Abstract
This study aimed to assess the human health risk of some toxic metals/metalloids [lead (Pb), mercury (Hg), cadmium (Cd), nickel (Ni), chromium (Cr), and arsenic (As)] on infants via consumption of the breast milk of women living in urban areas of Kermanshah city, west of Iran. After collecting milk samples, the carcinogenic and non-carcinogenic risk assessment as well as uncertainty analysis of toxic metal levels were carried out. The order of concentration of heavy metals/metalloids in the breast milk samples was Cr (41.07 ± 23.19) > Ni (19.25 ± 11.81) > Pb (11.5 ± 4.48) > As (1.96 ± 2.04) > Cd (.72 ± 0.42) > Hg (0.31 ± 0.26). The results revealed that the levels of Cr and Pb in the breast milk samples were exceeded the World Health Organization (WHO) tolerable daily intake. In the breast milk samples a high levels of one of the trace elements As, Cd, Cr, Pb, and Ni were observed (over 73%) and in 40% of them the levels of Cr, Pb, Cd, As, and Ni were all above WHO tolerable daily intake. Moreover, the As-related point assessment of target risk factor (THQ) was higher than the allowable limit only for 1-month-old male neonates and 2-month-old female neonates (THQ > 1). In addition, Cr-related THQ scores were higher at all age and gender groups (THQ > 1). In conclusion, our findings suggest a potential risk of some metals for infants via the consumption of mothers' breast milk.
Similar content being viewed by others
Introduction
Breastfeeding is essential to maintaine a good growth of the child1. The World Health Organization recommends that mother to breastfeed a child up to 6 months of age and then feeding with complementary foods for up to 2 years2. However, milk can transfer toxic elements from the mother to the baby via breastfeeding. The presence of these toxins does not necessarily cause health hazards in the infant, but since these hazards are biologically probable, they should be monitored3. The first stage of transmission of contaminants to the baby begins from the time of placement in the mother's womb and continues until breastfeeding4. Toxic heavy metals are one of the main chemical pollutants that enter the environment (water, soil, and air) naturally or through human activities and thay may enter the human body through inhalation, food, and skin transmission5,6,7 These heavy metals have toxic effects on human health and exposure to them can cause diffrent diseases7. Compared to adults, the infants are more sensitive to metal toxicity due to the rapid growth of body tissues, especially their nervous system4.
Among the heavy metals/metalloids, lead (Pb), cadmium (Cd), arsenic (As), and mercury (Hg) are of greater health importance to humans. These metals are not metabolized in the body which may lead to toxicity due to their accumulation in the tissues8,9. As one of the leading carcinogens, cadmium, transmit to humans body through water, food, inhalation, and smoking10. The average weekly Cd intake in European Food Safety Authority (EFSA) is set at 2.5 μg/kg11. Digestive absorption of Cd during lactation (37%) is higher than in adulthood (5%). Therefore, maternal exposure to Cd, even at low environmental concentrations, can lead to health risks, especially neurological changes and deformities in the infant's bones10,12.
Another heavy metal, lead, can also enter the body through water, food, inhalation, and the skin pathway5,13. If Pb consumed orally, about 5–10% of Pb is absorbed. This increases to 45% in children14. The neurological effects of Pb in children include movement disorders and behavioral problems12. The recommended daily intake of Pb for infants is 5 μg/kg15. The International Agency for Research on Cancer has placed Pb in the second category of carcinogenic compounds16.
Arsenic is also a metalloid that can be transmitted through inhalation of As-contaminated air, food contaminated with As-containing pesticides as well as contaminated water17. As is known to be a carcinogen that can cause skin, liver, kidney, lung, and bladder cancers and adversely affects the nervous, cardiovascular and respiratory systems18. The International Agency for Research on Cancer has placed As in the first group of carcinogenic compounds16. In 2010, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) set a weekly intake of 1.2 μg/kg for As11.
Humans are exposed to organic, inorganic, and metallic Hg mainly through the consumption of fish, medicinal and cosmetic compounds, and dental amalgam19. Mineral and elemental Hg are excreted mainly in the urine. Significant amounts of Hg are also excreted via sweat, tears, and breast milk20. JECFAsuggests a permissible weekly intake of 1.6 μg/kg for Hg21. Fetuses and infants are exposed to methylmercury, which is the most toxic form of Hg, that determines 5 μg/kg for total Hg22. Some studies have reported the presence of toxic metals in human milk around the world11,23,24,25,26,27.
On the contrary, the low concentrations of metals in human milk were determined in some reports28,29,30. There is little research available on the health risk of toxic metals/metalloids on infants transmitted via the breast milk using Iranian population. Therefore, this study aimed to measure the concentrations levels as well as the human health risk (non-carcinogenic and carcinogenic risk assessment) of six heavy metals/metalloids (Pb, Hg, Cd, Ni, Cr, and As) on infants via the breast milk of women in Kermanshah city, located in the west of Iran. We hypothesize that elevated heavy metal concentrations may have non-carcinogenic and carcinogenic risks for neonates.
Materials and methods
Study population
This study was a cross-sectional study in which after obtaining informed consent from participants, 100 samples were collected from lactating women living in urban areas of Kermanshah city during September to December 2021. This research was funded by Kermanshah University of Medical Sciences (ethic code: IR.KUMS.REC.1399.222). Participants were healthy breastfeeding mothers who were primiparous and breastfeed only one child after a normal pregnancy with no complications. They were residence of Kermanshah for at least five years. Mothers with chronic pre-pregnancy diseases (cardiac or autoimmune diseases) and also those living in areas with known emissions or suspected to the elevated levels of pollutants were excluded6.
