Abstract
Current research emphasizes the habitual dietary pattern without differentiating eating occasions. We aimed to assess meal-specific dietary patterns and insulin resistance indicators. This cross-sectional study was conducted on 825 Iranian adults. Dietary data were recorded by three 24-h dietary recalls. Dietary patterns were identified using principal component analysis (PCA) on main meals and an afternoon snack. Anthropometric measurements, blood pressure, and laboratory investigation, fasting plasma glucose (FPG), triglyceride, insulin, c-reactive protein (CRP) were done. Homeostatic model assessment for insulin resistance and sensitivity (HOMA-IR and HOMA-IS), Triglycerides and glucose (TyG-index), and Lipid accommodation product index were calculated. We used multivariate analysis of variance (MANOVA) analysis. Two major dietary patterns at the main meals and the afternoon were identified. Higher adherence to “Bread, vegetable, and cheese” dietary pattern at breakfast was related to lower FPG, and “Oil, egg, and cereals” showed a direct association with body mass index, FPG, and TyG-index at breakfast. The “Western” pattern in lunch and dinner directly related to waist circumference (WC) and body mass index, however, it was inversely related to HOMA-IS. This pattern at dinner was related to higher CRP. Higher adherence to “bread, cereals, and oil” pattern at afternoon snack was associated with a lower WC. These results indicated that unhealthy meal-specific dietary patterns are associated with a greater chance of obesity and insulin resistance risk. However, “Bread, vegetable, and cheese” dietary pattern at breakfast was related to lower FPG, and “bread, cereals, and oil” pattern in the afternoon was associated with lower WC.
Similar content being viewed by others
Introduction
Insulin resistance (IR) is one of the most important topics in nowadays medicine. IR generally refers to decreased insulin sensitivity in the human tissues1. Abnormal structure of insulin molecule, deteriorated signaling pathways, and declined function of insulin receptors may play a key role in the development of IR2. Accumulative evidence suggests that IR is an underlying cause of several cardiometabolic abnormalities such as dyslipidemia, impaired glucose tolerance, and hyperinsulinemia and thus, is associated with a higher risk of developing type 2 diabetes3, cardiovascular disease (CVD)4, and site-specific cancer risk5. There is also a bidirectional association between obesity and IR, in a way that obesity could lead to the incidence of IR and in return, IR could lead to the development of overweight and obesity6.
Given the global prevalence of non-communicable chronic disease and considering the underlying role of IR in developing chronic diseases, there is a pressing need to investigate modifiable risk factors implicated in developing IR. Studies have suggested a potential link between dietary habits and IR7. Evidence suggested that intake of some plant-based food groups such as whole grains8, vegetables9, and nuts10 may improve insulin resistance and in contrast, the consumption of red meat11 and soft drink12 may be associated with abnormal insulin sensitivity. Evidence from epidemiologic studies also suggest a potential link between healthy and unhealthy dietary patterns and IR13,14,15.
However, most of the studies addressing the association of dietary patterns and IR have focused on habitual dietary patterns13,14,15. Indeed, limited evidence is available about the association between meal-specific dietary patterns with IR and other cardiometabolic abnormalities16,17. Foods are consumed on different eating occasions across the day named meals. Recent studies have suggested that meal-specific dietary habits such as meal timing and frequency may be associated with multiple health outcomes18,19,20,21,22 Chrononutrition is an emerging field in nutrition research that focuses on the potential interaction between dietary habits and circadian rhythm and investigates how well meal timing and frequency and quality of foods consumed at each meal are associated with health consequences18,23.
Studies have indicated that breakfast skipping24,25,26, energy contribution by meals27,28 and the number of eating occasions across the day29 could have an influence on health outcomes. The 2017 American Heart Association scientific statement suggested that meal-specific eating styles such as meal timing and frequency may be associated with cardiometabolic health and suggested focusing on such meal-specific properties to achieve a healthier lifestyle and improved risk factor management30. However, limited evidence is available about the association between meal-specific eating styles and cardiometabolic abnormalities. To our knowledge, no study has examined the potential association between meal-specific data-driven dietary patterns and biomarkers of IR in Iran. To address this gap, we performed a cross-sectional study to investigate whether meal-specific data-driven dietary patterns, identified by a data-reduction statistical approach, are associated with biomarkers of IR among Iranian adults.
Subjects and methods
Study design and participants
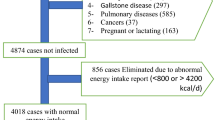
This cross-sectional study was conducted in apparently healthy men and women from Iran who attended health care centers of Tehran from February 2019 to August 2019. A sample size of 820 participants was calculated based on the following the formula n = ((zα + zβ)/(0.5 × In [(1 + r)/(1-r)]))2 + 331, where in r was the correlation between whole wheat bread and Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) (r = 0.098)32, and an α level of 0.05 and 1–β of 80%. Participants were recruited using a two-stage cluster sampling method within 25 healthcare centers across five different geographic areas of Tehran. A convenient sampling method was used to select the study participants from each health center, using the proportion-to-size approach. The inclusion criteria were having 18–59 years old and a body mass index (BMI) of 18.5–39.9 kg/m2. The exclusion criteria were pregnancy or lactation, and having a chronic disease.
Ethical approval
The study was ethically approved by the Ethics Committee of Tehran University of Medical Sciences (IR.TUMS.Medicine.REC.1399.295). The purpose of the study was explained to the participants, and all participants were given written informed consent precede to enter the study. The methods were conducted in accordance with the relevant Declaration of Helsinki guidelines and regulations.
