCs in ccRCC, MMP9 and TYROBP showed a significant positive correlation with the infiltration levels of B cells, CD4 + T cells, CD8 + T cells, macrophages, and other immune cells in ccRCC. The expression of these genes is significantly correlated with various immune markers of ccRCC, and the expression of these genes is different among different immune subtypes of ccRCC. These findings suggest that these 2 genes may affect the survival of CTCs by regulating the tumor immune microenvironment of ccRCC.
Previous studies have shown that the 2 genes MMP9 and TYROBP regulate the progression of a variety of tumor diseases. MMP9 is a type of matrix metalloproteinase that breaks down the extracellular matrix. The abnormal expression of MMP9 is related to the occurrence of many diseases such as cancers. Inhibitors of MMP9 can be used to treat cancers28. Hong et al. found that the positive expression level of MMP9 in CTCs of colorectal cancer was an independent prognostic factor for progression-free survival29. In addition, MMP9 also deeply regulates the immune microenvironment of tumors. The study has shown that MMP9 promotes tumor progression by regulating tumor immune infiltration in ccRCC30. TYROBP (Transmembrane Immune Signaling Adaptor TYROBP) contains an immunoreceptor tyrosine-based activation motif31. Lu et al. found that the high expression of TYROBP could affect the immune infiltration of glioma and predict poor prognosis32.
In this study, we found that DEGs between CTCs and primary tumors were mainly involved in the regulation of tumor immunity and the survival process of CTCs in blood circulation. At the same time, we verified the possibility of MMP9 and TYROBP as therapeutic and prognostic targets of ccRCC by constructing a CTCs model and detecting the gene expression of ccRCC tissue samples collected. However, we included a small number of ccRCC clinical specimens and lacked genetic testing for clinical CTCs. In the following study, we will continue to collect and detect the expression of CTCs genes in the peripheral blood of ccRCC patients and verify the function of these key genes through molecular and cellular experiments.
Conclusion
We found DEGs between CTCs and primary tumor cells based on data from the GEO database. Through the correlation analysis and enrichment analysis of these genes, the key genes in ccRCC were screened. GO and KEGG enrichment analysis suggested that these genes mainly affected the survival of CTCs by regulating the tumor immune microenvironment. Finally, we used the in vivo screening method to construct the ccRCC CTCs model and verified the expression of key genes at the cellular and tissue levels, proving that these genes can be used as potential therapeutic and prognostic targets of ccRCC.
Data availability
The datasets analyzed during the current study are available in the TCGA database (https://tcga-data.nci.nih.gov/tcga/) and GEO database (https://www.ncbi.nlm.nih.gov/geo/). All the data were available from the corresponding authors for reasonable request.
References
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 71(1), 7–33 (2021).
Jonasch, E., Walker, C. L. & Rathmell, W. K. Clear cell renal cell carcinoma ontogeny and mechanisms of lethality. Nat. Rev. Nephrol. 17(4), 245–261 (2021).
Gray, R. E. & Harris, G. T. Renal cell carcinoma: Diagnosis and management. Am. Fam. Physician 99(3), 179–184 (2019).
Agashe, R. & Kurzrock, R. Circulating tumor cells: From the laboratory to the cancer clinic. Cancers (Basel) 12(9), 2361 (2020).
Wu, J., Zhu, H., Gao, F., Wang, R. & Hu, K. Circulating tumor cells: A promising biomarker in the management of nasopharyngeal carcinoma. Front. Oncol. 11, 724150 (2021).
Broncy, L. & Paterlini-Brechot, P. Circulating tumor cells for the management of renal cell carcinoma. Diagnostics (Basel) 8(3), 63 (2018).
Sun, Y. F. et al. Circulating tumor cells: Advances in detection methods, biological issues, and clinical relevance. J. Cancer Res. Clin. Oncol. 137(8), 1151–1173 (2011).
Gorin, M. A. et al. Circulating tumour cells as biomarkers of prostate, bladder, and kidney cancer. Nat. Rev. Urol. 14(2), 90–97 (2017).
Sergeant, G., van Eijsden, R., Roskams, T., Van Duppen, V. & Topal, B. Pancreatic cancer circulating tumour cells express a cell motility gene signature that predicts survival after surgery. BMC Cancer 12, 527 (2012).
Mishra, D. K. et al. Ex vivo four-dimensional lung cancer model mimics metastasis. Ann. Thorac. Surg. 99(4), 1149–1156 (2015).
Sherman, B. T. et al. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. https://doi.org/10.1093/nar/gkac194 (2022).