Breast milk sample collection
The health center’s staff carried out all sampling procedures according to the study protocols. After the admission of lactating women to the study, 5 to 10 ml of milk was collected during morning hours by manual expression from each person. Mothers were instructed to wash their hands and chest areas before collecting samples. Collected samples were labeled inside sterile polyethylene tubes. The milk samples were then kept at − 20 °C until further analyses31.
Drinking water
In this study, the volume of water used was 5 cc. The sample locations were in Kermanshah city and similarly at the same time as the samples of mothers’ milk. We used sterilized plastic bottles that had already been washed with distilled water and 20% Nitric acid (HNO3). For sampling, the tap water was opened for few seconds to make sure the municipal water network is collected. To prevent adsorption and crystallization of the trace element, water samples were then filtered using a 0.45 mm Whatman’s membrane and acidified with 3 ml of nitric acid (HNO3, 65%, Merck, Germany)32. Samples were then transferred to cool, dark containers and kept at 4 °C until Inductively coupled plasma mass spectrometry (ICP-MS) analyses.
Heavy metals/metalloids in water and milk measurements
To prepare milk samples for digestion, they exposed to the room temperature. For each sample, 1.0 ml of milk was placed in a test tube and then 5 ml of nitric acid (HNO3, 65%, Merck, Germany) was added to the tube. The sample was placed at room temperature overnight to digest slowly. At the next day, 2 ml of 35% hydrogen peroxide (H2O2) was added to the tube and placed inside the water bath (TW12, Julabo GmbH, Germany) for 6 h or until the solution was clear at 98 °C. The samples were diluted with ultrapure water (18.2 MΩ-cm at 25 °C, Fistreem, WSC044, UK) up to 25 mL23. Concentrations of As, Cd, Cr, Hg, Ni, and Pb in the milk and water samples were measured with Inductively Coupled Plasma Mass Spectrometry (ICP-MS, Agilent 7900, Santa Clara, CA, USA). The recovery of As, Cd, Cr, Hg, Ni and Pb were 97, 102, 96, 98 and 99% respectively.
Human health risk assessment
Considering that the contaminants in breast milk, especially toxic metals, can cause acute and chronic health effects on infants, the health risk assessment of the toxic metals was carried out.
Non-Carcinogenic risk assessment
The non-carcinogenic health risk after exposure to a toxic element was determined using the ratio of the estimated daily intake (EDI) of the element over the target hazard quotient (THQ) of that element, that is
where the oral reference dose (RfD) of trace elements As, Cd, Pb, Ni, Hg, and Cr were 0.0003, 0.001, 0.0035, 0.02, 0.0001 and 0.003 mg/kg respectively33,34,35 and EDI was estimated using following equation:
where CHM denotes the concentration of heavy metals as reported in Table 1, BW is the neonatal body weight, and DCBM represents the daily consumption of breast milk. BW and DCBM by sex and age groups is given in Table S1 at Supplementary Materials.
The total target hazard quotients (TTHQ) was calculated by adding up the target hazard quotients of each trace element.
According to USEPA, the acceptable value is 1 for THQ (and TTHQ).
Carcinogenic risk assessment
The total carcinogenic risk from various metals was estimated using
where incremental lifetime cancer risk (ILCR) for a particular metal was obtained by EDI × CSF4,30. The cancer sloop factor (CSF) for As, Cd, Pb, Ni, and Cr were defined as 1.5, 6.3, 0.0085, 0.91, and 0.5, respectively. A carcinogenic risk is acceptable (tolerable) if TCR ≤ 10.
Uncertainty analysis approach
Since CHM, BW, and DCBM parameters vary between genders and trace elements (Supplementary Material, Table S1), the average number of these parameters is used in risk assessments36. As a consequence, the EDI is estimated as a point and may not reflect properly the actual exposure of the study population to the heavy metals11,37. Inappropriate estimation of EDI will affect THQ, TTHQ, ILCR, and also TCR parameters. Therefore, the uncertainty analysis using the Monte-Carlo simulation technique with 100,000 iterations was peformed using the Oracle Crystal Ball EXCEL plug-in (Ver.11.1.2.4). This allows us to vary HMC, BW, and DCBM parameters (according to Table S1) and estimate the uncertainty bound (5th to 95th percentile) for TTHQ and TCR.
Statistical analysis
The Excel and SPSS software (version 18) was used to analyze the data. Heavy metal concentrations were presented as mean and standard deviation. The Pearson correlation test was also used to investigate the relationship between heavy metal concentrations in drinking water samples and milk. A significance level of 5% was considered.
Compliance with ethical standards
This study was conducted by the World Medical Association Declaration of Helsinki. This study was approved by the Research and Ethics Committee of Kermanshah University of Medical Sciences (IR.KUMS.REC.1399.222).The informed consent was obtained from participants/ parents/legal guardian. We confirm that all methods were carried out in accordance with relevant guidelines and regulations.
