Abstract
Coccidiosis causes huge economic losses worldwide. Current study evaluated the effect of biosynthesized Zinc oxide nanoparticles (ZnONPs) using Nigella sativa, on Eimeria tenella infected broilers. Scanning electron microscopy showed spherical ZnONPs with 50–100 nm diameter, Fourier transforms infrared spectroscopy revealed the functional groups involved in the reduction of zinc acetate dihydrate to ZnONPs, UV–vis spectroscopy showed a peak at 354 nm, and Zeta potential exhibited stability at − 30 mV. A total of 150, a day-old broiler chicks were divided into 5 equal groups. Control negative: uninfected and untreated; Control positive: Infected and untreated; 3rd, 4th and 5th group were infected orally with 5 × 104 sporulated oocysts of Eimeria tenella and treated with 60 mg/kg ZnONPs, 1% Nigella sativa seeds and amprolium 125 ppm, respectively. ZnONPs significantly (p < 0.05) improved the growth performance in the infected birds and decreased the oocyst shedding and anti-coccidial index. A significant (p < 0.05) decrease in the level of aspartate transferase and alanine transferase, whereas, a significantly higher amount of antioxidants like catalase and superoxide dismutase in ZnONPs treated group was observed. Pro-inflammatory cytokines like IL-2 and TNF-α were significantly decreased by ZnONPs (p < 0.05). In conclusion, biogenic ZnONPs with Nigella sativa might have enhanced anticoccidial, antioxidant, and anti-inflammatory effects with improved growth performance.
Similar content being viewed by others
Introduction
The poultry business has become the fastest-growing business in many countries1. Parasitic infections are responsible for greater economic losses to the poultry farmers2. Coccidiosis is a very fatal protozoal disease characterized by bloody droppings, intestinal inflammation, poor growth performance, increased morbidity and mortality3. Coccidiosis affects nutrient digestibility by reducing the size of villi in the small intestine4, and alters the expression of genes regulating transport proteins and digestive enzymes of the small intestine5,6. In coccidiosis, there is increased production of reactive oxygen species (ROS) and nitrogenous species responsible for lower production of antioxidant enzymes including superoxide dismutase and catalase7. Also, liver enzymes like aspartate aminotransferase (AST) and alanine aminotransferase (ALT) increase notably. Financial losses in the diseased birds are linked to poor growth performance and increased death rates as a result of multiple changes in their physiological and metabolic system8,9. For control of coccidiosis different anticoccidials are used but resistance develops due to the widespread use of different anticoccidials like amprolium, clazuril, diclazuril, monensin, salinomycin, spiramycin, sulfadimethoxine, and toltrazuril10. Currently, natural remedies are adopted as alternatives to treat a variety of ailments11.
Anticoccidial drugs in birds have resulted in drug resistance as well as the presence of drug residues in chicken products like meat and eggs, ultimately posing a major public health risk12. However, the unavailability of new anticoccidial drugs demands novel anticoccidial alternatives13. Nanotechnology methods have currently been incorporated into the veterinary industry, not only for disease diagnosis but also for drug development and prevention of diseases14. Zinc is an essential mineral for poultry growth but too much zinc oxide (ZnO) in the feed leads to poor digestion and zinc excretion in the faeces, which harms the environment15. A good substitute for traditional zinc sources is the synthesis of zinc oxide nanoparticles (ZnONPs). Nowadays, trace elements such as zinc are manufactured as nanoparticles possessing higher absorption capacity and effectiveness than both natural and anthropogenic sources16.
ZnONPs exhibit greater efficacy because of having a large surface area, small particle size, efficient catalysts, more absorption and adsorption in comparison to old zinc sources17. Lower dosages of ZnONPs have better therapeutic effect on animals, good chemical activity, participation in oxidative reactions, whereas, these particles are environment friendly due to high bioavailability and good pharmacological activities than traditional zinc oxide18. ZnONPs have antimicrobial, antifungal, antiparasitic, anti-diabetic activities and are used as growth promotors, dietary supplements, immune- modulators, and catalysts for phytochemical reactions19,20. Furthermore, ZnONPs used in biological sensing, biological labeling, gene transport and targeted drug delivery21,22. Previous studies have reported that ZnONPs provided good results in terms of broiler productivity, antioxidant status, and gastrointestinal function23. Nanoparticles synthesized from physical and chemical methods have a limited range of size and morphology. These methods are very costly and the chemicals used cause environmental pollution and toxicity in the body. However, biogenic synthesis of nanoparticles with plants is an efficient, environmentally friendly, cost-effective, time saving, and energy saving method24. Nigella sativa is a medicinal plant of the Ranunculaceae family, its chief pharmacologically active ingredient is thymoquinone and it has anti-inflammatory, anti-oxidant, anti-parasitic, and anti-microbial properties25. ZnONPs loaded with garlic extract decreased the amount of ALT, AST, and ALP enzymes in rabbits infected with E. stiedae26. Some investigations have revealed that ZnONPs have antioxidant properties27,28. The current study was designed to check the anticoccidial, antioxidant, and anti-inflammatory effects of biosynthesized zinc oxide nanoparticles (ZnONPs) in Eimeria tenella-infected boilers.
