Abstract
Researchers are constantly searching for drugs to combat the coronavirus pandemic caused by SARS-CoV-2, which has lasted for over two years. Natural compounds such as phenolic acids are being tested against Mpro and AAK1, which are key players in the SARS-CoV-2 life cycle. This research work aims to study the ability of a panel of natural phenolic acids to inhibit the virus's multiplication directly through Mpro and indirectly by affecting the adaptor-associated protein kinase-1 (AAK1). Pharmacophore mapping, molecular docking, and dynamic studies were conducted over 50 ns and 100 ns on a panel of 39 natural phenolic acids. Rosmarinic acid (16) on the Mpro receptor (− 16.33 kcal/mol) and tannic acid (17) on the AAK1 receptor (− 17.15 kcal/mol) exhibited the best docking energy against both receptors. These favourable docking score values were found to be superior to those of the co-crystallized ligands. Preclinical and clinical research is required before using them simultaneously to halt the COVID-19 life cycle in a synergistic manner.
Similar content being viewed by others
Introduction
There are numerous potential sources of phenolic acids, such as fruits, vegetables, spices, and herbs1. These substances are naturally occurring by-products or secondary metabolites of plants, and they are found in food on a regular basis. They provide essential health benefits for people, including antioxidant, anti-inflammatory, anticancer, anti-allergic, antihypertensive, and antiviral capabilities2. Many studies have demonstrated the effectiveness of these substances in preventing viruses from causing serious health issues3,4,5. Natural phenolic acids and their derivatives have shown strong inhibitory effects on a variety of human viruses, including coronavirus (SARS-CoV-2)6,7,8,9,10. Based on the target, there are two groups of potential anti-coronavirus medications: one targets the human immune system or human cells, while the other focuses on the coronavirus itself11,12. SARS-CoV-2 has seven major structural proteins, and several human targets, such as transmembrane serine protease 2 (TMPRSS2), cathepsin L, and adaptor-associated protein kinase 1 (AAK1), are involved in the progression of symptoms linked to SARS-CoV-2 infections14,15,16. Phenolic acid products have been identified as excellent candidates as anti-SARS-CoV treatments, especially towards Mpro and AAK119,20. Inhibition of AAK1 could be a good strategy to prevent SARS-CoV-2 entry. The carbonyl and hydroxyl groups of phenolic acids play a major role in forming good binding interactions with both Mpro and AAK121,22,23. These findings have encouraged researchers to investigate natural phenolic acids against these two plausible targets in the SARS-CoV-2 life cycle, in an effort to find new natural and safe treatments for COVID-19. The selected library of natural phenolic acids is represented in Fig. 1.
Methods
In this study, molecular operating environment (MOE, 2019.0102) software was used for pharmacophoric generation, searching and molecular docking24,25,26, energy minimized structures were gained by applying MMFF94x force field until RMSD gradient of 0.1 kcal mol−1 Å−1 was reached. The protein data bank (PDB) was the source of the X-ray crystallographic structures of SARS CoV-2 main protease enzyme (Mpro) (ID: 7AEH) and adaptor-associated protein kinase 1 (AAK1) (ID: 4WSQ). Triangle matcher as a placement method and London dG as a scoring algorithm were used to determine the pharmacophoric properties of both enzymes and the binding site in each protein file using the co-crystallized ligand. AutoDock Vina 1.1.2. Software was also used for conducting the molecular docking study to confirm the results. SwissADME was used to evaluate the ADME properties of the promising compounds27.
Pharmacophore mapping
SARS CoV-2 main protease enzyme (Mpro)
The binding characteristics for the co-crystallized ligand were chosen, stored, and utilized to search for potential matches from the phenolic acids database using the PDB file (ID: 7AEH) that was obtained from the protein data bank.
Adaptor-associated protein kinase 1 (AAK1)
The PDB file (ID: 4WSQ) was obtained for mapping the pharmacophoric characteristics of AAK1 and examined to determine the most crucial binding properties. For the purpose of choosing the most appropriate phenolic acid structures, the created pharmacophore was preserved and utilized.
Molecular docking
SARS CoV-2 main protease enzyme (Mpro)
The PDB file (ID: 7AEH) was used to identify the required key binding features for the ligand through analysis of the binding interactions of the co-crystallized ligand and to perform the docking procedure.
Adaptor-associated protein kinase 1 (AAK1)
PDB file (ID: 4WSQ) was used for performing molecular docking simulations, the tested compounds were docked at the binding pocket of the enzyme.