Dietary intake assessment and meal timing
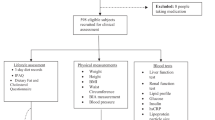
Dietary data were obtained based on three 24-h dietary recalls on non-consecutive days within the week. We conducted all recalls by trained dietitians during a private interview. The first 24-h dietary recall was recorded on the first visit in the health care center. The following recalls were collected via telephone on random days. A total of 2459 recalls were recorded. Subjects reported the following types of eating occasions in which food was consumed: breakfast, lunch, dinner, or snacks. The definition of main meals and afternoon snake according to the time of food intake was explained in a prior article23.
Daily intakes of all food items, derived from three 24-h dietary recalls, were converted into grams per day by using household measures33. Intake of food groups was adjuster for energy intake by using the residual method34. We used the Nutritionist IV software (First Databank, San Bruno, CA, USA), modified for Iranian foods, to obtain the values of energy and nutrients intake per day. A total of 420 food items were derived from 24-h dietary recalls and were classified into 26 food groups (Supplementary Table 1) based on the similarity of nutrient content in each food item and a literature search22,35,36,37. Every food group consumed at meals was used to extract meal-specific dietary patterns.
Data collection
Data were collected from each person by a face-to-face interview. Sociodemographic characteristics were collected by using pre-specified data extraction forms and included age, gender, marriage status (single, married, divorced), income (monthly income), smoking status (not smoking, ex-smoking, current smoking), education level (illiterate, under diploma and diploma, educated), occupation status (employed, unemployed, retired), supplement intake (yes or no) and living status (live alone or live with someone).
Physical activity
Physical activity was measured by the short form of the validated International Physical Activity Questionnaire (IPAQ)38. Participants reported the time spent walking or doing moderate- and/or vigorous-intensity activities within the previous seven days. The overall physical activity level was measured in the form of metabolic equivalent minutes per week (MET-minutes/week). MET scores were then categorized into three levels: point score < 600 MET-min/week as low physical activity, point score 600–3000 MET-min/week as moderate physical activity, and point score > 3000 MET-min/week as high physical activity39.
Assessment of blood pressure
Blood pressure was measured on the right hand by a digital barometer (BC 08, Beurer, Germany) after at least 10–15 min of rest and sitting. Blood pressure was measured twice for every person, and the average of the two measurements was reported for each person.
Anthropometric measurements
Weight was measured using a Seca weighing scale (Seca and Co. KG; 22 089 Hamburg, Germany; Model: 874 1321009; designed in Germany; made in China) with light clothing (without a coat and raincoat). A wall stadiometer board with a sensitivity of 0.1 cm was used to measure standing height, without shoes. BMI was calculated as weight (WT) in kilograms divided by height (HT) in meters squared (BMI: WT/HT2). Waist circumference (WC) was measured using a non-stretchable fiberglass measuring tape at the midpoint between the lower border of the rib cage and the iliac crest. Waist-hip ratio (WHR) was calculated for each person by dividing WC by hip circumference.
Laboratory investigations
All participants donated ten ml of blood between the hours 7–10 am in a fasted status. Following this, blood samples were collected in acid-washed test tubes without anticoagulants. Then, it was being stored at room temperature for thirty minutes and clot formation, blood samples were centrifuged at 1500 g for twenty minutes. Serums were stored at – 80 °C until future testing. Fasting plasma glucose (FPG) was assayed by the enzymatic (glucose oxidase) colorimetric method using a commercial kit (Pars Azmun, Iran, Pars Azmun Inc.). Serum total (TC) and high-density lipoprotein cholesterol (HDL-C) were measured using a cholesterol oxidase phenol aminoantipyrine method, and serum triglyceride (TG) was measured using a glycerol-3 phosphate oxidase phenol aminoantipyrine enzymatic method. Serum low-density lipoprotein cholesterol (LDL-C) was calculated using the Friedewald formula40. Serum insulin concentration was measured using the commercial kits (AccuBind Insulin ELIZA, USA, Monobind Inc.) and enzyme-linked immunosorbent assay (ELISA) method. Serum uric acid was measured by the calorimetry method using commercial kits (Bionic, Iran, Bionic Inc.) and biolysis 24. Serum C-reactive protein (CRP) was measured by a commercial kit (CRP LX (300 T) cobass c intergra, Germany, Roche Inc.) by the immunoturbidimetric method.
Definition of insulin resistant indicators
Triglycerides and glucose (TyG) index is a marker of insulin resistance41, which predicts the development of metabolic disorders and CVD42. TyG index was calculated based on the following formula: TyG Index: \(\frac{\mathrm{In }(\mathrm{Fasting triglycerides }\left(\mathrm{TG}\right) \left[\frac{\mathrm{mg}}{\mathrm{dL}}\right]*\mathrm{ Glucose }\left(\mathrm{ FPGPG}\right)\left[\frac{\mathrm{mg}}{\mathrm{dL}}\right]) }{2}\).
Lipid Accommodation Product (LAP) index, as a marker of CVD, is a simple indicator of high lipid accumulation in adults43, and has greater sensitivity and specificity than waist measures to show insulin resistance44. Based on values of WC and fasting TG, the LAP score was calculated using the following formula. Men: (WC (Cm)—65) * TG \(\left[\frac{\mathrm{mmol}}{\mathrm{L}}\right]\) and Women: (WC (Cm) -58)* TG \(\left[\frac{\mathrm{mmol}}{\mathrm{L}}\right]\).
HOMA is a measure of insulin resistance (HOMA-IR) and β-cell function among the diabetic and non-diabetic populations45. HOMA of \(\beta \)-cell function (HOMA-IS) is thought to be a good measure of \(\beta \)-cell function. High HOMA-IR and low HOMA-IS values were associated with glucose intolerance and subsequent risk of type 2 diabetes46,47. HOMA-IR: \(\frac{\mathrm{Fasting insulin }\left(\frac{\mathrm{\mu IU}}{\mathrm{mL}}\right)*\mathrm{ FPG }(\frac{\mathrm{mg}}{\mathrm{dL}})}{405}\) and HOMA-IS: \(\frac{20*\mathrm{Fasting insulin }\left(\frac{\mathrm{\mu IU}}{\mathrm{mL}}\right)}{\mathrm{FPG }\left(\frac{\mathrm{mg}}{\mathrm{dL}}\right)-3.5}\).