Results
Heavy metals/metalloids in drinking water and milk samples
The average concentrations of heavy metals/metalloids in milk and drinking water samples are presented in Table 1 and Supplementary Material, Table S2, respectively. The order of accumulation of heavy metals/metalloids in breast milk samples was Cr (mean = 41.07 µg L−1) > Ni (mean = 19.25 µg L−1) > Pb (mean = 11.5 µg L−1) > As (mean = 1.96 µg L−1) > Cd (mean = 0.72 µg L−1) > Hg (mean = 0.31 µg L−1). In drinking water concentration levels followed a decreasing order as Ni (mean = 16 µg L−1) > As (mean = 7.9 µg L−1) > Cr (mean = 6.2 µg L−1) > Pb (mean = 3.3 µg L−1) > Cd (mean = 0.1 µg L−1) > Hg (mean = 0.5 µg L−1). The box plot of heavy metal/metalloids concentration levels in breast milk and drinking water with corresponding WHO and EPA threshold values for each metal are presented by Fig. 1 and Supplementary Material, Figure S1 respectively. It can be seen clearly that the concentrations of Cr and Pb in all breast milk samples were well above WHO's tolerable daily intake (0.9 µg/L for Cr and 3.6 µg/L for Pb). The percentage of breast milk samples exceeded WHO tolerable daily intake of As (0.3 µg/L) was 75%. Moreover, 73% and 74% of breast milk samples exceeded TDIWHO for Cd (0.4 µg/L) and Ni (12 µg/L) respectively. Another notable point to mention is that in 40% of breast milk samples the levels of Cr, Pb, Cd, As, and Ni were all above WHO thresholds.
The mean concentration of Hg was 0.31 µg L−1 which was below WHO Maximum Tolerable Limit (MTL) (0.72 µg L−1) (only 9% of subjects exceeded WHO MTL). Heavy metal concentration levels in drinking water, presented by Supplementary Material, Fig. S1, were well below both WHO and EPA guideline values; All Cr, Pb, Ni, Cd, and Hg concentration values (10 of 10 concentrations values) were below the WHO standard threshold. Two values of As concentration levels (20%) were above the WHO recommended standard point.
Correlation of heavy metals/metalloids in water with breast milk
We also investigate the potential association between concentration levels of heavy metals/metalloids in breast milk and drinking water using Pearson correlation. Since only 10 water samples were obtained from the city water supply, but we had 100 breast milk samples available, the correlation was estimated using 10 water samples and 10 breast milk samples randomly selected out of 100 available samples. This procedure was repeated 100,000 times and each time the correlation and its corresponding p-value were obtained for each metal. The correlation between the Cr levels in breast milk and drinking water was only significant for 4963 samples (4.9%), for Ni at (4.7%), As (4.7%), Cd (4.9%), Hg (4.4%), and Pb (5.1%). We may therefore conclude that there was no strong evidence to claim the association between heavy metal levels in breast milk and drinking water.
Health risk assessment
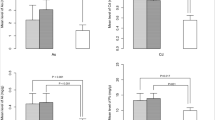
The results of the present study showed that the As-related point assessment of THQ was higher than the allowable limit for 1-month-old male neonates and 2-month-old female neonates (THQ = 1). This factor was lower for other age groups in terms of As metal (Table 2). In addition, Cr-related THQ scores were higher for all age groups and in both neonatal groups, while THQ was lower for other metals in all age groups and both neonates (Table 2). The point estimation of the ILCR parameter for all carcinogenic metals shows that this parameter was higher than the allowable limit for all age groups and both sexes of infants (ILCR = 1 × 10−4) (Table 2). In this study, to ensure the total amount of non-carcinogenic and carcinogenic effects of different metals, TTHQ and TCR factors by uncertainty analysis method for four age groups of infants including 3–1, 6–4, 7–9, and 10–12 months and it was calculated for both sexes (Figs. 2 and 3). The results of uncertainty analysis showed that TCR related to carcinogenic effect and TTHQ related to cumulative non-carcinogenic effect related to different metals in breast milk for all the above age groups and both sexes of infants, with a large difference from the acceptable level (TTHQ = 1 and TCR = 10−4) were higher (Figs. 2 and 3).
Discussion
Heavy metals/metalloids in milk samples
In this study, we characterized the concentrations of six heavy metals/metalloids (Pb, Hg, Cd, Ni, Cr, and As) in the breast milk samples of women living in urban areas of Kermanshah city. We found that the concentration levels of Cr and Pb in the breast milk samples were above WHO tolerable daily intake. Apart from these two trace elements, we also found a high levels of one of the trace elements As, Cd, and Ni (over 73%) in the breast milk samples. In 40% of breat milk samples, the levels of Cr, Pb, Cd, As, and Ni were all above WHO tolerable daily intake.
A summary of literature findings on concentration levels of toxic metals in the breast milk samples is presented in Table 3. The consistent presence of the selected toxic metals indicates the risk of contaminantion in the breast milk of lactating mothers which may have negative effects on the child's health. Infants are known more vulnerable if exposed to neurotoxins. Infants have a higher risk of heavy metal accumulation due to lower excretion rate and body weight, as well as a low immunity state12. It is also known that the toxicant uptake in the gastrointestinal system is greater for infants and newborns38. The higher levels of Pb and Cr and other metals in breast milk indicate exposure to various sources of these metals in Kermanshah province. Previous researches confirmed the presence of these toxic metals in soil39,40, street dust41, air42, rice43, vegetables 44, and cosmetics45 in this region.