Molecular dynamics simulations
The best docking postures were subjected to molecular dynamics (MD) computations to get insights into the stability of the protein–ligand interaction. The CHARMM-GUI solution builder created the input files for the MD calculations using the CHARMM force field parameters for proteins28. The simulation box dimensions of Mpro/Mpro-16 and AAK1/AAK1-16 were 98 × 98 × 98 nm and 132 × 132 × 132 nm, respectively, containing four sodium ions and seven chloride ions. The protein–ligand combination was subjected to the CHARMM36 forcefield. Prior to production simulation, the system's energy consumption was minimized using the steepest descent algorithm (5000 steps). The complex was then subjected to NVT and NPT ensemble, simulating for 125 ps at 300.15 K temperature utilizing 400 kJ mol−1 nm2 and 40 kJ mol−1 nm2 positional restrictions on the backbone and side chains, respectively, to equilibrate the complex for stabilizing its temperature and pressure. The complex is then put through a 100-ns production simulation run in an NPT ensemble at 300.15 K and 1 bar29.
ADMET study
SwissADME (http://www.swissadme.ch/index.php) web server was used the study the ADME properties of the promising compounds, it can evaluate the pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. The smiles of the selected library were generated and then the ADME properties were calculated. Lazar toxicity predictor web server (https://lazar.in-silico.ch/predict) was used to estimate the toxicity profile of (16) rosmarinic acid against Mpro and (17) tannic acid against AAK1.
Results and discussions
Pharmacophore mapping and molecular docking on SARS CoV-2 main protease (Mpro)
Pharmacophore generation
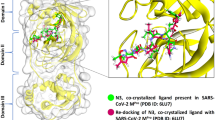
3D Pharmacophores represent the ensembles of the chemically defined interactions of the bioactive conformation of the ligand, accordingly, pharmacophore generation represents an elegant way to decode the chemically encoded ligand information and so becomes a valuable tool in drug design30,31. The selected PDB file for Mpro enzyme with its co-crystallized ligand; R8H, (2 ~ {R})-5-oxidanylidene- ~ {N}-[(2 ~ {R},3 ~ {S})-3-oxidanyl-4-oxidanylidene-1-phenyl-4-(pyridin-2-ylmethylamino)butan-2-yl]-1-(phenylmethyl)pyrrolidine-2-carboxamide [C28 H30 N4 O4] was used to identify the essential pharmacophoric features for better binding with the enzyme. The key amino acids in Mpro active site were HIS-41 and CYS-145 in the catalytic dyad32,33 in addition to GLY-143 that interacts with the oxygen atoms of the co-crystallized ligand. The pharmacophoric features were selected as shown in Fig. 2.
Virtual screening of the selected database against these pharmacophoric features revealed that six hit molecules, (9) gallic acid, (16) rosmarinic acid, (20) 2,5-dihydroxybenzoic-5-O-glucoside, (21) salicylic acid glucoside, (22) glucovanillic acid and (26) carnosic acid, they have showed RMSD value of 0.53, 0.56, 0.29, 0.18, 0.29 and 0.54 respectively, Fig. 3 is showing that these six hits are fitting the generated pharmacophoric features in Mpro.
Molecular docking on SARS Cov-2 Mpro
Molecular docking study was carried out for the hit molecules, their binding energy scores are summarized in Table 1, compared with the co-crystallized ligand (− 10.52 kcal./mol.).
According to the binding energy scores in Table 1, both compounds 16 (rosmarinic acid) and 20 (2,3-dihydroxybenzoic-5-O-glucoside) showed the highest scores, − 16.33 and − 12.28 kcal./mol. Both binding interactions are well demonstrated in Figs. 4 and 5, rosmarinic acid interacts with the catalytic dyad through HIS-41 with its carboxylic acid carbonyl oxygen, while compound 20 (2,5-dihydroxybenzoic-5-O-glucoside) showed the same binding interaction revealing its expected good inhibitory activity on Mpro enzyme. To validate the docking results, the best pose of the tested compounds (16) and (20) using MOE was aligned with the poses obtained from AutoDock Vina (Fig. 6).
Pharmacophore mapping and molecular docking on adaptor-associated protein kinase 1 (AAK1)
Pharmacophore generation
4WSQ PDB file was used to identify the key pharmacophoric features of AAK1 enzyme with its co-crystallized ligand (KSA, K-252A [C27H21N3O5]). The pharmacophoric features were selected (Fig. 7).