Statistical analyses
Dietary patterns at meals level (breakfast, lunch, afternoon, and dinner) were determined by principal component analysis (PCA). PCA is a data reduction statistical method that is frequently used to perform dietary pattern analysis and explore posteriori-defined eating patterns in nutrition epidemiologic research48. PCA extracts common patterns according to the correlation matrix of food intake49. The Kaiser–Meyer–Olkin (KMO) test was used to measure sampling adequacy and Bartlett’s test of sphericity was used to investigate the adequacy of test items and sample size for factor analysis. KMO values > 0.50 was considered as an adequate sample size50. The factor loading indicates the correlation between food groups and food patterns and varies from (\(-\) 1 to + 1). A positive loading score indicates a positive association with the factor, whereas a negative loading score indicates an inverse association with the factor. Larger positive or negative factor loadings for foods indicate which food groups are important in that component (dietary pattern). The factor loading with magnitude < − 0·2 or > 0.2 were indicated in the tables for simplicity51. The number of key dietary patterns to retain was determined based on scree plot analysis (factors with eigenvalues > 1.5) and the interpretability of the identified patterns. Adherence to the meal-based dietary patterns (breakfast, lunch, afternoon, and dinner) was determined based on pattern scores and was categorized into tertiles. Basal Metabolic Rate (BMR) was calculated using standard equations based on weight, age, and sex. Then, the BMR: EI (Basal Metabolic Rate to Energy Intake) is used to assess the validity of the reported amount of energy. Under-reporting of energy intake as BMR: EI < 1.35 and over-reporting as BMR: EI ≥ 2.40 was defined52.
Kolmogorov–Smirnov test was used to determine the normal distribution of the data. If the data were not normal, a logarithmic transform was used to normalize them; otherwise, non-parametric tests were used to analyze the data. Demographic, lifestyle characteristics, and health status of the study participants were compared between either sex by using χ2 for categorical variables and a t-test for continuous variables. To compare mean and variations of dependent variables across tertiles of meal-specific dietary patterns, we used multivariate analysis of variance (MANOVA) analysis in crude model and after controlling for confounders including age, sex, physical activity, smoking, marital status, income, supplementation, and education. Statistical analyses were conducted using SPSS version 22.0, and P-values < 0.05 were considered statistically significant.
Ethics approval and consent to participate
The study was ethically approved by the Ethics Committee of Tehran University of Medical Sciences (IR.TUMS.Medicine.REC.1399.295). Informed consent was obtained from all subjects involved in the study.
Results
The present cross-sectional study was conducted on 850 adults. To avoid misreporting, we excluded 25 participants due to the following reasons: two participants due to underreporting of energy intake (BMR: EI < 1.35), and the other 23 participants due to over-reporting of their energy intake (BMR: EI > 2.40). Finally, 825 participants including 140 men (19.96%) and 685 women (80.04%) with an age range of 20–59 years old and a mean (SD) age of 42.17 (10.5) years, were analyzed. The mean (SD) BMI was 27.1 (4.49) kg/m2, and the average calorie intake was 1681 (374) kcal/d. Of 825 participants, 50 participants had two 24-h dietary recalls and the other 775 participants had three 24-h dietary recalls. General characteristics of the study participants are presented in Table 1.
PCA identified two principal components (dietary patterns) at each meal (Table 2). Breakfast, lunch, afternoon, and dinner dietary patterns explained 26.97%, 24.97%, 26.34%, and 23.64% of the total variation of diet, respectively. (Supplement Figs. 1–5). The KMO index was 0.54 for breakfast, afternoon and dinner dietary patterns, and 0.57 for lunch dietary patterns. Also, Bartlett’s test was significant (P < 0.001) for all meals.
Table 2 presents characteristics of the dietary patterns identified at each meal. In breakfast, the pattern labeled “Oil, egg and cereals” was characterized by a high intake of liquid vegetable oils, egg, cereals and grains, legumes, potato, and low intake of milk and dairy products. The second pattern at breakfast was labelled “Bread, vegetable and cheese” and was characterized by high intake in bread, vegetables, cheese, and tea and herbal tea and low intake in butter, milk and dairy products, poultry and cereals and grains. At lunch, the pattern labeled “Western” was featured by bread, industrial beverages and juices, sugar and sweets, processed meat, liquid vegetable oils and low intake in poultry, milk and dairy products. The second pattern at lunch meal, labeled “Oil, dairy, potato and egg”, was characterized by high intake of liquid vegetable oils, milk and dairy products, potato, egg, vegetables, pickle, poultry and broth and low intake in sugar and sweets, butter and red meat and organ meats. In the afternoon, the pattern labelled “bread, cereals and oils” was identified by high intake of bread, cereals and grains, liquid vegetable oils, egg, legume, potato and butter. The second pattern in afternoon was labeled “dessert, soft drink and tee” and was characterized by a high intake in sugar and sweets, industrial beverages and juices, potato, tea and herbal tea. At dinner, the pattern labeled “Western” was featured by bread, industrial beverages and juices, sugar and sweets, processed meat, liquid vegetable oils and sauces. The second pattern labeled “Cereals, oil, poultry, and legume” pattern was characterized by a high intake of cereals and grains, liquid vegetable oils, vegetables, tea and herbal tea, poultry, pickles, and legumes and low intake of bread, egg, and, industrial beverages and juices.