This study showed higher Pb levels in breast milk compared to similar studies conducted in Iran. For example, Rahimi et al., Goudarzi et al., and Samiee presented mean Pb levels of 2.44, 7.11, and 75 μg/L, respectively23,46,47, these observations are comparable to previous literature in other countries that reported a similarly elevated level of Pb in breast milk over the lactation course25,27,28,38. Park et al. in the CHECK cohort study demonstrated that Pb levels of some breast milk samples were above the normal values suggested by WHO. The percentage of infants exceeding the standard value of Pb was 71% and 56% for 15-day, and 30-day-old infants, respectively25. Another study reported a higher reference value of 95% (RV 95) compared to the WHO/ International Atomic Energy Agency (IAEA) reference range for Pb. All the 203 lactating mothers in their study had a Pb concentration above the WHO/IAEA reference value in breast milk19. Another study on 100 lactating mothers in Hamedan showed that 94% of the breast milk samples exceeded the WHO standard range for Pb23. Lead exposure in the general population is via contaminated drinking water, food, and the inhalation of Pb particles. Morever, both active and passive smoking can also contribute to elevate Pb levels19. Breastfeeding can be associated with increased bone resorption in response to the calcium needs of breastfed infants25. In addition, during the mobilization of calcium in the mother’s bones in the second half of pregnancy for fetal skeletal growth, Pb enters the mother's bloodstream23. The proportion of infants who have exceeded the reference ranges of studied metals, especially for Pb, is alarming. Lead can cause neurological complications in children, including movement and behavioral problems12. A study suggestes that neurodevelopment and walking age are negatively affected in infants living near smelters and fishing villages and therefore are exposed to high levels of Pb via breast milk48. Low intelligence quotient (IQ) was observed in infants who were breastfed with Pb-contaminated milk49. Longer-term follow-up studies for infants exposed to high Pb levels are necessary to understand the consequences of neurodevelopment in the early life stage.
Chromium (Cr) is a heavy metal that is naturally distributed in the environment and some industrial activities can release large amounts of Cr compounds of different forms in the living environment23. The Cr and As contents of breast milk of 100 lactating mothers in hamedan, Iran were above the limit of detection (LOD) in 19% and 76% of samples, respectively. The breast milk Cr content in 74.8% of samples were above the safe range recommended by the WHO23. An study using a Nigerian population showed that the levels of the Cr in the breast mike was higher than the allowable limit28. Postnatal feeding infants through hexavalent Cr-contaminated breast milk can expose them to excessive oxidative stress50.
Cadmium (Cd) is another toxic element that has unknown function in the human body28. The primary sources of Cd exposure in the general population are diet and smoking. It can exert its toxic effects on the kidney, respiratory and skeletal systems51. There is a potent association between Cd concentration in breast milk and the secretion of calcium in breast milk52. This indicates that there may not be enough calcium in breast milk due to maternal exposure to Cd. The concentration of Cd in breast milk observed in the current study is generally comparable to the previous studies28,31. Feizi el al. study showed that 87% of the cosmetic samples exceeded the WHO limit for Cd levels in Pakistan27. Al-Saleh reported that the reference value in Saudi Arabia was higher than the WHO/IAEA reference range of less than 1 μg/L for Cd in breast milk in normal condition. Of the 203 mothers who participated in their study, 190 (93.6%) mothers exceeded the WHO/IAEA limits for Cd in breast milk19.
As, a well-documented toxic metalloid, is particularly dangerous for infants and young children who have chronic exposure, associated with low IQ, poor mental function, cognitive decline, and cancer53. In a study on Iranian women, Samiee et. al. (2019) reported that the concentration levels of As in 19% of breast milk samples was higher than the WHO recommended level for As23. Released reports from Taiwan and Sweden, both of which found milk As concentrations less than 1 μg/L38,54. The mechanism of excretion for As through breast milk is not known. Some findings indicate that inorganic As are not easily transmitted through breast milk. A study in Bangladesh (2008), claimed that even among women exposed to the high levels of As via groundwater the levels of inorganic As in their milk was relatively low55. This is in agreement with our finding of no strong evidence for the association between heavy metal/metalloid concentration levels in breast milk and drinking water.
The current study showed that the mean concentration of Hg was 0.31 µg L−1 which was below the WHO Maximum Tolerable Limit (only 9% of subjects exceeded WHO MTL). A global and regional review of trends in total mercury concentrations in human breast milk and whole blood suggested that South America, followed by Africa and Asia had the highest concentrations of total mercury while Europe and North America had the lowest values in the selected biological matrices56. In the countryside of Tehran, Tabriz, and Noushahr, Iran, human milk samples gave a mean mercury level of 0.12, 0.86, and 0.15 g/L, respectively. When compared to the WHO-recommended limit, only 3.7% of samples had mercury concentrations higher than normal57. A study in Saudi Arabia detailed that mercury levels in human breast milk were 2.19 μg l_1 and 4.15 μg l_1 for Al-Ehssa and Riyadh residents, respectively58. In 68 colostrum samples collected in Taiwan, the geometric mean mercury level was 2.03 μg l_1(ranged from: 0.24–9.45 μg l_1)59.
