Abstract
Parageobacillus thermoglucosidasius is a thermophilic bacterium characterized by rapid growth, low nutrient requirements, and amenability to genetic manipulation. These characteristics along with its ability to ferment a broad range of carbohydrates make P. thermoglucosidasius a potential workhorse in whole-cell biocatalysis. The phosphoenolpyruvate:carbohydrate phosphotransferase system (PTS) catalyzes the transport and phosphorylation of carbohydrates and sugar derivatives in bacteria, making it important for their physiological characterization. In this study, the role of PTS elements on the catabolism of PTS and non-PTS substrates was investigated for P. thermoglucosidasius DSM 2542. Knockout of the common enzyme I, part of all PTSs, showed that arbutin, cellobiose, fructose, glucose, glycerol, mannitol, mannose, N-acetylglucosamine, N-acetylmuramic acid, sorbitol, salicin, sucrose, and trehalose were PTS-dependent on translocation and coupled to phosphorylation. The role of each putative PTS was investigated and six PTS-deletion variants could not grow on arbutin, mannitol, N-acetylglucosamine, sorbitol, and trehalose as the main carbon source, or showed diminished growth on N-acetylmuramic acid. We concluded that PTS is a pivotal factor in the sugar metabolism of P. thermoglucosidasius and established six PTS variants important for the translocation of specific carbohydrates. This study lays the groundwork for engineering efforts with P. thermoglucosidasius towards efficient utilization of diverse carbon substrates for whole-cell biocatalysis.
Similar content being viewed by others
Introduction
In the past decades, industrial biotechnology and biocatalysis have become an increasingly integrated part of the synthesis and manufacturing of therapeutics and food additives, providing a selective and sustainable solution for modern production1. However, this solution is constantly under economic pressure caused by the high demand and competing performance of heterogeneous catalysis in the chemical industry2. A challenge of biocatalysis is the use of bacterial strains grown under mild conditions allowing the growth of unwanted microorganisms3. Here, extremophilic microorganisms have gained increasing interest due to their resilience towards extreme conditions—leading to the decreased risk of contamination—along with their ability to produce proteins and enzymes that retain function under extreme conditions4. Enzymes from thermophilic microorganisms, or thermozymes, have previously been highlighted for not only their thermostability but also their resistance towards chemical denaturing agents, wide pH tolerance, and non-aqueous solvents making them relevant for industrial applications3. The use of higher temperatures in industrial processes would also increase substrate/product solubility, reduce hydrolysis time, reduce cooling costs, and decrease the viscosity of the liquids used in the process5. Thermozymes have been utilized for in vitro single-step reactions, but more complex multistep chemical conversions are primarily done in an intact cellular host, and this approach has been halted by the lack of genetic tools for thermophilic organisms.
Parageobacillus spp. are thermophilic aerobic or facultatively anaerobic Gram-positive bacilli capable of growth between 40 and 70 °C with an optimum temperature of 60–65 °C6,7,8. Members of the genus can ferment both hexose and pentose monosaccharides and oligosaccharides to generate lactate, formate, acetate, and ethanol as products6. As type species of the genus (Parageobacillus), P. thermoglucosidasius DSM 25429 (P. thermoglucosidasius henceforth), has been engineered and utilized for industrial bioethanol production from lignocellulosic feedstocks6. Different works have also engineered this bacterium for the production of isobutanol10, riboflavin11, (S)-lactic acid12, terpenes13 and (2R, 3R)-butanediol14. The strain demonstrates a rapid growth rate and the ability to ferment a broad range of monosaccharides, cellobiose, and short-chain oligosaccharides. Its success in bioethanol production, the possibility of genetic manipulation, and recent whole-genome sequencing have highlighted P. thermoglucosidasius as a potential future cell factory for other valuable small molecules. To further increase the understanding and leveraging of P. thermoglucosidasius, it is necessary to characterize the metabolism of the microorganism, including its carbohydrate metabolism and transportation. In prokaryotes, the transport of carbohydrates is mainly catalyzed by the phosphoenolpyruvate(PEP):carbohydrate phosphotransferase system (PTS)15. The PTS couples the transport of carbohydrates with subsequent phosphorylation through a four-step phosphoryl transfer system15,16. Each PTS consist of two cytoplasmatic proteins, the PTS-general component Enzyme I (EI) that receives the phosphate from PEP, and the histidine-containing phosphocarrier protein (HPr), which is phosphorylated by EI along with a substrate-specific Enzyme II (EII) complex (Fig. 1)15,16. Generally, EI and HPr are common to all PTSs of a cell, meaning that they perform the phosphoryl transfer to all the different EII complexes. Each EII complex is formed by two cytoplasmatic domains; EIIA, which is phosphorylated by HPr, and EIIB, which is phosphorylated by EIIA; and one or two integral membrane domains (EIIC/EIID) that are necessary for substrate translocation (Fig. 1). Furthermore, the three or four EII domains could be either encoded in a single multi-domain protein, or in distinct single-domain proteins15.