We used MANOVA test to assess the association between meal-specific dietary patterns and biomarkers of obesity, FPG, insulin resistance and inflammation at each meal. Greater adherence to the “bread, vegetables, and cheese” dietary pattern was associated with a lower FPG concentration in crude model (P value = 0.02) and adjusted model (P value = 0.04), in contrast, greater adherence to the “oil, egg, and cereals” dietary pattern was accompanied by a higher BMI in adjusted model (P value = 0.01), a higher FPG concentration in crude model (P value = 0.04) and in adjusted model (P value = 0.01), and a higher TyG-index crude model (P value = 0.007) and adjusted model (P value = 0.004) in adjusted model Table 3. At lunch meal, greater adherence to the “Western” dietary pattern was associated with higher WC (P value = 0.04) and BMI values (P value = 0.04) and a lower HOMA-IS (P value = 0.049) in adjusted model (Table 4).
At afternoon meal, a greater adherence to the “bread, cereals, and oil” dietary pattern was associated with a lower WC (P value = 0.02) in adjusted model (Table 5). We also found significant associations between adherence to the Western dietary pattern at dinner meal and some CVD risk factors, in ways that greater adherence to the Western dietary pattern was associated with higher WC (P value = 0.04), BMI (P value = 0.04), and serum CRP concentrations (P value = 0.04) and in contrast, was associated to a lower HOMA-IS value (P value = 0.04) in adjusted model (Table 6).
Discussion
In the present cross-sectional study, we used PCA to derive meal-specific dietary patterns and then, investigated how well values of CVD risk factors and insulin indices change along with the increase in adherence to meal-specific dietary patterns. The analyses suggested a significant association between dietary patterns at breakfast meal and some CVD risk factors, in ways that greater adherence to the “bread, vegetables, and cheese” dietary pattern was associated with a lower FPG and in contrast, adherence to the “oil, egg, and cereals” dietary pattern was associated to a higher FPG concentration, BMI, and TyG index. There were also some suggestions of a positive association between adherence to the “Western” dietary pattern at lunch and dinner meals and BMI and WC and an inverse association with insulin sensitivity, as assessed by HOMA-IS. We also found that adherence to the “bread, cereals, and oil” dietary pattern as afternoon snack was associated to a lower WC. The characteristics of the "Western" dietary pattern at lunch and dinner meals in our study were similar to the Western-style dietary patterns, derived from habitual or meal-specific dietary intake, in other studies conducted in Iran35,53,54,55, Germany36 and Brazil56,57. A high factor loading of sugar and sweet drinks, processed meat and butter found in the Western pattern was similar to those found in previous studies among Iranian adults35,53,58. At breakfast meal, the “bread, vegetable and cheese” dietary pattern identified in our study was similar to a dietary pattern labeled “Mediterranean” found at a breakfast meal in a cross-sectional study of European adolescents59, as well as the “Brazilian southeastern” dietary pattern found at a breakfast meal in a large cross-sectional study in Brazil60. Our “oil, egg and cereals” dietary pattern at breakfast meal shared similarities with those found in previous studies among European adolescents59 and Brazilian adults61. In addition, our “cereals, oil, poultry and, legume” pattern found at dinner meal shared similar food groups to dietary patterns labeled “other grains and fat”62, “cereals and legumes”36, “traditional”63, “healthy”35, “fruits and vegetables”61, “balanced”64 found at dinner meals in previous research. However, dietary patterns could be different because of differences in sex55, social and economic status, ethnicity65, marriage status, and food security66 across diverse populations. Also, dissimilarity in beliefs, religions, cultures, educational status and employment status could lead to dissimilarity in dietary patterns 15,67.
The association of habitual dietary patterns with cardiometabolic risk factors and obesity has been well established in several studies across different countries15,68,69,70,71,72. Totally, these studies showed that a “healthy” dietary pattern that is rich in fish, poultry, nuts, legume, vegetables and fruits is negatively associated with cardiometabolic risk including insulin resistance and inflammation15,68,73, whereas a “unhealthy” dietary pattern that is rich in foods such as red meat, processed food, and fried food is directly associated with cardiometabolic risk15,70. Previous studies also showed that the “Western” dietary pattern as a unhealthy pattern, is associated with higher insulin resistance15,70, metabolic syndrome15 and inflammation74,75. In contrast, a “Healthy” dietary pattern high in low-fat dairy products, fruit, whole grains, poultry, fish and vegetables was associated with greater insulin sensitivity and lower systemic inflammation68. Higher adherence to healthy food patterns such as the "Mediterranean" pattern, with high consumption of olive oil, fruits, vegetables, legumes, and low-fat dairy products was significantly associated with lower CRP75. Saghafi-Asl et al. have previously demonstrated that greater adherence to “Traditional” dietary pattern characterized by high intake of fruits and green vegetable, other vegetable poultry, organ meat, red meat and hydrogenated fat was inversely related to lower HOMA-IR76. Amini and et al. reported that higher adherence of “Healthy” dietary pattern by higher factor loading of legume, poultry, vegetable, fruits, egg, fish and nut related to lower LAP but not significantly related to TyG index73. The same as habitual intake, there are evidence that meal patterns play an important role in the development of cardiometaolic risk factors. A previous study has shown that snacking could alter postprandial glucose, insulin concentrations and satiety77. Additionally, eating at the right or wrong time, the duration of calorie restriction, the time of meals and the time of macronutrient intake during a day and even on different days can play an important role in the amount of daily calorie intake and regulating the body weight and fat mass78 that are responsible for both insulin resistance and chronic low-grade systemic inflammation79. Eating breakfast and consuming fruits and vegetables at breakfast had also a significant relationship with less daily calorie intake80. Moreover, the time of meals intake is considered to be an effective factor in health and metabolic function, so that, late dinner increases the risk of obesity/overweight and inflammatory biomarkers20.