In this study, 72% of subjects exceeded the maximum tolerable limit for concentration levels of Ni in breast milk. Ni is known to be toxic to several body systems. It is defined as a nephrotoxic, neurotoxic, hepatotoxic, and carcinogenic agent and it can be also toxic to the pulmonary and reproductive systems30. International Agency for Research on Cancer (IARC) classified Ni compounds as Group 1agents30. Gürbay reported mean nickel concentrations in 64 breast milk samples collected in Turkey as 43.94 ± 33.82 μg/l. they reported that nickel Levels were also partly exceeded the recommended provisional tolerable weekly dietary intake (PTWI) limits30. In a study from Yazd, Iran, the mean Ni levels in breast milk samples on days 3–5, 16, and 30 after delivery were 47.3 ± 7.40, 49.9 ± 8.05, and 54.8 ± 7.38 μg/l, respectively. Also, its concentration in more than 86% of samples was higher than the permissible limits60. Osmar (2014) also found that breast milk from 48 Brazilian women living in Minas Gerais contained lower levels of nickel than WHO reference levels61. Consuming food, drinking water, and coming into direct contact with nickel-containing items like coins, stainless steel, and jewelry are all possible explanations for the higher concentration of nickel in breast milk. Due to the high levels of nickel in some foods like coffee, tea, chocolate, nuts, potatoes, cabbage, oatmeal, and spinach, these foods can be a major source of nickel exposure30.
Health risk assessment
Our results showed that AS-related point assessment of THQ was higher than the allowable limit only for 1-month-old male neonates and 2-month-old female neonates. In addition, Cr-related THQ scores were higher at all age groups and in both neonatal groups.
In line with our results, Samiee et al. reported unacceptable non-cancer health risk levels or hazard quotient (HQ) for Pb and As in 61% and 10% of breast milk samples, respectively23.
Another study in Saudi Arabia showed that HQ of Cd and Hg was more than 1 indicating exposure to these toxic metals through breast milk feeding has noncarcinogenic effects. They also reported a neurotoxic risk of Pb for breastfed infants19. Many changes in toxic metal concentrations and their health effects, may be due to differences in environmental exposures and dietary differences among populations. In our study, the point estimation of the ILCR parameter for all carcinogenic metals showed that this parameter was higher than the acceptable limits for both gender at all age groups. Moreover, our study revealed that the risk of trace elements in younger infants was higher than that of in older infants. This outcome may be due to the greater vulnerability of the growing nervous system to the toxic effects of metals.
In the study of Al-Saleh et al. (2021) the mean CR for Pb from breast milk consumption was 3.7 × 10−5 with two values higher than the US EPA acceptable level of 1 × 10−4. In addition, the CR was significantly higher in younger infants (< 6 months) (4.7 × 10−5) than in infants aged 6 to 12 months (3.1 × 10−5)19. Agency for Research on Cancer (IARC) has defined Pb and its inorganic compounds as possible human carcinogens (group 2B and 2A, respectively). It also classified As as Group 1 and Cd as class B162. Hexavalent Cr is a known carcinogen strongly correlated with lung cancer. It has also been reported as having a detrimental effect on embryonic development for both Cr (III) and Cr (IV). Beaumont et al. (2008) conducted a study in Liaoning Province, China, and reported an increased risk of cancer following the consumption of Cr (VI) via drinking water. In this regard, an increased risk of gastric cancer was shown, but some evidence also indicated an increased risk of lung cancer63. Therefore, the potential carcinogenic risk to infants through breastfeeding should not be ignored. Our results highlight the need to monitor the concentration of toxic metals in breast milk in the first 6 months of life as it is the primary food source for infants. However, this should be kept in mind that since the risk assessment approaches assume 100% of the toxic elements are absorbed and biologically effective, they are inherently noisy and may underestimate or overestimate the risk prediction.
Limitation
Several limitations should be considered in this study. The volume of milk consumption by infants was calculated based on the published data. Longitudinal measurements of the metal concentration levels during the lactation course didn’t carried out in this study. No information was obtained about the dietary habits of mothers. In the current study, we assessed the risk of toxic heavy metals only from breast milk consumption and all other possible related sources were not assessed. The health risk assessment does not consider interactions between metals as a mixture, and therefore, if the interactions are more than additive, the health risk may be underestimated or if the interactions are less than additives, it may be overestimated.
Conclusion
In this study, we assessed the concentrations of six heavy metals/metalloids (Pb, Hg, Cd, Ni, Cr, and As) in the breast milk samples. We found that in all samples the concentration levels of both Cr and Pb were well above WHO tolerable daily intake. We also found a high levels of one of the trace elements As, Cd, and Ni (over 73%) in the breast milk samples. In 40% of breat milk samples, the levels of Cr, Pb, Cd, As, and Ni were all above WHO tolerable daily intake. We also found no strong evidence to establish an association between heavy metal/metalloid concentration levels in breast milk and drinking water. Our results suggest that there may be a potential risk of some studied metals for infants via the consumption of mothers' breast milk.
Our results demonstrated that the As-related point assessment of THQ was higher than the allowable limit only for 1-month-old male neonates and 2-month-old female neonates. In addition, Cr-related THQ scores were higher for all age groups and in both neonatal groups. The point estimation of the ILCR parameter for all carcinogenic metals showed that this parameter was higher than the allowable limit for all age groups and both sexes of infants. Also, our study revealed that the risk of metals in younger infants was higher than in older infants. The observations of the current study may not represent the nationwide status of breast milk contamination with heavy metals. However, our study can add information about the range of studied toxic metal concentrations. Given the benefits of breast milk for the health and nutrition of the infant, this study should be repeated periodically with a larger sample size and in other parts of the world. This study is an update on the current status of toxic metal contamination in the breast milk of women in western Iran. According to our results, it is suggested that routine monitoring of potential sources of heavy metal contaminations in mothers and their infants should be performed to explore the contributing factors and develop appropriate interventions for mitigating the exposure.