Schematic representation of the general bacterial phosphotransferase system (PTS). The two general cytoplasmatic components, which are used for all putative PTS systems are indicated in pink. These are the PTS-general component Enzyme I (EI) and the histidine-containing phosphocarrier protein (HPr). The area marked in tan is a representation of an Enzyme II (EII) complex. EII complexes confer the carbohydrate specificity and are specific for each PTS system. Each EII complex is formed either by distinct proteins or by a single multidomain protein and consists of two hydrophilic domains (EIIA and EIIB), and one or two transmembrane domains (EIIC and EIID).
The PTS participates in complex regulatory mechanisms, including both carbon and nitrogen metabolisms. In summary, in low G+C DNA Gram-positives, HPr also works as a sensor of glycolytic intermediates, especially for fructose-1,6-bisphosphate (FBP). High concentrations of FBP increase phosphorylation of HPr on the conserved serine-46 (different to the histidine involved in the PTS phosphorelay) triggering carbon catabolite repression (CCR), generally through the carbon catabolite repressor CcpA15. Furthermore, CcpA also regulates the synthesis of branched-chain amino acids which directly stimulates the global regulator CodY, linked to both carbon and nitrogen metabolism17. For detailed explanations of the regulatory networks refer to Deutscher et. al (2006) and Sonenshein (2007).
In this study, we investigated the role of the PTSs in the transport of common carbon substrates in P. thermoglucosidasius. By constructing PTS knockouts and measuring the growth of P. thermoglucosidasius on fifteen common carbon substrates, we were able to determine some of the PTSs specificities in P. thermoglucosidasius and show that there is a complex redundancy between different PTS systems. Of the fifteen substrates, thirteen showed a dependence on active PTS-mediated transport. Knockouts of a minimum of one PTS element in each of the fifteen putative PTS gene clusters in P. thermoglucosidasius revealed five PTSs solely responsible for the translocation of arbutin, mannitol, N-acetylglucosamine, sorbitol, and trehalose, respectively, and the main PTS responsible for the translocation of N-acetylmuramic acid. This study establishes the basis for further metabolic- and strain engineering of P. thermoglucosidasius for novel biotechnological solutions.
Results
Genome analysis and variant design
To assess the capacity of P. thermoglucosidasius DSM 2542 to metabolize carbon substrates through PTS and associated elements thereof, we searched on the available genome sequence (GenBank Accession No. CP012712)9 for genes encoding putative PTSs. The genomic analysis, performed as indicated in Bioinformatic analysis in the Materials and Methods section, revealed that P. thermoglucosidasius contains 15 genomic regions with at least one such PTS element (Fig. 2), scattered throughout the bacterial genome. A minimum of one PTS-associated gene from each gene cluster was knocked out by a scarless-genome edition method based on allelic replacement6, yielding 15 deletion strains. In addition, the gene encoding the EI (ptsI; AOT13_08105) (Fig. 1) was also knocked out, thereby yielding a total of 16 deletion variants of P. thermoglucosidasius (Fig. 2 and Supplementary Fig. 16).
Knockout of ptsI
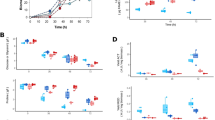
After the mutants were constructed, we focused on quantitative physiology experiments towards identifying the associated growth phenotypes. To this end, 15 carbon sources were selected for their potential as substrates for P. thermoglucosidasius. Besides the carbon sources typically used for bacterial growth experiments (e.g., glucose, fructose, mannose, and xylose), other substrates were likewise included (N-acetylglucosamine, N-acetylmuramic acid, glycerol, mannitol, sorbitol, cellobiose, maltose, sucrose, trehalose, arbutin, and salicin). To determine which of the 15 substrates were transported by the PTS in P. thermoglucosidasius, the WT strain along with the common enzyme knockout strain, ∆ptsI (strain GTS17 in Table 1), were grown in minimal media supplemented with the 15 carbon substrates, respectively. Comparing the growth profiles of these two strains revealed that the WT strain grew in all conditions (Fig. 3A), while the ∆ptsI deletion mutant had abolished growth with 12 out of the 15 feedstocks (Fig. 3B). The ∆ptsI strain had no significant growth when the medium was supplemented with arbutin, cellobiose, fructose, glucose, glycerol, mannitol, mannose, N-acetylglucosamine, sorbitol, salicin, sucrose, or trehalose. These results indicate PTS-dependent processing of the 12 molecules. Although we cannot exclude that other functional elements could be involved in the transport of the substrates tested herein (e.g., hexose permeases or facilitators, which could promote growth on glucose or fructose)18,19,20. In addition to the 12 carbon substrates on which growth was abolished, N-acetylmuramic acid yielded diminished growth indicating that this substrate is mainly, but not exclusively, metabolized through PTS. Interestingly, the ∆ptsI strain was still able to grow when the media was supplemented with maltose or xylose. These results indicate that the transport of these sugars is either independent of phosphorylation processes catalyzed by PTSs or, as indicated above, could be mediated by another transport mechanism, such as a permease.