Lanczky, A. & Gyorffy, B. Web-based survival analysis tool tailored for medical research (KMplot): Development and implementation. J. Med. Internet Res. 23(7), e27633 (2021).
Tang, Z. et al. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 45(W1), W98–W102 (2017).
Li, T. et al. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 48(W1), W509–W514 (2020).
Ru, B. et al. TISIDB: An integrated repository portal for tumor-immune system interactions. Bioinformatics 35(20), 4200–4202 (2019).
Sato, T., Muramatsu, T., Tanabe, M. & Inazawa, J. Identification and characterization of transforming growth factor beta-induced in circulating tumor cell subline from pancreatic cancer cell line. Cancer Sci. 109(11), 3623–3633 (2018).
Yue, J. et al. Downregulation of NDR1 contributes to metastasis of prostate cancer cells via activating epithelial-mesenchymal transition. Cancer Med. https://doi.org/10.1002/cam4.1532 (2018).
Bagaev, A. et al. Conserved pan-cancer microenvironment subtypes predict response to immunotherapy. Cancer Cell 39(6), 845-865 e847 (2021).
Prasetyanti, P. R. & Medema, J. P. Intra-tumor heterogeneity from a cancer stem cell perspective. Mol. Cancer 16(1), 41 (2017).
Umer, M., Vaidyanathan, R., Nguyen, N. T. & Shiddiky, M. J. A. Circulating tumor microemboli: Progress in molecular understanding and enrichment technologies. Biotechnol. Adv. 36(4), 1367–1389 (2018).
Palmela Leitao, T. et al. Circulating tumor cell detection methods in renal cell carcinoma: A systematic review. Crit. Rev. Oncol. Hematol. 161, 103331 (2021).
Espejo-Cruz, M. L. et al. Circulating tumor cells in hepatocellular carcinoma: A comprehensive review and critical appraisal. Int. J. Mol. Sci. 22(23), 13073 (2021).
Castro-Giner, F. & Aceto, N. Tracking cancer progression: From circulating tumor cells to metastasis. Genome Med. 12(1), 31 (2020).
Mohme, M., Riethdorf, S. & Pantel, K. Circulating and disseminated tumour cells - mechanisms of immune surveillance and escape. Nat. Rev. Clin. Oncol. 14(3), 155–167 (2017).
Wang, W. C. et al. Survival mechanisms and influence factors of circulating tumor cells. Biomed. Res. Int. 2018, 6304701 (2018).
Zhang, Y. & Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 17(8), 807–821 (2020).
Li, W. et al. Anti-cancer nanomedicines: A revolution of tumor immunotherapy. Front. Immunol. 11, 601497 (2020).
Mondal, S., Adhikari, N., Banerjee, S., Amin, S. A. & Jha, T. Matrix metalloproteinase-9 (MMP-9) and its inhibitors in cancer: A minireview. Eur. J. Med. Chem. 194, 112260 (2020).
Hong, X. C. et al. PRL-3 and MMP9 expression and epithelial-mesenchymal transition markers in circulating tumor cells from patients with colorectal cancer: Potential value in clinical practice. Front. Oncol. 12, 878639 (2022).
Xu, T. et al. MMP9 and IGFBP1 regulate tumor immune and drive tumor progression in clear cell renal cell carcinoma. J. Cancer 12(8), 2243–2257 (2021).
Audrain, M. et al. Reactive or transgenic increase in microglial TYROBP reveals a TREM2-independent TYROBP-APOE link in wild-type and Alzheimer’s-related mice. Alzheimers Dement. 17(2), 149–163 (2021).
Lu, J. et al. Elevated TYROBP expression predicts poor prognosis and high tumor immune infiltration in patients with low-grade glioma. BMC Cancer 21(1), 723 (2021).
Author information
Authors and Affiliations
Contributions
L.Y.G. performed the experiments and wrote a manuscript. T.C. and L.Y.G. conceived and designed the study. T.A. analyzed the data and checked the manuscript. H.B.Z. collected clinical specimens. Z.Y.W. and Z.X.H. did the analysis and interpretation of data. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, L., An, T., Zhou, H. et al. MMP9 and TYROBP affect the survival of circulating tumor cells in clear cell renal cell carcinoma by adapting to tumor immune microenvironment. Sci Rep 13, 6982 (2023). https://doi.org/10.1038/s41598-023-34317-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-34317-2
This article is cited by
-
Low TYROBP expression predicts poor prognosis in multiple myeloma
Cancer Cell International (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.