Virtual screening was performed on the generated pharmacophore model, it has showed three hit molecules: (16) rosmarinic acid, (17) tannic acid and (20) 2,3-dihydroxybenzoic-5-O-glucoside with RMSD values of 0.45, 0.20, 0.28, respectively (Fig. 8).
Molecular docking against AAK1
Molecular docking study was carried out for the hit molecules, their binding energy scores are summarized in Table 2, compared to the co-crystallized ligand (− 13.28 kcal./mol.).
Analysis of molecular docking results revealed that all three tested compounds have the main binding interaction with CYS-19334. Compound (17), tannic acid showed the highest binding energy score (− 17.15 kcal./mol.) with strong H-bond interactions through its hydroxyl groups to CYS-129, GLU-180 and CYS-193 amino acid residues. On the other hand, compound (16), rosmarinic acid showed H-bond interaction through its carboxylic acid OH group to CYS-193 in addition to binding of phenolic OH group to ASP-127 in the binding vicinity. Finally, compound (20), 2,3-dihydroxybenzoic-5-O-glucoside showed the H-bond interactions with GLU-180 and CYS-193 residues through its hydroxyl groups. All obtained results are illustrated in Figs. 9, 10 and 11.
Molecular dynamics (MD) simulation
The GROMACS software was used to run an MD simulation in order to compare the behaviour of compound (16) rosmarinic acid with MPRO and compound (17) tannic acid with AAK1 across simulation times of 50 and 100 ns, respectively35,36,37.
Simulation time over 50 ns
Analysis of the root mean square deviation (RMSD)
The root mean square deviation (RMSD) was investigated quantitatively to assess the degree of divergence of each complex protein structure with each ligand from its baseline behaviour. The system's stability is evaluated during the simulation with the help of the RMSD. In two different MD simulations, a control system (a ligand-free structure) and a complex were put up for this38. The stability and convergence of compound 16 in the target Mpro and compound 17 in the target AAK1 were investigated using a 50-ns molecular dynamics (MD) simulation, where the backbone atoms' RMSD value was obtained, as shown in Fig. 12. The findings indicated that the complex-maintained equilibrium for the course of the experiment. The RMSD values of the apoprotein and the compound 17-bound complex were 0.14–0.36 nm. Furthermore, the RMSD values of the apoprotein and the compound 16-bound complex ranged from 0.12 to 0.23 nm. Compounds 16 and 17 behaved consistently inside their pockets throughout the simulation and went farther toward the binding pocket. This could explain why both 16 and 17 have potent inhibitory effects against Mpro and AAK1, respectively.
Analysis of the root mean square fluctuation (RMSF)
The local changes in the protein structure brought on by the presence of the suggested inhibitor were examined using the root mean square fluctuation (RMSF)39. Throughout the simulation period, it showed how flexible the protein was. The ranges of 0.03–0.48 nm and 0.15–1.2 nm showed the highest variation. The similar residues in the compounds 16 or 17-bound complexes were less flexible than the native, unbound targets in general. These low-fluctuating residues contributed to the stability of the docked molecules at the binding site (Fig. 13).
Analysis for the radius of gyration (Rg)
The radius of gyration provides information regarding the size and compactness of protein molecules (Rg). The Rg can be used to track the folding and unfolding of protein structures when ligands are bound40. Rg values for the drug-bound complexes were typically closer to the native unbound Mpro and AAK1 values (Fig. 14). Compound 16 and Mpro had average Rg values of 2.18–2.26 nm, whereas compound 17 and AAK1 had values of 3.22–3.40 nm. A less compact or more unfolded protein–ligand interaction is indicated by a greater Rg. A protein is considered securely folded, though, if its Rg value holds steady during the MD simulation. It is viewed as unfolded if the value of Rg changes with time. Figure 14 illustrates how, as compared to the unbound protein, each complex had very identical properties in terms of compactness and almost constant values of Rg.
Analysis of solvent-accessible surface area (SASA)
Both with and without ligands, the protein's solvent-accessible surface area (SASA) was studied. The protein–ligand complex's SASA computation is used to forecast the number of conformational changes that the aqueous solvent can access41. As a result, the SASA was used to evaluate interactions between the complex and the solvent throughout the 50-ns MD simulation. For the unbound protein and protein–ligand complexes, Fig. 15 shows the SASA vs. simulation time curve. In addition to compound 17 and AAK1, the SASA averages for compound 16 and Mpro ranged from 141 to 155 nm2 and 290 to 320 nm2, respectively. Each compound binding caused the SASA to slightly increase due to the expanded surface generated by a portion of the bound ligand surface poking out from the protein surface.