Growth curves of P. thermoglucosidasius DSM 2542 wild type strain (A) and ∆ptsI (B) on TMMYE medium without (control) or with supplemented carbohydrates (Fructose, GlcNAc, Glucose, Mannose, MurNAc, Xylose; Cellobiose, Maltose, Sucrose, Trehalose; Arbutin, Salicin; Glycerol, Mannitol or Sorbitol). Data presented are average values based on at least three replicates. Error bars indicate standard deviations.
Knockout of individual PTS gene clusters
To understand the importance of each individual PTS gene cluster in the carbohydrate metabolism of P. thermoglucosidasius, a minimum of one PTS element was knocked out of each cluster (Fig. 2). The resulting 15 deletion variants were subjected to the same growth assays as ∆ptsI and WT described above (see Supplementary Figs. 1–15). Cultures of the six knockout strains (AOT13_10525-10530; AOT13_11075; AO13_12555; AO13_15110; AO13_15920 and AO13_18760-18770) had no noticeable growth (or deficient growth in the case of AOT13_11075) when supplementing with a specific carbohydrate suggesting a direct relationship between the knocked-out PTS and the carbohydrate (Fig. 4). Strains ∆10525-10530, ∆11075, ∆12555, ∆15110, ∆15920, and ∆18760-18770 exhibited significantly impaired growth when supplemented with mannitol, N-acetylmuramic acid, trehalose, arbutin, N-acetylglucosamine, and sorbitol, respectively. Strain ∆10525-10530 showed inhibited growth on xylose as well as the mentioned mannitol, a phenotype that was not observed for the strain ∆ptsI. In addition, the growth of this mutant when supplemented with most of the carbohydrates showed to be lower compared with the WT strain (see Supplementary Fig. 7).
Growth curves of P. thermoglucosidasius DSM 2542 ∆10525–10530, ∆11075, ∆12555, ∆15110, ∆15920, and ∆18760–18770 on TMMYE medium without (control) or with supplemented carbohydrates. Only strain:carbohydrate pairs with significantly impaired growth are displayed. The full set of growth curves can be found in the supplementary material. Data presented are mean values based on at least three replicates. Error bars indicate standard deviations.
Discussion
P. thermoglucosidasius-related species hold potential to produce valuable compounds from a broad variety of carbon sources. Rapid growth, availability of genetic tools, and, most importantly, its thermophilic nature, make this strain a prominent workhorse for large-scale industrial production. In light of the features, in this study, we systematically investigated the role of the PTSs of P. thermoglucosidasius in the transport of 15 common molecules used as carbon feedstock by knocking out both common and specific PTS components. The elimination of some components of the PTS systems affected carbon substrate utilization on the host strain. The extent of this impact is variable, and carbon substrate-dependent.
Knockout of the shared EI enzyme resulted in abolished growth for all tested carbohydrates except maltose, xylose, and N-acetylmuramic acid. Although many carbohydrates depend on PTS-mediated translocation with subsequent phosphorylation, other transporters are also known for transporting carbohydrates in bacteria. Here, the ATP-binding cassette (ABC) and the major facilitator superfamily (MFS) transporters also play a major role in the sugar uptake in prokaryotes21,22,23. The uptake of maltose has previously been described to occur through an ABC transporter in other bacterial species24,25,26,27. However, previous works on Bacillus have had contradictory results regarding maltose transport. Reizer et al.27 showed that inactivation of the putative EIIBC (malP) in B. subtilis resulted in a seven-fold increase of the doubling time in a minimal medium supplemented with maltose. Similarly, Schönert et al.28 have shown a lack of [14C]maltose uptake in cell suspensions of B. subtilis ΔmalP after growth in LB supplemented with 1% maltose. Contrary, both in B. subtilis and in B. licheniformis, another major industrial Gram-positive bacteria, it has been suggested that maltose is transported by a proton symport mechanism, which does not occur via the PTS but is regulated by the PTS, and further metabolized through a maltose phosphorylase29,30. Furthermore, complementation of an E. coli strain deficient for maltose transport genes, with the MFS transporter MalA from Geobacillus stearothermophilus suppressed the growth defects on maltose31. A homologous genomic region encoding the same putative genes, including an MFS transporter homologue to MalA, is also found in P. thermoglucosidasius (AOT13_18465). In addition, a homologue of the maltose/maltodextrin transport system permease protein MalG from E. coli, part of the MalEFGK ABC transporter complex32, is also found in P. thermoglucosidasius (AOT13_07020). The results presented in our work, the presence of a MalA homologue, and the presence of a putative maltodextrin phosphorylase in the genome of P. thermoglucosidasius DSM 2542 (AOT13_18705), suggest a PTS-independent pathway.