Materials and methods
Preparation of Nigella sativa seed extract
Nigella sativa seeds were purchased from the local market of Lahore, Pakistan for green synthesis of Zinc oxide nanoparticles. Seeds were grounded to powder form and then dissolved in distilled water and boiled for 30 min. The mixture was filtered using Whatman filter paper No.1, and the extract was stored at 4 °C29.
Green synthesis of zinc oxide nanoparticles
Zinc acetate dihydrate salt (0.25 M) solution was prepared by adding 22.92 g zinc acetate dihydrate salt to 500 ml of distilled water in a flask. Further, 10 ml of Nigella sativa seed extract added dropwise into the zinc acetate solution under continuous stirring at 60 ℃ for 2 h on a magnetic stirrer. While stirring, NaOH was added dropwise in the above solution to maintain the pH at 12. Plant extract acted as a reducing agent. The formation of light yellow color suspended particles and visible color change of the solution from brown to yellow indicated the formation of zinc oxide nanoparticles. This mixture was then centrifuged at 6000 × g for 15 min and supernatant was discarded to collect the pellet in a Petri plate. Afterwards, it was placed overnight in a hot air oven for drying at 60 ℃. Calcination of yellow-colored ZnONPs was done in a muffle furnace at 400 ℃ for 4 h to remove impurities (Figs. 1, 2)30.
Influence of the plant extract on production of ZnONPs was investigated by varying the ratio of plant extract to salt (v/w) and yield of prepared ZnONPs was calculated by following formula
Characterization of zinc oxide nanoparticles
The optical properties of Zinc oxide nanoparticles (ZnONPs) were analyzed by using a UV- spectrophotometer. Firstly, water was run as a blank, and then the ZnONPs sample was set in a spectrophotometer. UV light passed at 354 nm and confirmed the formation of ZnONPs31. Scanning Electron Microscopy was used to identify the morphology of ZnONPs. The sample was observed at 20kv with a frequency of 2838cps. For determination of shape and size, ZnONPs was analyzed at different resolution and magnification power. Fourier transform infrared spectroscopy (FTIR) analysis was performed to know the functional groups involved in the synthesis of ZnONPs. The solution was dried at 75˚C and characterization was done at a wavenumber ranging from 4000 to 400 cm−129. Stability of green synthesized ZnONPs were analyzed by Zeta potential32. To determine the purity and structural characteristics of ZnONPs, diffraction intensities was recorded at 30 kV and 10 mA current with the range 2θ from − 3 to 160°by using X-ray diffractometer (Bruker D2 Phaser).
Preparation of Eimeria tenella infection
Feces from E.tenella infected bird’s ceca were collected and examined for the presence of oocysts by sedimentation technique. By floatation technique, E.tenella oocysts were collected from the supernatant and washed with tap water by centrifugation at 1500 rpm for 10 min. For sporulation, washed oocysts were incubated at room temperature in 2.5% potassium dichromate for 72 h. Sporulated oocysts were washed with PBS by centrifugation at 1500rom for 10 min. Beading was performed by vortexing the sporulated oocysts with 0.5 mm sterilized glass beads33.
Ethical approval
The experiments were performed in accordance with comprehensive animal welfare guide (FASS, 2010) and approved by the Ethical Review Committee (ERC) of the University of Veterinary and Animal Sciences (UVAS), Lahore, Pakistan (permit No. DR/ 357).