In the present study, the pattern of “oil, egg and cereals” at breakfast meal had a significant positive relationship with BMI, FPG and TyG-index. In contrast, a higher adherence to the “bread, vegetable and cheese” pattern at breakfast meal was related to a lower BMI. In line with our findings, the pattern labeled "breakfast" among European girls and boys, characterized by high consumption of bread, fruit, cheese and dairy products, indicated an inverse relationship with obesity after controlling for confounders. However, contrary to our findings, the "Mediterranean" and "plant-based and eggs" dietary patterns at breakfast meal did not show a significant association with obesity59. Similar to our findings, the “processed-food” dietary pattern in German adults showed a positive association with BMI and WC but not with glycated hemoglobin81.
In a recent study, a higher adherence to the "Western" pattern at lunch and dinner was related to a higher WC and BMI and lower HOMA-IS. Also, higher adherence to the "Western" dietary pattern at dinner associated with higher CRP. A positive association between "Western" habitual dietary pattern and obesity was similar to our previous findings69,82, although some studies did not find a significant relationship at dinner pattern17, in the lunch63 and daily pattern83. Similar to our findings, previous studies indicated “Western” dietary patterns associated with higher insulin resistance15,70 and inflammation74.
Strengths and limitations
We used 24-h dietary recalls, a short-term dietary assessment method, that includes more detailed information about types and amounts of food than long-term assessment methods. All self- reported dietary assessment methods have measurement error, but 24-h dietary recalls are a better measure than FFQ and also, different to FFQ, allow for meal-specific analysis84. Most previous studies assessed the association between habitual dietary pattern and disease. According to our knowledge, this is the first study that investigated the relationship between meal-specific dietary pattern and insulin resistance indices. However, under or over reporting of dietary intake is a serious problem related to self-reported dietary assessment methods, particularly in a population with overweight or obese84. In addition, subjective decision listed of food groups in PCA, the number of factors extracted and the definition of eating occasions might have a kind of inconsistency in results. Moreover, this is a cross-sectional study which is not able to determine cause and effect relationships. Then, longitudinal study design is recommended to better understanding of causality.
Conclusion
These results provide evidence that major meal-specific dietary patterns were associated with insulin resistance biomarkers in a sample of Iranian adults. These findings may help inform designing dietary interventions for improving dysmetabolic risk factors. Further prospective studies are required to confirm such relationships.
Data availability
The datasets generated and analyzed in current study are available from the corresponding author (SSb) upon request with reasonable justification. The data are not publicly available because they contain confidential information that may compromise the privacy/consent of the participants.
References
Wallace, T. M. & Matthews, D. R. The assessment of insulin resistance in man. Diabet. Med. 19, 527–534. https://doi.org/10.1046/j.1464-5491.2002.00745.x (2002).
de Luca, C. & Olefsky, J. M. Inflammation and insulin resistance. FEBS Lett. 582, 97–105. https://doi.org/10.1016/j.febslet.2007.11.057 (2008).
Orgel, E. & Mittelman, S. D. The links between insulin resistance, diabetes, and cancer. Curr. Diab. Rep. 13, 213–222. https://doi.org/10.1007/s11892-012-0356-6 (2013).
Ginsberg, H. N. Insulin resistance and cardiovascular disease. J. Clin. Investig. 106, 453–458. https://doi.org/10.1172/jci10762 (2000).
Arcidiacono, B. et al. Insulin resistance and cancer risk: An overview of the pathogenetic mechanisms. Exp. Diabetes Res. 2012, 789174. https://doi.org/10.1155/2012/789174 (2012).
Hardy, O. T., Czech, M. P. & Corvera, S. What causes the insulin resistance underlying obesity?. Curr. Opin. Endocrinol. Diabetes. Obes. 19, 81–87. https://doi.org/10.1097/MED.0b013e3283514e13 (2012).
Isharwal, S., Misra, A., Wasir, J. S. & Nigam, P. Diet & insulin resistance: A review & Asian Indian perspective. Indian J. Med. Res. 129, 485–499 (2009).
Liese, A. D. et al. Whole-grain intake and insulin sensitivity: The Insulin Resistance Atherosclerosis Study. Am. J. Clin. Nutr. 78, 965–971. https://doi.org/10.1093/ajcn/78.5.965 (2003).
Wallace, I. R. et al. Dose-response effect of fruit and vegetables on insulin resistance in people at high risk of cardiovascular disease: A randomized controlled trial. Diabetes Care 36, 3888–3896. https://doi.org/10.2337/dc13-0718 (2013).
Casas-Agustench, P., Bulló, M. & Salas-Salvadó, J. Nuts, inflammation and insulin resistance. Asia Pac. J. Clin. Nutr. 19, 124–130 (2010).
Zelber-Sagi, S. et al. High red and processed meat consumption is associated with non-alcoholic fatty liver disease and insulin resistance. J. Hepatol. 68, 1239–1246. https://doi.org/10.1016/j.jhep.2018.01.015 (2018).
Rivera-Paredez, B. et al. Cumulative soft drink consumption is associated with insulin resistance in Mexican adults. Am. J. Clin. Nutr. 112, 661–668. https://doi.org/10.1093/ajcn/nqaa169 (2020).
McNaughton, S. A., Mishra, G. D. & Brunner, E. J. Dietary patterns, insulin resistance, and incidence of type 2 diabetes in the Whitehall II Study. Diabetes Care 31, 1343–1348. https://doi.org/10.2337/dc07-1946 (2008).
Villegas, R., Salim, A., Flynn, A. & Perry, I. J. Prudent diet and the risk of insulin resistance. Nutr. Metab. Cardiovasc. Dis. 14, 334–343. https://doi.org/10.1016/s0939-4753(04)80023-1 (2004).