Xylose is transported across the cell membrane either mediated by a PTS-independent mechanism or by a PTS-dependent but phosphorylation-independent mechanism. Facilitated diffusion of xylose catalyzed by the enzyme II complex (EII) of the PTS specific to mannose, has previously been reported in three species of lactobacilli (L. pentosus, L. plantarum, and L. casei)33. The transport was demonstrated to be independent of phosphorylation, which could explain why the growth of ∆ptsI supplemented with xylose remains unaffected. Genome analysis of six Geobacillus strains showed that xylose transport and metabolism are encoded in a gene cluster containing ABC transporters34. It has also been shown that xyl genes in thermophilic Bacillus sp. are clustered encoding xylO (ATP-binding protein), xylP (xylose permease), xylA (xylose isomerase), xylB (xylulose kinase)35, and although P. thermoglucosidasius DSM 2542 has an operon with both homologues to xylA and xylB (AOT13_11570 and AOT13_11575 respectively), it lacks homologues of xylO and xylP. Since strain ∆10525–10530 is not able to grow when supplemented with xylose, the EII complex encoded in that gene cluster probably catalyzes the facilitated diffusion of xylose with a similar mechanism as reported for lactobacilli, which would further be metabolized through the operon xylAB. In addition to the described mechanisms, xylose has been shown to be transported by AraE (part of the MFS) in Bacillus subtilis23. However, no homologue of AraE is found in P. thermoglucosidasius to our knowledge.
In both Gram-negative and -positive bacteria, N-acetylmuramic acid is mainly transported across the membrane by the PTS MurP and subsequently phosphorylated yielding the 6-phospho sugar utilized for peptidoglycan formation, or as carbon source through the N-acetylglucosamine-6P degradation pathway36,37. This seems to be the case also for P. thermoglucosidasius DSM 2542, which contains a putative operon encoding homologues to the regulator MurR, the etherase MurQ and the transporter MurP. Moreover, this is supported by the diminished growth of the strain ∆11075 on this carbon source.
Still remain to be determined which mechanism allows the partial metabolism of N-acetylmuramic acid observed on the strains ∆ptsI and ∆11075. In bacteria, the first PTS-independent N-acetylmuramic acid transporter has been identified in the periodontal pathogen Tannerella forsythia38. This organism contains an operon encoding a specific N-acetylmuramic acid transporter, a sugar kinase, and a MurQ etherase. No homologues of T. forsythia transporter and kinase are found in the genome of P. thermoglucosidasius, however, some of its many uncharacterized transporters and kinases could have unspecific activity towards N-acetylmuramic acid.
For all carbon sources tested, except for the three discussed above, growth was inhibited by the deletion of ptsI. While this is not generally surprising, we could have expected PTS-independent growth on glycerol. This triol is known to be transported both in Gram-negative and Gram-positive bacteria by energy-independent diffusion mediated by GlpF, a conserved glycerol uptake facilitator39,40. Although P. thermoglucosidasius DSM 2542 encodes the corresponding homologue (AOT13_09870), the mutant ∆ptsI had impaired growth when the medium was supplemented with glycerol. Previous studies also report inhibited growth on glycerol by mutants of Gram-positive and Gram-negative bacteria defective in one of the common enzymes of the PTS41,42,43,44,45,46. The mechanisms involved in this regulation are different in Gram-negative compared to Gram-positive bacteria but both are mediated by the glycerol kinase responsible for the formation of glycerol-3-phosphate trapping the substrate in the cell upon uptake41. For Gram-positive bacteria, the glycerol kinase was found to be phosphorylated by PEP and the common enzymes of the PTS; EI and HPr, causing an increase in glycerol kinase activity41,43,46,47. In fact, the His-232 of glycerol kinase from Enterococcus casseliflavus has been identified as the site of PEP-dependent PTS-catalyzed phosphorylation47. Given that glycerol kinase from P. thermoglucosidasius DSM 2542 (AOT13_09875) shares 64% homology to the enzyme from E. casselliflavus, and it contains the highly conserved histidine-232 residue, we suggest the same regulatory mechanism.
The findings of this study could have important implications for the future scalability and industrial applications of P. thermoglucosidasius as a cell factory or whole-cell biocatalysis. By identifying the specific PTS systems responsible for the transport and phosphorylation of various carbon substrates, this study lays the groundwork for future engineering efforts aimed at enhancing the strain's ability to efficiently utilize diverse carbon sources. Such efforts could potentially lead to the development of a highly versatile whole-cell biocatalyst capable of converting a wide range of substrates into valuable products. Overall, this study provides valuable insights into the metabolic capabilities of P. thermoglucosidasius.
Materials and methods
Bacterial strains and plasmids
The strains and plasmids used in this study are listed in Table 1 and Supplemental table 1 respectively. P. thermoglucosidasius DSM 2542 strains were routinely grown at 60 °C under agitation (200 rpm) on SPY medium (per litre: 16 g soy peptone, 10 g yeast extract, 5 g NaCl) and plated on Trypticase Soy Agar (TSA) plates (Becton Dickinson, US) unless stated differently. E. coli DH5αTM subcloning efficiencyTM (ThermoFisher Scientific, Germany), was used as host in cloning experiments and grown in Luria–Bertani medium at 37 °C under agitation. E. coli DH5αTM and P. thermoglucosidasius DSM 2542 transformants were selected with kanamycin (6.25 μg mL−1 and 12.5 μg mL−1 respectively, given the resulting promotor strength in each particular host).