Analysis of hydrogen bond
The protein–ligand complex is stabilized by hydrogen bonds that form between the receptor and ligand. It also impacts the design of medications and their specificity, metabolization, and adsorption42. Therefore, the hydrogen bonds in each ligand–protein combination were studied. Figure 16 displays the total number of hydrogen bonds discovered in the complex after a 50-ns simulation. Each complex had between one and six hydrogen bonds, with two of them remaining constant over the course of the simulation. Additionally, each compound showed a similar hydrogen-bonding pattern throughout the simulation, as seen in Fig. 16.
Simulation time over 100 ns
Analysis of the root mean square deviation (RMSD)
When the simulation period was extended to 100 nm, we found that the complex still maintained equilibrium for the course of the experiment (Fig. 17). The RMSD values of the apoprotein and the compound 17-bound complex reached 0.58 nm. Furthermore, the RMSD values of the apoprotein and the compound 16-bound complex reached 0.29 nm. So, compounds 16 and 17 still behaved consistently inside their pockets throughout the simulation and went farther toward the binding pocket.
Analysis of the root mean square fluctuation (RMSF)
Throughout the extended simulation period to 100 ns, it still showed how flexible the proteins were. The similar residues in the compounds 16- and 17-bound complexes were less flexible than the native, unbound targets. These low-fluctuating residues contributed to the stability of the docked molecules at the binding site (Fig. 18).
Analysis for the radius of gyration (Rg)
Rg values for the drug-bound complexes were still typically closer to the native unbound Mpro and AAK1 values. Figure 19 illustrates how, as compared to the unbound protein, each complex had very identical properties in terms of compactness and almost constant values of Rg.
Analysis of solvent-accessible surface area (SASA)
Each compound binding still caused SASA to increase slightly due to the expanded surface resulting from a portion of the binding surface bound from the protein surface (Fig. 20).
Analysis of hydrogen bond
The complex formed by compound 17 with AAK1 (in black color) still had between one and six hydrogen bonds, with two of them remaining constant over the course of the simulation (100 ns). But the complex formed by compound 16 with Mpro (in red color) had between one and three hydrogen bonds, without making any hydrogen bonding around 30–70 ns period (Fig. 21).
Principle component analysis (PCA)
The conformational distribution during the simulation time was investigated using the PCA approach, as well as the large-scale collective motions of the protein in protein–ligand complexes on the simulation-generated trajectories. It was assumed that the complex that takes up less phase space with a stable cluster is more stable than the complex that takes up more space with a nonstable cluster43. During simulations of compound 16 bound to Mpro and compound 17 bound to AAK1 proteins, the first two principal components (PC1 and PC2) were chosen to investigate their projection of trajectories in phase space. The results clearly showed that Mpro-16 complex occupied smaller regions of phase space than AAK1-17 complex (Fig. 22).
Binding energy estimation by MM/PBSA method
The Molecular Mechanics/Poisson Boltzmann Surface Area (MM/PBSA) method throughout the 100 ns simulations was selected for rescoring complexes because it is the fastest force field-based method that computes the free energy of binding, as compared to the other computational free energy methods, such as free energy perturbation (FEP) or thermodynamic integration (TI) methods. The MM/PBSA calculation was performed using g-mmpbsa software. The calculated binding free energies are shown in Table 3. The average overall binding free energy of the Mpro-16 and AAK1-17 complexes are − 39.776 (± 41.068) kJ/mol and − 171.265 (± 53.984) kJ/mol, respectively.
ADME study
Selected compounds that showed good binding activity to both tested enzymes (9, 16, 17, 20, 21, 22 and 26) were studied and tested through the SwissADME web tool. First, we studied the bioavailability through a radar chart that tests six parameters, Lipophilicity (LIPO), Size, Polarity (POLAR), Insolubility (INSOL), Unsaturation (UNSAT) and Flexibility (FLEX). Compounds 22 and 26 showed no violation, compounds 9, 20 and 21 showed only one violation in the chart, while compounds 16 and 17 showed more than one violation due to their high hydrophilic characters of both compounds revealing their expected oral bioavailability (Fig. 23).