Experimental design
The experiments were performed in accordance with comprehensive animal welfare guide (FASS, 2010) and approved by the Ethical Review Committee (ERC) of the University of Veterinary and Animal Sciences (UVAS), Lahore, Pakistan (permit No. DR/ 357). This study also followed the ARRIVE guidelines. A total of 150, day-old broiler chicks were purchased from a nearby hatchery and kept under standard conditions. Birds were divided into five equal groups. On the 28th day except for group 1, all groups were infected orally through a stomach tube, with 5 × 104 sporulated oocysts of Eimeria tenella. Group 1 was control negative, which was kept un-infected and un-medicated. Group 2 was control positive, infected, and un-medicated. Group 3 was fed with 60 mg/kg ZnONPs orally through a stomach tube. Group 4 was fed with 1% Nigella sativa crushed seeds mixed in feed. Group 5 was given amprolium at a dose rate of 125 ppm orally. Treatment was given from 28 to 35th day of trial.
Anticoccidial activity analysis
Anticoccidial activity of ZnONPs was analyzed by recording the body weight gain (BWG), FCR, OPG, mortality rate, lesion scores, and anticoccidial index of the experimentally infected birds.
Determination of weight gain and feed conversion ratio (FCR)
Growth performance, body weight, and feed intake of the birds were recorded on weekly basis34.
Fecal examination
Fecal samples of birds were collected daily, post challenge till 35th day. McMaster oocyst counting technique was used for quantitative examination of feces, while qualitative examination of feces was done by using the direct smear and flotation techniques35.
Mortality rate and lesion score
Birds were observed daily for mortality. At the end of the trial, birds from each group were slaughtered and examined for cecal lesions, and scored on the basisof severity of lesions. 0 score for –ve group and 4 for severely affected and dead birds36.
Anticoccidial index (ACI)
Anticoccidial index of each group was calculated by the formula: (Relative ratio of the body weight gain + survival rate)–(lesion index + oocyst value). Anti-coccidial index greater than 180 is perceived to have a very effective anticoccidial action. ACI in 160–180 range indicated marked anticoccidial action and ACI less than 120 indicated no anticoccidial activity37.
Blood sampling and serum biochemical analysis
Blood samples (2 ml) were collected on 35th day from the wing vein of all the birds in plain tubes, allowed to clot and then centrifuged at 4000 rpm for 10 min. The obtained serum was stored at − 20 °C for biochemical analysis38. Serum biochemical profile including aspartate transferase (AST), and alanine transferase (ALT) was evaluated by using a Diasys kit following the manufacturer’s guidelines39.
Determination of antioxidant enzymes activity
Antioxidants like catalase (CAT) and superoxide dismutase (SOD) activities were evaluated by using Fine test Elisa kits following guidelines given by the manufacturer40.
Determination of serum inflammatory cytokines
The determination of tumor necrosis factor-alpha (TNF-α) was evaluated by using the BTlab ELISA kit and level of Interleukins-2 (IL-2) was determined by using a Fine test ELISA kit following the manufacturer’s guidelines41.
Statistical analysis
The data were analyzed by using one-way ANOVA using SPSS version 20.0 and difference between mean was determined using Tukey’s test at (p < 0.05) level.
Results
Ultra violet analysis of zinc oxide nanoparticles
Ultraviolet spectroscopy confirmed the synthesis of ZnONPs and showed the optical activity and stability of nanoparticles. After the formation of yellow suspended particles, this solution was run in an ultraviolet spectrophotometer. The light passed and spectra observed at 354 nm indicated ZnONPs as shown in (Fig. 3).
Scanning electron microscopy
Scanning electron microscopy images showed spherical-shaped nanoparticles of average size ranging from 50–100 nm (Fig. 4).
X-ray diffraction analysis
XRD peaks showed 2θ values at 31.9, 35.1, 36.4, 47.6, 56.6 and 63 corresponding to the (100), (002), (101), (102), (110) and (103) planes showing hexagonal wurtzite crystalline ZnONPs using JCPDS (Fig. 5).
Fourier transform infra-red spectroscopy
Fourier transform infrared spectrum of ZnONPs showed several peaks at 3861, 3746, 2367, 1395, 1087, 850, 760, and 490 cm−1 (Fig. 6).
Zeta potential
Results of zeta potential at − 30 mV indicated the colloidal stability of green synthesized ZNONPs (Fig. 7).
Growth performance
An increase in body weight was noted in birds of all groups and there was no significant difference (p > 0.05) among different groups till the onset of clinical signs of the coccidiosis. Growth performance decreased significantly (p < 0.05) due to Eimeria infection. Treatment with ZnONPs and Nigella sativa significantly (p > 0.05) enhanced the growth performance and the results were comparable to that of the amprolium-treated group (Table 1).