Bioinformatic analysis
To identify PTS-related genes in the genome of P. thermoglucosidasius DSM 2542, the hmmscan tool from the HMMer suite48 was used with the following profiles downloaded from the Pfam database49. PF00358.23; PF00359.25; PF00367.23; PF02255.19; PF02378.21; PF02896.21; PF03608.16; PF03611.17; PF03612.17; PF03829.16; PF05524.16; PF07663.14, using the trusted cutoffs provided within the individual profiles.
DNA manipulation, oligonucleotides and sequencing
PCR primers (Supplemental table 2) were synthesized by IDT (USA). 11M2 and 12 backbone primers or custom primers hybridizing within the appropriate DNA fragments were used for checking plasmid assembly, integration site and verifying genome deletions. All PCR reactions were performed with the Phusion U Hot Start polymerase (ThermoFisher Scientific, Germany), and colony PCR reactions with OneTaq® (New England Biolabs, US). DNA sequencing was carried out by Eurofins Scientific (Luxembourg). Sequence analyses were carried out with SnapGene Viewer (Dotmatics) and sequence similarities were analyzed with BLAST50.
Preparation of P. thermoglucosidasius DSM 2542 electrocompetent cells
To make electrocompetent cells, P. thermoglucosidasius DSM 2542 was initially inoculated into 50 mL of SPY and incubated at 60 °C with shaking (200 rpm) until an OD600 of ~1.5. The culture was then diluted to an OD600 of 0.5 in a new flask with 30 mL of fresh SPY and incubated at 60 °C with shaking (200 rpm) until an OD600 of ~1.7. After 10 minutes of incubation on ice, the culture was divided into two aliquots. The aliquots were washed consecutively four times in 15, 10, 10, and 5 mL of ice-cold electroporation buffer (0.5 M mannitol, 0.5 M sorbitol, 10% glycerol), and finally resuspended in 2 mL of electroporation buffer. The final cell suspensions were aliquoted (60 µl) in pre-chilled Eppendorf tubes and stored frozen at −80 °C.
Construction of recombinant strains
Construction of the mutant strains was performed by two-step allelic exchange through homologous recombination, exploiting the native machinery of P. thermoglucosidasius DSM 254251. DNA fragments containing flanking regions of the targeted genes designed to include only the start and stop codons of the knockout-target genes were obtained by PCR using P. thermoglucosidasius DSM 2542 chromosomal DNA and the corresponding oligonucleotide pairs. The PCR products were purified using a NucleoSpin Gel and PCR kit (Macherey-Nagel, Germany) and cloned into the backbone of pMTL61110 (obtained by PCR using primers 23 and 24d) by USER cloning (New England Biolabs, US). Chemically competents E. coli DH5α were transformed by heat shock with 3 μl of the USER reactions and after 1h recovery cells were plated on LB with kanamycin. The resulting plasmids were purified using a NucleoSpin Plasmid kit (Macherey-Nagel, Germany), and its sequence verified (Eurofin Genomics). P. thermoglucosidasius DSM 2542 electrocompetents6 were transformed with each of these plasmids using a single electric pulse in a Bio-Rad GenePulser Xcell (10 μF, 600 Ω, 25 kV/cm) and recovered in 1mL of pre-warmed SPY supplemented with 1% glycerol at 52 °C with agitation (200 rpm) for 3 hours. Selection of kanamycin-resistant colonies was done on TSA plates with 12.5 μg ml−1 kanamycin overnight at 52 °C. A kanamycin-resistant colony from each transformation was incubated overnight at 62 °C on SPY supplemented with kanamycin to force the first recombination and later plated on TSA plates with 12.5 μg ml−1 kanamycin. After confirming the right integration site by colonyPCR, the selected colonies were grown 3 days in 5mL of SPY without kanamycin at 60 °C with agitation (200 rpm), doing subcultures in fresh media each morning and evening. Cells were plated on TSA and incubated at 60 °C overnight. The next day the plates were replicaplated on TSA plus kanamycin. Antibiotic-sensitive clones were isolated and, among them, one for each gene was selected (GTS17-GTS32 strains) in which a second recombination event led to the excision of the plasmid and deletion of the targeted gene. DNA sequencing reactions of the appropriate PCR products (see Supplementary Fig. 16) were performed by Eurofins Genomics.