Esmaillzadeh, A. et al. Dietary patterns, insulin resistance, and prevalence of the metabolic syndrome in women. Am. J. Clin. Nutr. 85, 910–918. https://doi.org/10.1093/ajcn/85.3.910 (2007).
Shi, Z., Riley, M., Taylor, A. & Noakes, M. Meal-specific food patterns and the incidence of hyperglycemia in a Chinese adult population. Br. J. Nutr. 118, 53–59. https://doi.org/10.1017/s000711451700174x (2017).
Akbarzade, Z. et al. The association between major dietary patterns at dinner and obesity in adults living in Tehran: A population-based study. J. Cardiovasc. Thorac. Res. 12, 269–279. https://doi.org/10.34172/jcvtr.2020.45 (2020).
Oda, H. Chrononutrition. J. Nutr. Sci. Vitaminol. (Tokyo) 61(Suppl), S92-94. https://doi.org/10.3177/jnsv.61.S92 (2015).
Almoosawi, S., Prynne, C., Hardy, R. & Stephen, A. Time-of-day and nutrient composition of eating occasions: Prospective association with the metabolic syndrome in the 1946 British birth cohort. Int. J. Obes. 37, 725–731 (2013).
Martínez-Lozano, N. et al. Late eating is associated with obesity, inflammatory markers and circadian-related disturbances in school-aged children. Nutrients https://doi.org/10.3390/nu12092881 (2020).
Molzof, H. E. et al. The impact of meal timing on cardiometabolic syndrome indicators in shift workers. Chronobiol. Int. 34, 337–348. https://doi.org/10.1080/07420528.2016.1259242 (2017).
Manoogian, E. N. C., Chaix, A. & Panda, S. When to eat: The importance of eating patterns in health and disease. J. Biol. Rhythms 34, 579–581. https://doi.org/10.1177/0748730419892105 (2019).
Lesani, A., Djafarian, K., Akbarzade, Z., Janbozorgi, N. & Shab-Bidar, S. Meal-specific dietary patterns and their contribution to habitual dietary patterns in the Iranian population. Br. J. Nutr. https://doi.org/10.1017/s0007114521005067 (2022).
Goyal, R. & Julka, S. Impact of breakfast skipping on the health status of the population. Indian J. Endocrinol. Metab. 18, 683 (2014).
Hopkins, L. C., Sattler, M., Steeves, E. A., Jones-Smith, J. C. & Gittelsohn, J. Breakfast consumption frequency and its relationships to overall diet quality, using healthy eating index 2010, and body mass index among adolescents in a low-income urban setting. Ecol. Food Nutr. 56, 297–311 (2017).
Ahola, A. J., Mutter, S., Forsblom, C., Harjutsalo, V. & Groop, P.-H. Meal timing, meal frequency, and breakfast skipping in adult individuals with type 1 diabetes–associations with glycaemic control. Sci. Rep. 9, 1–10 (2019).
Rosato, V. et al. Energy contribution and nutrient composition of breakfast and their relations to overweight in free-living individuals: A systematic review. Adv. Nutr. 7, 455–465 (2016).
Madjd, A. et al. Beneficial effect of high energy intake at lunch rather than dinner on weight loss in healthy obese women in a weight-loss program: A randomized clinical trial. Am. J. Clin. Nutr. 104, 982–989 (2016).
Yoo, K.-B. et al. Breakfast eating patterns and the metabolic syndrome: The Korea National Health and Nutrition Examination Survey (KNHANES) 2007–2009. Asia Pac. J. Clin. Nutr. 23, 128–137 (2014).
St-Onge, M. P. et al. Meal timing and frequency: Implications for cardiovascular disease prevention: A scientific statement from the American Heart Association. Circulation 135, e96–e121. https://doi.org/10.1161/cir.0000000000000476 (2017).
Hulley, S. B., Cummings, S. R., Browner, W. S., Grady, D. & TB, N. Designing Clinical Research: An Epidemiologic Approach Vol. Appendix 6C (Lippincott Williams & Wilkins, 2013).
Ehrampoush, E. et al. Association between dietary patterns with insulin resistance in an Iranian population. Clin. Nutr. ESPEN 36, 45–52. https://doi.org/10.1016/j.clnesp.2020.02.011 (2020).
Ghaffarpour, M., Houshiar-Rad, A. & Kianfar, H. The manual for household measures, cooking yields factors and edible portion of foods. Tehran 7, 213 (1999).
Willett, W. C., Howe, G. R. & Kushi, L. H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr., 65, 1220S-1228S; discussion 1229S-1231S. https://doi.org/10.1093/ajcn/65.4.1220S (1997).
Aghayan, M. et al. Secular trend in dietary patterns of Iranian adults from 2006 to 2017: Tehran lipid and glucose study. Nutr. J. 19, 110. https://doi.org/10.1186/s12937-020-00624-x (2020).
Schwedhelm, C., Iqbal, K., Knüppel, S., Schwingshackl, L. & Boeing, H. Contribution to the understanding of how principal component analysis-derived dietary patterns emerge from habitual data on food consumption. Am. J. Clin. Nutr. 107, 227–235. https://doi.org/10.1093/ajcn/nqx027 (2018).
Akbarzade, Z. et al. The association between lunch composition and obesity in Iranian adults. Br. J. Nutr. 127, 1517–1527. https://doi.org/10.1017/S0007114521002543 (2022).
Committee, I. R. Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ)-short and long forms (2005).
Ainsworth, B. E. et al. 2011 compendium of physical activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 43, 1575–1581. https://doi.org/10.1249/mss.0b013e31821ece12 (2011).
Friedewald, W. T., Levy, R. I. & Fredrickson, D. S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18, 499–502 (1972).