Data availability
The datasets used and analyzed during the current research are available from the corresponding author on request.
References
Gupta, A., Suri, S., Dadhich, J., Trejos, M. & Nalubanga, B. The world breastfeeding trends initiative: Implementation of the global strategy for infant and young child feeding in 84 countries. J. Public Health Policy 40, 35–65 (2019).
Syeda, B., Agho, K., Wilson, L., Maheshwari, G. K. & Raza, M. Q. Relationship between breastfeeding duration and undernutrition conditions among children aged 0–3 Years in Pakistan. Int. J. Pediatr. Adolesc. Med. 8, 10–17 (2021).
Mandiá, N. et al. Human milk concentrations of minerals, essential and toxic trace elements and association with selective medical, social, demographic and environmental factors. Nutrients 13, 1885 (2021).
Dursun, A. et al. Maternal risk factors associated with lead, mercury and cadmium levels in umbilical cord blood, breast milk and newborn hair. J. Matern. Fetal Neonatal Med. 29, 954–961 (2016).
Fu, Z. & Xi, S. The effects of heavy metals on human metabolism. Toxicol. Mech. Methods 30, 167–176 (2020).
Mohammadi-Moghadam, F. et al. Toxic and essential elements in drinking water, blood, hair and intestinal tissues of ulcerative colitis patients: Probabilistic health risk assessment for drinking water consumers. Toxin Rev. 41, 487–495 (2022).
Bagherzadeh, F. et al. Influence of metal ions concentration in drinking water in the development of ulcerative colitis. Int. J. Environ. Sci. Technol. 19, 3539–3546 (2022).
Norouzi, M., Mansouri, B., Hamidian, A. H., Zarei, I. & Mansouri, A. Metal concentrations in tissues of two fish species from Qeshm Island, Iran. Bull. Environ. Contam. Toxicol. 89, 1004–1008 (2012).
Rezaei, M. et al. Thyroid dysfunction: how concentration of toxic and essential elements contribute to risk of hypothyroidism, hyperthyroidism, and thyroid cancer. Environ. Sci. Pollut. Res. 26, 35787–35796 (2019).
Olszowski, T. et al. Cadmium concentration in mother’s blood, milk, and newborn’s blood and its correlation with fatty acids, anthropometric characteristics, and mother’s smoking status. Biol. Trace Elem. Res. 174, 8–20 (2016).
Rebelo, F. M. & Caldas, E. D. Arsenic, lead, mercury and cadmium: Toxicity, levels in breast milk and the risks for breastfed infants. Environ. Res. 151, 671–688 (2016).
Winiarska-Mieczan, A. Cadmium, lead, copper and zinc in breast milk in Poland. Biol. Trace Elem. Res. 157, 36–44 (2014).
Baramaki Yazdi, R., Ebrahimpour, M., Mansouri, B., Rezaei, M. R. & Babaei, H. Contamination of metals in tissues of Ctenopharyngodon idella and Perca fluviatilis, from Anzali Wetland, Iran. Bull. Environ. Contam. Toxicol. 89, 831–835 (2012).
Sipahi, H., Eken, A., Aydın, A., Şahin, G. & Baydar, T. Safety assessment of essential and toxic metals in infant formulas. Turkish Journal of Pediatrics 56, 385–391 (2014).
Mandour, R. A., Ghanem, A.-A. & El-Azab, S. M. Correlation between lead levels in drinking water and mothers’ breast milk: Dakahlia, Egypt. Environ. Geochem. Health 35, 251–256 (2013).
IARC. International Agency for Research on Cancer. Classified by the IARC Monographs. (2016).
Salmani, M. H., Rezaie, Z., Mozaffari-Khosravi, H. & Ehrampoush, M. H. Arsenic exposure to breast-fed infants: Contaminated breastfeeding in the first month of birth. Environ. Sci. Pollut. Res. 25, 6680–6684 (2018).
Carignan, C. C. et al. Estimated exposure to arsenic in breastfed and formula-fed infants in a United States cohort. Environ. Health Perspect. 123, 500–506 (2015).
Al-Saleh, I. Health risk assessment of trace metals through breast milk consumption in Saudi Arabia. Biol. Trace Elem. Res. 199, 4535–4545 (2021).
Manohar, K., Mutya, V., Irfan, I. & Srikanth, V. Acute local mercury toxicity induced by self injection: A rare occurrence. J. Clin. Toxicol. 11, 468 (2021).
Ariyaee, M., Azadi, N. A., Majnoni, F. & Mansouri, B. Comparison of metal concentrations in the organs of two fish species from the Zabol Chahnimeh Reservoirs, Iran. Bull. Environ. Contam. Toxicol. 94, 715–721 (2015).
Rebelo, F. M. et al. Mercury in breast milk from women in the Federal District, Brazil and dietary risk assessment for breastfed infants. J. Trace Elem. Med Biol. 44, 99–103 (2017).
Samiee, F., Vahidinia, A., Javad, M. T. & Leili, M. Exposure to heavy metals released to the environment through breastfeeding: A probabilistic risk estimation. Sci. Total Environ. 650, 3075–3083 (2019).