Culture of P. thermoglucosidasius strains with 15 carbon substrates
Precultures of P. thermoglucosidasius strains were grown overnight at 60 °C under agitation (200 rpm) on sugar-free Thermophile Minimal Medium (TMM) supplemented with 3 g/L yeast extract (TMMYE). TMM is adapted from Fong et al.52 and contained the following sterile solutions, per litre: 930 mL Six Salts Solution (SSS), 40 mL of 1 M MOPS solution (pH = 8.2), 10 mL of 1 mM FeSO4 in 0.4 M tricine, 10 mL of 0.132 M K2HPO4, 10 mL of 0.953 M NH4Cl, 0.5 mL of 1 M CaCl2, 1x trace elements solution, and 1x Wolfe’s vitamin solution, with the final pH adjusted to 6.8. SSS contained, per litre: 4.95 g NaCl, 1.45 g Na2SO4, 0.25 g KCl, 0.04 g KBr, 1.85 g MgCl2·6H2O, and 0.89 g NaNO3. The trace element solution contains 1 g FeCl3·6H2O, 0.18 g ZnSO4·7H2O, 0.12 g CuCl2·2H2O, 0.12 g MnSO4·H2O, and 0.18 g CoCl2·6H2O. Finally, Wolfe’s vitamin solution contained, per litre: 10 mg Pyridoxine HCl, 5 mg Thiamine HCl, 5 mg Riboflavin, 5 mg Nicotinic acid, 5 mg Ca-D-(+)pantothenate, 5 mg p-Aminobenzoic acid, 5 mg Thiotic acid (Dithiolane Pentanoic acid), 2 mg Biotin, 2 mg Folic acid, and 0.1 mg Vitamin B12. Overnight cultures were diluted 1:12,5 in 200 μL of TMMYE with or without the following carbon substrates (80mM glycerol; 48mM xylose; 40 mM glucose, fructose, mannose, mannitol or sorbitol; 30mM N-acetyl-glucosamine; 26mM N-acetyl-muramic acid; 20mM cellobiose, maltose, sucrose, or trehalose; 8mM arbutin or salicin). All carbohydrates/glucosides were purchased from Sigma-Aldrich (USA) or Carbosynth (Compton, Berkshire, UK). Cultures were incubated in 96-well plates sealed with Titer-Tops® (Sigma-Aldrich, USA) at 60 °C with agitation (567 cpm) in an Epoch2 microplate spectrophotometer (BioTek, Agilent, USA), and bacterial growth was monitored every 10 minutes for 24 h measuring OD600 nm. At least three independent biological replicates for each growth curve were obtained. Results were expressed as means ± standard deviations.
Figures and data analysis
Figure 1 was created with BioRender (https://www.BioRender.com). Data analysis and illustrations were prepared in R (https://www.R-project.org/) using RStudio (https://www.RStudio.com).
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
References
Sheldon, R. A. & Woodley, J. M. Role of biocatalysis in sustainable chemistry. Chem. Rev. 118, 801–838 (2018).
Hauer, B. Embracing nature’s catalysts: A viewpoint on the future of biocatalysis. ACS Catal. 10, 8418–8427 (2020).
Chen, G.-Q. & Jiang, X.-R. Next generation industrial biotechnology based on extremophilic bacteria. Curr. Opin. Biotechnol. 50, 94–100 (2018).
Adams, M. W. W., Perler, F. B. & Kelly, R. M. Extremozymes: Expanding the limits of biocatalysis. Nat. Biotechnol. 13, 662–668 (1995).
Atalah, J., Cáceres-Moreno, P., Espina, G. & Blamey, J. M. Thermophiles and the applications of their enzymes as new biocatalysts. Bioresour. Technol. 280, 478–488 (2019).
Cripps, R. E. et al. Metabolic engineering of geobacillus thermoglucosidasius for high yield ethanol production. Metab. Eng. 11, 398–408 (2009).
Nazina, T. N. et al. Taxonomic study of aerobic thermophilic bacilli: Descriptions of geobacillus subterraneus gen. nov., sp. nov. and geobacillus uzenensis sp. nov. from petroleum reservoirs and transfer of bacillus stearothermophilus, bacillus thermocatenulatus, bacillus thermoleovorans, bacillus kaustophilus, bacillus thermodenitrificans to geobacillus as the new combinations G. stearothermophilus, G. th. Int. J. Syst. Evol. Microbiol. 51, 433–446 (2001).
Najar, I. N. & Thakur, N. A systematic review of the genera geobacillus and parageobacillus: Their evolution, current taxonomic status and major applications. Microbiology (U.K.) 166, 800–816 (2020).
Chen, J., Zhang, Z., Zhang, C. & Yu, B. Genome sequence of Geobacillus thermoglucosidasius DSM2542, a platform hosts for biotechnological applications with industrial potential. J. Biotechnol. 216, 98–99 (2015).
Lin, P. P. et al. Isobutanol production at elevated temperatures in thermophilic geobacillus thermoglucosidasius. Metab. Eng. 24, 1–8 (2014).
Yang, Z. et al. Engineering thermophilic Geobacillus thermoglucosidasius for riboflavin production. Microb. Biotechnol. 14, 363–373 (2021).