Unger, G., Benozzi, S. F., Perruzza, F. & Pennacchiotti, G. L. Triglycerides and glucose index: A useful indicator of insulin resistance. Endocrinol. Nutr. 61, 533–540. https://doi.org/10.1016/j.endonu.2014.06.009 (2014).
Sánchez-Íñigo, L., Navarro-González, D., Fernández-Montero, A., Pastrana-Delgado, J. & Martínez, J. A. The TyG index may predict the development of cardiovascular events. Eur. J. Clin. Investig. 46, 189–197. https://doi.org/10.1111/eci.12583 (2016).
Maturana, M. A., Moreira, R. M. & Spritzer, P. M. Lipid accumulation product (LAP) is related to androgenicity and cardiovascular risk factors in postmenopausal women. Maturitas 70, 395–399. https://doi.org/10.1016/j.maturitas.2011.09.012 (2011).
Marcadenti, A., Fuchs, F. D., Moreira, L. B., Gus, M. & Fuchs, S. C. Adiposity phenotypes are associated with type-2 diabetes: LAP index, body adiposity index, and neck circumference. Atherosclerosis 266, 145–150. https://doi.org/10.1016/j.atherosclerosis.2017.09.022 (2017).
Matthews, D. R. et al. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419. https://doi.org/10.1007/bf00280883 (1985).
Wallace, T. M., Levy, J. C. & Matthews, D. R. Use and abuse of HOMA modeling. Diabetes Care 27, 1487–1495. https://doi.org/10.2337/diacare.27.6.1487 (2004).
Song, Y. et al. Insulin sensitivity and insulin secretion determined by homeostasis model assessment and risk of diabetes in a multiethnic cohort of women: the Women’s Health Initiative Observational Study. Diabetes Care 30, 1747–1752. https://doi.org/10.2337/dc07-0358 (2007).
Hoffmann, K., Schulze, M. B., Schienkiewitz, A., Nöthlings, U. & Boeing, H. Application of a new statistical method to derive dietary patterns in nutritional epidemiology. Am. J. Epidemiol. 159, 935–944. https://doi.org/10.1093/aje/kwh134 (2004).
Newby, P. K. & Tucker, K. L. Empirically derived eating patterns using factor or cluster analysis: A review. Nutr. Rev. 62, 177–203. https://doi.org/10.1301/nr.2004.may.177-203 (2004).
Kline, P. An Easy Guide to Factor Analysis 1st edn. (Routledge, 1994). https://doi.org/10.4324/9781315788135.
Castelló, A. et al. Reproducibility of data-driven dietary patterns in two groups of adult Spanish women from different studies. Br. J. Nutr. 116, 734–742. https://doi.org/10.1017/s000711451600252x (2016).
Mirmiran, P., Esmaillzadeh, A. & Azizi, F. Under-reporting of energy intake affects estimates of nutrient intakes. Asia Pac. J. Clin. Nutr. 15, 459–464 (2006).
Esmaillzadeh, A. & Azadbakht, L. Major dietary patterns in relation to general obesity and central adiposity among Iranian women. J. Nutr. 138, 358–363. https://doi.org/10.1093/jn/138.2.358 (2008).
Asadi, Z. et al. Association of dietary patterns and risk of cardiovascular disease events in the MASHAD cohort study. J. Hum. Nutr. Diet. 32, 789–801. https://doi.org/10.1111/jhn.12669 (2019).
Kafeshani, O., Sarrafzadegan, N., Nouri, F. & Mohammadifard, N. Major dietary patterns in Iranian adolescents: Isfahan healthy heart program. Iran. ARYA Atheroscler. 11, 61–68 (2015).
de Oliveira Santos, R., Fisberg, R. M., Marchioni, D. M. & Troncoso Baltar, V. Dietary patterns for meals of Brazilian adults. Br. J. Nutr. 114, 822–828. https://doi.org/10.1017/s0007114515002445 (2015).
Antunes, A. B. S. et al. Dietary patterns of Brazilian adults in 2008–2009 and 2017–2018. Rev. Saude Publica 55, 8s. https://doi.org/10.11606/s1518-8787.2021055003437 (2021).
Abdollahi, S., Zeinali, F., Azam, K., Toupchian, O. & Djafarian, K. Identifying major dietary patterns among the elderly in tehran health homes. Jundishapur J. Health Sci. 7, e30395. https://doi.org/10.17795/jjhs-30395 (2015).
Cacau, L. T. et al. Breakfast dietary pattern is inversely associated with overweight/obesity in european adolescents: The HELENA study. Children https://doi.org/10.3390/children8111044 (2021).
Baltar, V. T., Cunha, D. B., Santos, R. O., Marchioni, D. M. & Sichieri, R. Breakfast patterns and their association with body mass index in Brazilian adults. Cad Saude Publica 34, e00111917. https://doi.org/10.1590/0102-311x00111917 (2018).
Drehmer, M. et al. Brazilian dietary patterns and the dietary approaches to stop hypertension (DASH) diet-relationship with metabolic syndrome and newly diagnosed diabetes in the ELSA-Brasil study. Diabetol. Metab. Syndr. 9, 13. https://doi.org/10.1186/s13098-017-0211-7 (2017).
Murakami, K., Livingstone, M. B. E. & Sasaki, S. Meal-specific dietary patterns and their contribution to overall dietary patterns in the Japanese context: Findings from the 2012 National Health and Nutrition Survey, Japan. Nutrition 59, 108–115. https://doi.org/10.1016/j.nut.2018.07.110 (2019).
de Oliveira Santos, R. et al. The traditional lunch pattern is inversely correlated with body mass index in a population-based study in Brazil. BMC Public Health 18, 33. https://doi.org/10.1186/s12889-017-4582-3 (2017).
Xia, Y. et al. Association between dietary patterns and metabolic syndrome in Chinese adults: A propensity score-matched case-control study. Sci. Rep. 6, 34748. https://doi.org/10.1038/srep34748 (2016).