Motas, M., Jiménez, S., Oliva, J., Cámara, M. Á. & Pérez-Cárceles, M. D. Heavy metals and trace elements in human breast milk from industrial/mining and agricultural zones of southeastern Spain. Int. J. Environ. Res. Public Health 18, 9289 (2021).
Park, Y. et al. Exposure to lead and mercury through breastfeeding during the first month of life: A CHECK cohort study. Sci. Total Environ. 612, 876–883 (2018).
Tahboub, Y. R., Massadeh, A. M., Al-Sheyab, N. A., El Shrafat, D. & Nsserat, I. A. Levels of trace elements in human breast milk in Jordan: A comparison with infant formula milk powder. Biol. Trace Elem. Res. 199, 4066–4073 (2021).
Khan, S., Ismail, A., Gong, Y. Y., Akhtar, S. & Hussain, M. Concentration of Aflatoxin M1 and selected heavy metals in mother milk samples from Pakistan. Food Control 91, 344–348 (2018).
Ekeanyanwu, C. L., Alisi, C. S. & Ekeanyanwu, R. C. Levels of Aflatoxin M1 and selected heavy metals (Pb, Cd, Cr, Cu, Zn, Fe, As, and Hg) in the breast milk of lactating mothers in South Eastern, Nigeria. Food Control 112, 107150 (2020).
Kunter, İ et al. Assessment of aflatoxin M1 and heavy metal levels in mothers breast milk in Famagusta, Cyprus. Biol. Trace Elem. Res. 175, 42–49 (2017).
Gürbay, A. et al. Toxic metals in breast milk samples from Ankara, Turkey: Assessment of lead, cadmium, nickel, and arsenic levels. Biol. Trace Elem. Res. 149, 117–122 (2012).
Tahboub, Y. R., Massadeh, A. M., Al-Sheyab, N. A. & Nsserat, I. A. Levels of trace elements in human breast milk in Jordan: A comparison with infant formula milk powder. Biol. Trace Elem. Res. 199, 4066–4073 (2021).
Rajaei, G., Mansouri, B., Jahantigh, H. & Hamidian, A. H. Metal concentrations in the water of Chah nimeh reservoirs in Zabol, Iran. Bull. Environ. Contam. Toxicol. 89, 495–500 (2012).
Environmental Protection Agency US. Guidelines for the health risk assessment of chemical mixtures. Federal Register 51, 34014–34025. http://www.epa.gov/risk/guidelines-health-risk-assessment-chemical-mixtures (1986).
United State Environmental Protection Agency (USEPA). Role of the Baseline Risk Assessment in Superfund Remedy Selection Decisions (Memorandum from D. R. Clay, OSWER 9355.0–30, April 1991) US Environmental Protection Agency, Washington DC, USA. www.epa.gov/oswer/riskassessment/baseline.htm (1991).
US-EPA. US Environmental Protection Agency Washington, DC, USA. www.epa.gov/iris. (1993).
WHO. Global strategy for infant and young child feeding. WHO Library Cataloguing-in-Publication Data, Geneva. (2003).
García-Esquinas, E. et al. Mercury, lead and cadmium in human milk in relation to diet, lifestyle habits and sociodemographic variables in Madrid (Spain). Chemosphere 85, 268–276 (2011).
Chao, H.-H. et al. Arsenic, cadmium, lead, and aluminium concentrations in human milk at early stages of lactation. Pediatr. Neonatol. 55, 127–134 (2014).
Ghassemi Dehnavi, A., Sarikhani, R., Moradpour, A. & Amiri, M. Distribution and source identification of heavy metals in the soil surrounding Kermanshah Refinery, Iran. J. Adv. Environ. Health Res. 7, 169–177 (2019).
Rastegari Mehr, M., Shakeri, A., Amjadian, K., Khalilzadeh Poshtegal, M. & Sharifi, R. Bioavailability, distribution and health risk assessment of arsenic and heavy metals (HMs) in agricultural soils of Kermanshah Province, west of Iran. J. Environ. Health Sci. Eng. 19, 107–120 (2021).
Roshanak Manesh, A., Rastegari Mehr, M. & Shakeri, A. Investigating distribution, contamination and health risk of heavy metals in street dust of Kermanshah Metropolis. Adv. Appl. Geol. 11, 557–571 (2021).
Nazari, Z., Khorasani, N., Feiznia, S. & Karami, M. Investigation of emission sources of suspended elements air in city of Kermanshah. J. Arid Biome 8, 65–76 (2019).
Mohammadi, P. et al. Evaluation of lead and cadmium levels of Iranian and imported rice in Kermanshah, 2016 (Iran). Arch. Hyg. Sci. 7, 106–111 (2018).
Ahmadi-Jouibari, T., Ahmadi Jouybari, H., Sharafi, K., Heydari, M. & Fattahi, N. Assessment of potentially toxic elements in vegetables and soil samples irrigated with treated sewage and human health risk assessment. Int. J. Environ. Anal. Chem. https://doi.org/10.1080/03067319.2021.1893704 (2021).
Feizi, R., Jaafarzadeh, N., Akbari, H. & Jorfi, S. Evaluation of lead and cadmium concentrations in lipstick and eye pencil cosmetics. Environ. Health Eng. Manag. J. 6, 277–282 (2019).
Rahimi, E., Hashemi, M. & Baghbadorani, Z. T. Determination of cadmium and lead in human milk. Int. J. Environ. Sci. Technol. 6, 671–676 (2009).