The SwissADME server also provides a BOILED EGG chart to indicate the human intestinal absorption (white part), blood–brain barrier penetration (the yellow part)44, and the probability of the tested compound acting as a substrate for permeability glycoprotein (PGP) which is an efflux pump for many drugs (blue color if it’s a possible substrate or red color if it’s not)45. Only three of the tested compounds showed good gastrointestinal absorption (compounds 9, 22 and 26), which exhibited a good balance between lipophilic and hydrophilic characters. Other tested phenolic acid derivatives exhibited poor gastrointestinal absorption due to their high hydrophilic characters, especially for tannic acid (compound 17), which did not appear in the chart due to extreme hydrophilic properties. All the seven tested compounds are not substrates for PGP, so they will not be susceptible to cell efflux (Fig. 24).
Eventually, applying Lipinski’s rule of five46 to predict the probability of the tested compounds being orally active, all showed no violations in the four parameters (log P, molecular weight, number of H-bond donor groups and number of H-bond acceptor groups) except for compound 17 (three violations) and compound 20 (one violation), this was because compound 17 (tannic acid) is extremely hydrophilic with high molecular weight (Table 4).
The overall data shows that compounds 16 (rosmarinic acid) and 17 (tannic acid) require structural modifications to improve their pharmacokinetic properties and to be suitable for oral administration. Toxicity profile of our most promising natural acids 16 and 17 against Mpro and was investigated through lazar toxicity predictor, the obtained results revealed that both (16) rosmarinic acid and (17) tannic acid are non-carcinogenic in mice, rats and rodents model, additionally, AAK1 showed to be non-mutagenic in Salmonella typhimurium model. With Maximum Recommended Daily Dose in Human equal 4.35 (mg/kg_bw/day) and 137.0 (mg/kg_bw/day) respectively.
Conclusion
The objective of this study was to find natural phenolic acids that can target SARS-CoV-2's main protease (Mpro) and adaptor-associated protein kinase 1 (AAK1) enzymes. The researchers employed several molecular modelling techniques such as pharmacophore mapping, molecular docking, molecular dynamics, and ADME studies. Rosmarinic acid (16) and 2,5-dihydroxybenzoic-5-O-glucoside (20) demonstrated remarkable binding affinities for both target enzymes and, as such, could act as dual inhibitors. Tannic acid (17) showed good potential in fitting all the required pharmacophoric features in AAK1 with the lowest docking score. These results were confirmed through pharmacophore mapping, molecular docking, and dynamic studies over 50 ns and extended to 100 ns. In terms of the ADME study, Rosmarinic acid has low bioavailability due to its high polarity and thus requires structural modification to improve its bioavailability as a promising dual target for COVID-19 prevention via main protease inhibition and endocytosis inhibition.
Data availability
All data generated or analyzed during this study are included in this article.
References
Prabhu, S., Molath, A., Choksi, H., Kumar, S. & Mehra, R. Classifications of polyphenols and their potential application in human health and diseases. Int. J. Physiol. Nutr. Phys. Educ 6, 293–301 (2021).
El-Nashar, H. A., Mostafa, N. M., Abd El-Ghffar, E. A., Eldahshan, O. A. & Singab, A. N. B. The genus Schinus (Anacardiaceae): A review on phytochemicals and biological aspects. Nat. Prod. Res. 36, 4839–4857 (2022).
Kumar, N. & Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 24, 1–10 (2019).
Sehrawat, R. et al. Phenolic acids-versatile natural moiety with numerous biological applications. Curr. Top. Med. Chem. 22, 1472–1484 (2022).
Montenegro-Landívar, M. F. et al. Polyphenols and their potential role to fight viral diseases: An overview. Sci. Total Environ. 801, 1–14 (2021).
Wu, Y.-H. et al. Structure properties and mechanisms of action of naturally originated phenolic acids and their derivatives against human viral infections. Curr. Med. Chem. 24, 4279–4302 (2017).
Kowalczyk, M. et al. Drug design strategies for the treatment of viral disease. Plant phenolic compounds and their derivatives. Front. Pharmacol. 1, 1–21 (2021).
Sabet, R., Sisakht, M., Emami, L. & Sabahi, Z. Comparison of COVID-19 virus main protease inhibition activities of phenolic acids by molecular docking. Trends Pharmaceut. Sci. 7, 117–126 (2021).