Nicklas, T. A., Webber, L. S., Thompson, B. & Berenson, G. S. A multivariate model for assessing eating patterns and their relationship to cardiovascular risk factors: The Bogalusa Heart Study. Am. J. Clin. Nutr. 49, 1320–1327. https://doi.org/10.1093/ajcn/49.6.1320 (1989).
Rezazadeh, A. et al. Major dietary patterns in relation to demographic and socio-economic status and food insecurity in two Iranian ethnic groups living in Urmia. Iran. Public Health Nutr. 19, 3337–3348. https://doi.org/10.1017/S1368980016001634 (2016).
Hosseyni Esfahani, F., Jazayeri, A., Mirmiran, P., Mehrabi, Y. & Azizi, F. Dietary patterns and their association with socio-demographic and lifestyle factors among Thehrani adults: Tehran Lipid and Glucose Study. J. School Public Health Inst. Public Health Res. 6, 23–36 (2008).
Anderson, A. L. et al. Dietary patterns, insulin sensitivity and inflammation in older adults. Eur. J. Clin. Nutr. 66, 18–24. https://doi.org/10.1038/ejcn.2011.162 (2012).
Naja, F. et al. A Western dietary pattern is associated with overweight and obesity in a national sample of Lebanese adolescents (13–19 years): A cross-sectional study. Br. J. Nutr. 114, 1909–1919. https://doi.org/10.1017/s0007114515003657 (2015).
Romero-Polvo, A. et al. Association between dietary patterns and insulin resistance in Mexican children and adolescents. Ann. Nutr. Metab. 61, 142–150. https://doi.org/10.1159/000341493 (2012).
Petersen, K. S. et al. Healthy dietary patterns for preventing cardiometabolic disease: The role of plant-based foods and animal products. Curr. Dev. Nutr. https://doi.org/10.3945/cdn.117.001289 (2017).
Qin, C. et al. Dietary patterns and cardiometabolic diseases in 0.5 million Chinese adults: A 10-year cohort study. Nutr. J. 20, 74. https://doi.org/10.1186/s12937-021-00730-4 (2021).
Amini, M. R. et al. Association of dietary patterns with visceral adiposity, lipid accumulation product, and triglyceride-glucose index in Iranian adults. Clin. Nutr. Res. 9, 145–156. https://doi.org/10.7762/cnr.2020.9.2.145 (2020).
Khayyatzadeh, S. S. et al. A Western dietary pattern is associated with elevated level of high sensitive C-reactive protein among adolescent girls. Eur. J. Clin. Investig. https://doi.org/10.1111/eci.12897 (2018).
Nanri, H. et al. Association between dietary pattern and serum C-reactive protein in Japanese men and women. J. Epidemiol. 21, 122–131. https://doi.org/10.2188/jea.je20100110 (2011).
Saghafi-Asl, M. et al. The association of dietary patterns with dietary inflammatory index, systemic inflammation, and insulin resistance, in apparently healthy individuals with obesity. Sci. Rep. 11, 7515. https://doi.org/10.1038/s41598-021-86993-7 (2021).
Chapelot, D. The role of snacking in energy balance: A biobehavioral approach. J. Nutr. 141, 158–162. https://doi.org/10.3945/jn.109.114330 (2011).
Johnston, J. D. Physiological responses to food intake throughout the day. Nutr. Res. Rev. 27, 107–118. https://doi.org/10.1017/s0954422414000055 (2014).
Qatanani, M. & Lazar, M. A. Mechanisms of obesity-associated insulin resistance: Many choices on the menu. Genes Dev. 21, 1443–1455. https://doi.org/10.1101/gad.1550907 (2007).
Cho, S., Dietrich, M., Brown, C. J., Clark, C. A. & Block, G. The effect of breakfast type on total daily energy intake and body mass index: Results from the Third National Health and Nutrition Examination Survey (NHANES III). J. Am. Coll. Nutr. 22, 296–302. https://doi.org/10.1080/07315724.2003.10719307 (2003).
Iqbal, K. et al. Breakfast quality and cardiometabolic risk profiles in an upper middle-aged German population. Eur. J. Clin. Nutr. 71, 1312–1320. https://doi.org/10.1038/ejcn.2017.116 (2017).
Murtaugh, M. A. et al. Diet composition and risk of overweight and obesity in women living in the southwestern United States. J. Am. Diet Assoc. 107, 1311–1321. https://doi.org/10.1016/j.jada.2007.05.008 (2007).
Rutayisire, E. et al. Dietary patterns are not associated with overweight and obesity in a sample of 8900 Chinese preschool children from four cities. J. Nutr. Sci. 7, e24. https://doi.org/10.1017/jns.2018.15 (2018).
Livingstone, M. B. & Black, A. E. Markers of the validity of reported energy intake. J. Nutr. 133(Suppl 3), 895s–920s. https://doi.org/10.1093/jn/133.3.895S (2003).
Acknowledgements
We thank all those who participated in this study.
Funding
The current manuscript has been granted by Tehran University of Medical Sciences (Grant Number: 45553).
Author information
Authors and Affiliations
Contributions
A.L., M.K. and S.S.b. contributed to the conception/design of the research; Z.A., M.K. and A.L. contributed to acquisition, analysis, or interpretation of the data; A.L., A.J. and B.B. drafted the manuscript; S.S.b. and K.Dj. critically revised the manuscript; and S.S.b. agree to be fully accountable for ensuring the integrity and accuracy of the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lesani, A., Jayedi, A., Karimi, M. et al. Meal-specific dietary patterns and biomarkers of insulin resistance in a sample of Iranian adults: a cross-sectional study. Sci Rep 13, 7423 (2023). https://doi.org/10.1038/s41598-023-34235-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-34235-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.