Van Kranenburg, R., Verhoef, A. & Petrus Machielsen, M. Genetic modification of (S)-lactic acid producing thermophilic bacteria. (2019).
Styles, M. Q. et al. The heterologous production of terpenes by the thermophile parageobacillus thermoglucosidasius in a consolidated bioprocess using waste bread. Metab. Eng. 65, 146–155 (2021).
Zhou, J., Lian, J. & Rao, C. V. Metabolic engineering of Parageobacillus thermoglucosidasius for the efficient production of (2R, 3R)-butanediol. Appl. Microbiol. Biotechnol. 104, 4303–4311 (2020).
Deutscher, J., Francke, C. & Postma, P. W. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol. Mol. Biol. Rev. 70, 939–1031 (2006).
Kotrba, P., Inui, M. & Yukawa, H. Bacterial phosphotransferase system (PTS) in carbohydrate uptake and control of carbon metabolism. J. Biosci. Bioeng. 92, 502–517 (2001).
Sonenshein, A. L. Control of key metabolic intersections in bacillus subtilis. Nat. Rev. Microbiol.. 5(12), 917 (2007).
Cerisy, T. et al. ABC transporters required for hexose uptake by clostridium phytofermentans. J. Bacteriol. 201, 15 (2019).
Van Wezel, G. P. et al. GlcP constitutes the major glucose uptake system of streptomyces coelicolor A3(2). Mol. Microbiol. 55, 624–636 (2004).
Fiegler, H., Bassias, J., Jankovic, I. & Brückner, R. Identification of a gene in staphylococcus xylosus encoding a novel glucose uptake Protein. J. Bacteriol. 181, 4929–4936 (1999).
Higgins, C. F. ABC transporters: From microorganisms to man. Annu. Rev. Cell Biol. 8, 67–113 (1992).
Pao, S. S., Paulsen, I. T. & SaierJr, M. H. Major facilitator superfamily. Microbiol. Mol. Biol.Rev. 62, 1–34 (1998).
Krispin, O. & Allmansberger, R. The Bacillus subtilis arae protein displays a broad substrate specificity for several different sugars. J. Bacteriol. 180, 3250–3252 (1998).
Davidson, A. L., Shuman, H. A. & Nikaido, H. Mechanism of maltose transport in Escherichia coli: Transmembrane signaling by periplasmic binding proteins. Proc. Nat. Acad. Sci. 89, 2360–2364 (1992).
Hülsmann, A., Lurz, R., Scheffel, F. & Schneider, E. Maltose and maltodextrin transport in the thermoacidophilic gram-positive bacterium Alicyclobacillus acidocaldarius Is mediated by a high-affinity transport system that includes a maltose binding protein tolerant to low pH. J. Bacteriol. 182, 6292–6301 (2000).
Morbach, S., Tebbe, S. & Schneider, E. The ATP-binding cassette (ABC) transporter for maltose/maltodextrins of Salmonella typhimurium. Characterization of the ATPase activity associated with the purified MalK subunit. J. Biol. Chem. 268, 18617–21 (1993).
Reizer, J. et al. Novel phosphotransferase system genes revealed by genome analysis—The complete complement of PTS proteins encoded within the genome of bacillus subtilis. Microbiology (N Y) 145, 3419–3429 (1999).
Schönert, S. et al. Maltose and maltodextrin utilization by bacillus subtilis. J. Bacteriol. 188, 3911–3922 (2006).
Tangney, M., Smith, P., Priest, F. G. & Mitchell, W. J. Maltose transport in bacillus licheniformis NCIB 6346. J. Gen. Microbiol. 138, 1821–1827 (1992).
Tangney, M., Buchanan, C. J., Priest, F. G. & Mitchell, W. J. Maltose uptake and its regulation in bacillus subtilis. FEMS Microbiol. Lett. 76, 191–196 (1992).
Liong, E. C. & Ferenci, T. Molecular cloning of a maltose transport gene from bacillus stearothermophilus and its expression in Escherichia coli K-12. MGG Mol. Gen. Genet. 243, 343–352 (1994).
Dassa, E. & Hofnung, M. Sequence of gene malG in E. coli K12: Homologies between integral membrane components from binding protein-dependent transport systems. EMBO J. 4, 2287–2293 (1985).
Chaillou, S., Pouwels, P. H. & Postma, P. W. Transport of d-xylose in lactobacillus pentosus, lactobacillus casei, andlactobacillus plantarum: evidence for a mechanism of facilitated diffusion via the phosphoenolpyruvate: mannose phosphotransferase system. J. Bacteriol. 181, 4768–4773 (1999).
Brumm, P. J., De Maayer, P., Mead, D. A. & Cowan, D. A. Genomic analysis of six new Geobacillus strains reveals highly conserved carbohydrate degradation architectures and strategies. Front. Microbiol. 6, 430 (2015).