Sohail, M.I., Siddiqui, A., Erum, N., Kamran, M. Phytomedicine and the COVID-19 pandemic. in: Phytomedicine, Elsevier, 2021, pp. 693–708.
Liu, H. et al. COVID-19 pandemic between severity facts and prophylaxis. Nat. Prod. Commun. 16, 1270 (2021).
Gil, C. et al. COVID-19: Drug targets and potential treatments. J. Med. Chem. 63, 12359–12386 (2020).
Eldeeb, E. & Belal, A. Two promising herbs that may help in delaying corona virus progression. Int. J. Trend Sci. Res. Dev. 4, 764–766 (2020).
Raj, R. Analysis of non-structural proteins, NSPs of SARS CoV-2 as targets for computational drug designing. Biochem. Biophys. Rep. 25, 1–10 (2021).
Qayed, W. S., Ferreira, R. S. & Silva, J. R. A. In silico study towards repositioning of FDA-approved drug candidates for anticoronaviral therapy: Molecular docking, molecular dynamics and binding free energy calculations. Molecules 27, 1–22 (2022).
Pillaiyar, T. & Laufer, S. Kinases as potential therapeutic targets for anti-coronaviral therapy. J. Med. Chem. 65, 955–982 (2021).
Ayele, A. G., Enyew, E. F. & Kifle, Z. D. Roles of existing drug and drug targets for COVID-19 management. Metabolism Open 11, 1–10 (2021).
Cantini, F. et al. Baricitinib therapy in COVID-19: A pilot study on safety and clinical impact. J. Infect. 81, 318–356 (2020).
Conner, S. D. & Schmid, S. L. Identification of an adaptor-associated kinase, AAK1, as a regulator of clathrin-mediated endocytosis. J. Cell Biol. 156, 921–929 (2002).
Islam, M. T. et al. Natural products and their derivatives against coronavirus: A review of the non-clinical and pre-clinical data. Phytother. Res. 34, 2471–2492 (2020).
Sayed, A. M. et al. Nature as a treasure trove of potential anti-SARS-CoV drug leads: A structural/mechanistic rationale. RSC Adv. 10, 19790–19802 (2020).
Souid, I., Korchef, A. & Souid, S. In silico evaluation of Vitis amurensis Rupr. polyphenol compounds for their inhibition potency against CoVID-19 main enzymes Mpro and RdRp. Saudi Pharm. J. 30, 1–15 (2022).
Gligorijevic, N. et al. Molecular mechanisms of possible action of phenolic compounds in COVID-19 protection and prevention. Int. J. Mol. Sci. 22, 1–29 (2021).
Belal, A. et al. Toward the discovery of SARS CoV-2 main protease inhibitors: exploring therapeutic potentials of evodiamine and its derivatives, virtual screening, molecular docking, and molecular dynamic studies. Nat. Prod. Commun. 17, 3082 (2022).
Vilar, S., Cozza, G. & Moro, S. Medicinal chemistry and the molecular operating environment (MOE): Application of QSAR and molecular docking to drug discovery. Curr. Top. Med. Chem. 8, 1555–1572 (2008).
Mehany, A. et al. Biological effect of quercetin in repairing brain damage and cerebral changes in rats: Molecular docking and in vivo studies. BioMed Res. Int. 1, 1 (2022).
Mehany, A. B., Belal, A., Mohamed, A. F., Shaaban, S. & Abdelhamid, G. Apoptotic and anti-angiogenic effects of propolis against human bladder cancer: Molecular docking and in vitro screening. Biomarkers 27, 138–150 (2022).
Daina, A., Michielin, O. & Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 7, 1–13 (2017).
Tawfik, H. O. et al. Dependence on linkers’ flexibility designed for benzenesulfonamides targeting discovery of novel hCA IX inhibitors as potent anticancer agents. J. Enzyme Inhib. Med. Chem. 37, 2765–2785 (2022).
Farouk, F. et al. Investigating the potential anticancer activities of antibiotics as topoisomerase II inhibitors and DNA intercalators: In vitro, molecular docking, molecular dynamics, and SAR studies. J. Enzyme Inhib. Med. Chem. 38, 2171029 (2023).
Šribar, D. et al. Next generation 3D pharmacophore modeling. WIREs Comput. Mol. Sci. 10, 1–20 (2020).