Oocysts shedding and lesion scores
On the 4th day post-infection, there was frequent bloody diarrhea in the infected groups and generalized weakness was present in the birds. No. of oocysts per gram (OPG) of feces was counted by McMaster’s chamber on, 32th, 33th, 34th, and 35th days. On the 4th day post-infection, a large number of oocysts was shedding in the feces and maximum OPG was found in the control positive group, which was significantly higher (p < 0.05) than in other groups. The non-infected group was free from any signs and oocyst shedding. In treatment groups, OPG of ZnONPs groups was lower than in NS seeds and amprolium treated groups. Lesion score was significantly higher in control + ve group indicating the cecal damage. Lesion scores in ZnONPs were significantly low (p < 0.05) and comparable to that of amprolium treated group (Table 2).
Anticoccidial index (ACI)
There was 16% mortality in the control positive group and no mortality in the treated and control negative group. Lesion index was calculated by multiplying lesion score by 10. ACI of the control positive group was < 120 indicating no anticoccidial effect. ZnONPs treated group showed ACI > 180 comparable to that of amprolium treated group (p > 0.05) (Fig. 8).
Serum biochemical analysis
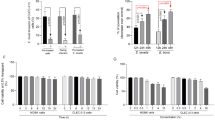
The current study observed an increased level of ALT and AST in the serum due to tissue damage 7 days post-infection (Fig. 8). Amount of ALT and AST showed a significant (p < 0.05) increase in an infected group as compared to the non-infected group. Group treated with ZnONPs has shown a significant effect (p < 0.05) on the amount of ALT and AST comparable to amprolium treated group.
Antioxidant enzyme activities
The current study observed a significant (p < 0.05) decrease in the amount of SOD and CAT in control positive group. The level of SOD and CAT was significantly increased (p < 0.05) in infected but treated groups. ZnONPs showed a significant effect (p < 0.05) as compared to other treatment groups (Fig. 8).
Serum inflammatory cytokines
The amount of IL-2 and TNF-α was significantly higher (p < 0.05) in the infected group than control negative and other treated groups. ZnONPs and NS seed groups showed a significant decline in the level of IL-2 and TNF-α. The level of IL-2 and TNF-α in amprolium group was significantly lower (p < 0.05) than that of control positive group but higher than other groups (Fig. 9).
Serum biochemical profile, Antioxidant status, and level of inflammatory cytokines of the broilers challenged with Eimeria tenella and given different treatments (A) Aspartate transferase, (B) Alanine transferase, (C) Catalase, (D) Superoxide dismutase, (E) Interleukins-2, (F) Tumor necrosis factor-α.
Discussion
Poultry coccidiosis is a very harmful disease that damages the gastrointestinal tract of the birds causing huge economic losses. Prevailing resistance against available anticoccidials provokes the use of alternative treatments for coccidiosis like natural products42. Moreover, the drugs used against coccidiosis like toltrazuril cause the problem of drug residues in poultry products i.e. meat and eggs43. That’s why nanoparticles and herbal plants are being used as a new and effective remedy against coccidiosis with maximum efficacy and no residue problem44.
In this study, anticoccidial effect of green synthesized ZnONPs was evaluated as compared to traditional zinc. During green synthesis, a color change from light brown to yellow was observed indicating the reduction of zinc45. Bioactive components like quinones, alkaloids, phenolics and flavonoids present in Nigella sativa extract reduced the zinc acetate salt and control the size of ZnONPs. Carboxylic and amino groups of these bioactive compounds binds to the surface of zinc (Zn+2) and amides from capping proteins may act as a stablizer46. Plant extract concentration is critical in the production of ZnO NPs. Hence, 3 ml/gm and 6 ml/gm don’t have sufficient bioactive components that can reduce Zinc acetate and lead to low yield of NPs. Whereas, 10 ml/gm has given highest yield of ZnONPs47 (Fig. 2). Confirmation of synthesis of ZnONPs was done by Uv- analysis. In Uv-spectrum, a clear absorption peak was formed at 354 nm, indicating the presence of ZnONPs. In another study by48, ZnONPs were synthesized by using Cassia fistula and Melia azedarach which revealed absorption peak at 320 nm and 324 nm, respectively49 reported that ZnONPs synthesized by leaf extract of Piper betle showed absorption peaks at 358, 368, and 378 nm. This low absorbance is due to presence of complex biomolecules in the plants50.