Liao, W.-X., Earnest, L., Kok, S. L. & Jeyaseelan, K. Organization of the XYL genes in a thermophilic bacillus species. IUBMB Life 39, 1049–1062 (1996).
Dahl, U., Jaeger, T., Nguyen, B. T., Sattler, J. M. & Mayer, C. Identification of a phosphotransferase system of Escherichia coli required for growth on N -acetylmuramic acid. J. Bacteriol. 186, 2385–2392 (2004).
Litzinger, S. et al. Muropeptide rescue in Bacillus subtilis involves sequential hydrolysis by β- N -acetylglucosaminidase and N -acetylmuramyl- <scp>l</scp> -alanine amidase. J. Bacteriol. 192, 3132–3143 (2010).
Ruscitto, A. et al. Identification of a novel N -acetylmuramic acid transporter in tannerella forsythia. J. Bacteriol. 198, 3119–3125 (2016).
Heller, K. B., Lin, E. C. & Wilson, T. H. Substrate specificity and transport properties of the glycerol facilitator of escherichia coli. J. Bacteriol. 144, 274–278 (1980).
Beijer, L., Nilsson, R.-P., Holmberg, C. & Rutberg, L. The glpP and glpF genes of the glycerol regulon in bacillus subtilis. J. Gen. Microbiol. 139, 349–359 (1993).
Darbon, E. et al. Glycerol transport and phosphoenolpyruvate-dependent enzyme I- and HPr-catalysed phosphorylation of glycerol kinase in thermus flavus. Microbiology (N Y) 145, 3205–3212 (1999).
Simoni, R. D., Roseman, S. & Saier, M. H. Sugar transport. Properties of mutant bacteria defective in proteins of the phosphoenolpyruvate: Sugar phosphotransferase system. J. Biol. Chem. 251, 6584–6597 (1976).
Reizer, J., Novotny, M. J., Stuiver, I. & Saier, M. H. Regulation of glycerol uptake by the phosphoenolpyruvate-sugar phosphotransferase system in Bacillus subtilis. J. Bacteriol. 159, 243–250 (1984).
Romano, A. H., Saier, M. H., Harriott, O. T. & Reizer, J. Physiological studies on regulation of glycerol utilization by the phosphoenolpyruvate: Sugar phosphotransferase system in enterococcus faecalis. J. Bacteriol. 172, 6741–6748 (1990).
Beijer, L. & Rutberg, L. Utilisation of glycerol and glycerol 3-phosphate is differently affected by the phosphotransferase system in Bacillus subtilis. FEMS Microbiol. Lett. 100, 217–220 (1992).
Deutscher, J. & Sauerwald, H. Stimulation of dihydroxyacetone and glycerol kinase activity in streptococcus faecalis by phosphoenolpyruvate-dependent phosphorylation catalyzed by enzyme I and HPr of the phosphotransferase system. J. Bacteriol. 166, 829–836 (1986).
Charrier, V. et al. Cloning and sequencing of two enterococcal glpk genes and regulation of the encoded glycerol kinases by phosphoenolpyruvate-dependent, phosphotransferase system-catalyzed phosphorylation of a single histidyl residue. J. Biol. Chem. 272, 14166–14174 (1997).
Eddy, S. R. Accelerated profile HMM searches. PLoS Comput. Biol. 7, e1002195 (2011).
Mistry, J. et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 49, D412–D419 (2021).
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Sheng, L., Kovács, K., Winzer, K., Zhang, Y. & Minton, N. P. Development and implementation of rapid metabolic engineering tools for chemical and fuel production in geobacillus thermoglucosidasius NCIMB 11955. Biotechnol. Biofuels 10, 5 (2017).
Fong, J. C. N. et al. Isolation and characterization of two novel ethanol-tolerant facultative-anaerobic thermophilic bacteria strains from waste compost. Extremophiles 10, 363–372 (2006).
Suzuki, Y. et al. Bacillus thermoglucosidasius sp. nov., a new species of obligately thermophilic bacilli. Syst. Appl. Microbiol. 4, 487–495 (1983).
Aliyu, H., Lebre, P., Blom, J., Cowan, D. & De Maayer, P. Phylogenomic re-assessment of the thermophilic genus geobacillus. Syst. Appl. Microbiol. 39, 527–533 (2016).
Acknowledgements
We thank Ivan Pogrebnyakov and Alex Nielsen for sharing research materials and technical assistance. To The Novo Nordisk Foundation for grants NNF19OC0055620 and NNF20CC0035580.
Author information
Authors and Affiliations
Contributions
D.H.W. and G.N.B. conceived and designed the overall study. G.N.B. constructed strain variants. G.N.B. and H.G. collected and analyzed the wet lab data. H.G. designed and prepared the figures. All authors compiled relevant text parts, reviewed the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bidart, G.N., Gharabli, H. & Welner, D.H. Functional characterization of the phosphotransferase system in Parageobacillus thermoglucosidasius. Sci Rep 13, 7131 (2023). https://doi.org/10.1038/s41598-023-33918-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-33918-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.