Belal, A., Elanany, M. A., Raafat, M., Hamza, H. T. & Mehany, A. B. Calendula officinalis phytochemicals for the treatment of wounds through matrix metalloproteinases-8 and 9 (MMP-8 and MMP-9): in silico approach. Nat. Prod. Commun. 17, 848 (2022).
Lockbaum, G. J. et al. Pan-3C protease inhibitor rupintrivir binds SARS CoV-2 main protease in a unique binding mode. Biochemistry 60, 2925–2931 (2021).
Refaey, R. H., El-Ashrey, M. K. & Nissan, Y. M. Repurposing of renin inhibitors as SARS COV-2 main protease inhibitors: A computational study. Virology 554, 48–54 (2021).
Sorrell, F. J., Szklarz, M., Azeez, K. R. A., Elkins, J. M. & Knapp, S. Family-wide structural analysis of human numb-associated protein kinases. Structure 24, 401–411 (2016).
Yousef, R. G. et al. Discovery of new nicotinamides as apoptotic VEGFR-2 inhibitors: Virtual screening, synthesis, anti-proliferative, immunomodulatory, ADMET, toxicity, and molecular dynamic simulation studies. J. Enzyme Inhib. Med. Chem. 37, 1389–1403 (2022).
Elrazaz, E. Z. et al. Discovery of potent thieno [2, 3-d] pyrimidine VEGFR-2 inhibitors: Design, synthesis and enzyme inhibitory evaluation supported by molecular dynamics simulations. Bioorg. Chem. 113, 1–16 (2021).
Belhassan, A. et al. Camphor, Artemisinin and Sumac Phytochemicals as inhibitors against COVID-19: Computational approach. Comput. Biol. Med. 136, 1–17 (2021).
Guterres, H. & Im, W. Improving protein-ligand docking results with high-throughput molecular dynamics simulations. J. Chem. Inf. Model. 60, 2189–2198 (2020).
Jena, A. B., Kanungo, N., Nayak, V., Chainy, G. & Dandapat, J. Catechin and curcumin interact with S protein of SARS-CoV2 and ACE2 of human cell membrane: Insights from computational studies. Sci. Rep. 11, 1–14 (2021).
Ali, A. et al. Toward the discovery of a novel class of leads for high altitude disorders by virtual screening and molecular dynamics approaches targeting carbonic anhydrase. Int. J. Mol. Sci. 23, 1–20 (2022).
Costa, R. A. D. et al. A computational approach applied to the study of potential allosteric inhibitors protease NS2B/NS3 from Dengue virus. Molecules 27, 1–20 (2022).
Lee, S., Wong, A. R., Yang, A. W. H. & Hung, A. Interaction of compounds derived from the Chinese medicinal formula Huangqi Guizhi Wuwu Tang with stroke-related numbness and weakness targets: An in-silico docking and molecular dynamics study. Comput. Biol. Med. 146, 1–9 (2022).
Elmaaty, A. A. et al. In a search for potential drug candidates for combating COVID-19: Computational study revealed salvianolic acid B as a potential therapeutic targeting 3CLpro and spike proteins. J. Biomol. Struct. Dyn. 40, 8866–8893 (2022).
Daina, A. & Zoete, V. A boiled-egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem 11, 1117–1121 (2016).
Sharom, F. The P-glycoprotein efflux pump: How does it transport drugs?. J. Membr. Biol. 160, 161–175 (1997).
Lipinski, C. A. Lead-and drug-like compounds: the rule-of-five revolution. Drug Discov. Today Technol. 1, 337–341 (2004).
Acknowledgements
The researchers would like to acknowledge Deanship of scientific research, Taif University for funding this work.
Funding
Deanship of scientific research, Taif University, Saudi Arabia has supported this research work.
Author information
Authors and Affiliations
Contributions
H.I.G.: validation, revision and writing. A.B.: conceptualization, methods, writing. M.K.E.-A.: wrote the draft of the manuscript text, methods and review. H.O.T.: prepared figures, methods and writing. R.I.A.: methods, figures check and writing. A.J.O.: methodology, validation, editing. A.A.E.-M.: data curation, writing and revision. D.A.: methods, data analysis and revision. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ghamry, H.I., Belal, A., El-Ashrey, M.K. et al. Evaluating the ability of some natural phenolic acids to target the main protease and AAK1 in SARS COV-2. Sci Rep 13, 7357 (2023). https://doi.org/10.1038/s41598-023-34189-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-34189-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.