Goudarzi, M., Parsaei, P., Nayebpour, F. & Rahimi, E. Determination of mercury, cadmium and lead in human milk in Iran. Toxicol. Ind. Health 29, 820–823 (2013).
Marques, R. C., Bernardi, J. V., Dórea, J. G., Maria de Fatima, R. M. & Malm, O. Perinatal multiple exposure to neurotoxic (lead, methylmercury, ethylmercury, and aluminum) substances and neurodevelopment at six and 24 months of age. Environ. Pollut. 187, 130–135 (2014).
Isaac, C., Sivakumar, A. & Kumar, C. Lead levels in breast milk, blood plasma and intelligence quotient: A health hazard for women and infants. Bull. Environ. Contam. Toxicol. 88, 145–149 (2012).
Stanley, J. A. et al. Postnatal exposure to chromium through mother’s milk accelerates follicular atresia in F1 offspring through increased oxidative stress and depletion of antioxidant enzymes. Free Radical Biol. Med. 61, 179–196 (2013).
Jurowski, K., Krośniak, M., Fołta, M., Cole, M. & Piekoszewski, W. The toxicological analysis of lead and cadmium in prescription food for special medical purposes and modified milk products for newborns and infants available in Polish pharmacies. J. Trace Elem. Med. Biol. 51, 73–78 (2019).
ATSDR. (Agency for Toxic Substances and Disease Registry). Case studies in environmental medicine. Cadmium toxicity. Course WB 1096. http://www.Atsdr.cdc.gov/csem/cadmium/docs/cadmium.pdf Accessed September 2021.
Tyler, C. R. & Allan, A. M. The effects of arsenic exposure on neurological and cognitive dysfunction in human and rodent studies: a review. Curr. Environ. Health Rep. 1, 132–147 (2014).
Björklund, K. L. et al. Metals and trace element concentrations in breast milk of first time healthy mothers: A biological monitoring study. Environ. Health 11, 1–8 (2012).
Fängström, B. et al. Breast-feeding protects against arsenic exposure in Bangladeshi infants. Environ. Health Perspect. 116, 963–969 (2008).
Sharma, B. M., Sáňka, O., Kalina, J. & Scheringer, M. An overview of worldwide and regional time trends in total mercury levels in human blood and breast milk from 1966 to 2015 and their associations with health effects. Environ. Int. 125, 300–319 (2019).
Dahmardeh Behrooz, R., Esmaili-Sari, A., Peer, F. E. & Amini, M. Mercury concentration in the breast milk of Iranian women. Biol. Trace Elem. Res. 147, 36–43 (2012).
Al-Saleh, I., Shinwari, N. & Mashhour, A. Heavy metal concentrations in the breast milk of Saudi women. Biol. Trace Elem. Res. 96, 21–37 (2003).
Chien, L.-C. et al. Analysis of the health risk of exposure to breast milk mercury in infants in Taiwan. Chemosphere 64, 79–85 (2006).
Salmani, M. H., Mozaffari-Khosravi, H. & Rezaei, Z. The nickel concentration in breast milk during the first month of lactation in Yazd, Center of Iran. Biol. Trace Elem. Res. 174, 65–70 (2016).
Cardoso, O. O. et al. Concentration profiles of metals in breast milk, drinking water, and soil: Relationship between matrices. Biol. Trace Elem. Res. 160, 116–122 (2014).
IARC. Monographs on the evaluation of carcinogenic risks to humans: Cadmium and cadmium compounds. Vol. 100 C. International Agency for Research on Cancer, Lyon, France. http://monographs.iarc.fr/ENG/Monographs/vol100C/mono100C-8.pdf (2012).
Beaumont, J. J. et al. Cancer mortality in a Chinese population exposed to hexavalent chromium in drinking water. Epidemiology 19(1), 12–23 (2008).
Nazlıcan, E. et al. The risk estimation and assessment of heavy metal exposure by biomonitoring in the breast milk of mothers in the Cukurova Region, Turkey. Environ. Sci. Pollut. Res. 29, 13963–13970 (2022).
Almeida, A. A., Lopes, C. M., Silva, A. M. & Barrado, E. Trace elements in human milk: Correlation with blood levels, inter-element correlations and changes in concentration during the first month of lactation. J. Trace Elem. Med. Biol. 22, 196–205 (2008).
Acknowledgements
This project was generously supported financially by the Kermanshah University of Medical Sciences (Grant number: 1399/990217). The authors would like to acknowledge Kermanshah Health Center and the Vice Chancellor for Research and Technology of Kermanshah University of Medical Sciences for their assistance. We are also very grateful to all of the mothers who participated in this project.
Funding
This project was generously supported financially by the Kermanshah University of Medical Sciences (Grant number: 1399/990217).
Author information
Authors and Affiliations
Contributions
K.S., S.N., N.A., B.M., S.M.K., M.P. and Y.H. contributed to the design of the study, the interpretation of the results, and the drafting of the manuscript. B.M., M.P. and Y.H. conducted the collection of the data. K.S,. N.A. and B.M. conducted the statistical analyses. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sharafi, K., Nakhaee, S., Azadi, N.A. et al. Human health risk assessment of potentially toxic elements in the breast milk consumed by infants in Western Iran. Sci Rep 13, 6656 (2023). https://doi.org/10.1038/s41598-023-33919-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-33919-0
This article is cited by
-
The effects of active and passive smoking on selected trace element levels in human milk
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.