Scanning Electron Microscopy images showed more or less spherical NPs of 50–100 nm size range51 reported that the diameter of ZnONPs synthesized by Mentha Spicata extract was 11–80 nm. The difference in the size of the NPs is because of the difference in temperature conditions provided during the synthesis of NPs. If a high temperature is provided to the reaction mixture, small-sized NPs will be synthesized52,53. The volume of the plant extract also plays a role in controlling the size and shape of NPs. Using a large volume of extract during synthesis will reduce the size of NPs54,55. FTIR spectrum showed a peak at 3861 cm−1 and 3746 cm−1 representing the stretching vibration of hydroxyl OH-bond from phenolic compounds present in the plant29. The peak at 2367 cm−1 indicated N–H bond of the primary and secondary amides. The band at 1395 cm−1 indicated C=O stretching of amides and C=C stretching of alkenes56. The band at 1087 cm−1 corresponded with stretching of C-O bond57,58. The peaks at 850 cm−1 and 760 cm−1 indicated C–N stretch of the amine group59. The absorption peak at 490 cm−1 showed the ZnO bond as reported previously60. The presence of alkenes is confirmed by FTIR spectra that have a role in antioxidant activity61. Hydroxyl bonds confirmed the presence of flavonoids, having antioxidant and anti-inflammatory properties62 and C=O groups indicateed the presence of thymoquinone responsible for anti-coccidial activity63. Zeta potential of − 30 mV showed good stability of green synthesized ZnONPs. Negative surface charge is due to the strong binding affinity of extract chemicals for the NPs which increased their stability and reduced their tendency to aggregate64. Distinct narrow peaks of XRD showed that ZnONPs are free from impurities and have hexagonal wurtzite crystals47.
In this study, a significant decrease in growth performance was observed in an infected group similar to the studies reported by65. The reduction in the growth performance may occur due to intestinal damage and decreased length of the villi which may have resulted in poor nutrient absorption66. Infected but treated groups, with ZnONPs, NS seeds, and amprolium respectively, showed improvement in growth performance because of decreased intestinal damage36 reported that the supplementation of the Nigella sativa reduced the adverse effects of Eimeria and increased the growth performance. ZnONPs are better than the traditional Zn sources in improving feed utilization and growth because Zn is involved in many enzymatic reactions of metabolism67. ZnONPs promote growth because they increase the intestinal villi length and crypt depth and hence resulting in increased nutrient absorption68.
The results of this study showed that there was severe bloody diarrhea in the control positive group and a maximum number of oocysts shedding in the feces. Bloody diarrhea occurred due to penetration of spores inside the cecal epithelium and spores of protozoans complete their developmental stages inside the epithelium till the schizogony stage, ultimately damaging the intestinal blood vessels and causing hemorrhages69,70. Current study revealed that ZnONPs significantly decreased the number of E. tenella oocysts in the feces, caecum lesion score and anticoccidial index at a dose of 60 mg/kg similar to71. This may be due to the reason that ZnONPs diminished the growth and development of protozoa inside the intestinal cells before the inactive oocysts were formed and shed44. Furthermore, NS seeds given in diet also decreased the OPG in E.tenella-infected birds36.
Current study showed a significant increase level of AST and ALT in the serum of infected birds. Similar results were reported by10 and they proposed that this rise in enzyme activity occurs due to cecal cell wall damage, inflammation, and heavy blood loss. In this study, ZnONPs and NS seeds caused a reduction in the level of these enzymes. It is thought that quinone component present in NS seeds may have a role in reducing cecal lesions72. Our results are in line with the study of36 which showed that the treatment with NS seeds reduced the level of these enzymes. In contrast73, reported that there was no significant change in the AST and ALT levels in infected and treated groups. Moreover74, stated that the level of ALT was enhanced after E. tenella infection, but there was no effect of infection on level of AST. This contradiction in results may occur because of variations in the degree of intestinal inflammation, intestinal damage and hemorrhages75.
Results of antioxidant activity showed a significant decrease in the amount of SOD and CAT, indicating oxidative damage because of Eimeria infection76. While the level of SOD and CAT was high in treatment groups similar to the findings of77 who stated that Nigella sativa significantly increased the level of SOD and CAT. In our study, the antioxidant activity of ZnONPs was higher than the other treated groups similar to the results of78,79 who stated that administering ZnONPs in the broiler diet significantly increases both SOD and CAT activity in serum. However, in contrast to our study, (El Katcha et al. 2017) reported that there was no significant change in antioxidant activity upon feeding 15–60 ppm nano-Zinc to the broilers. Moreover, studies have reported that 60 mg/kg ZnONPs have good Cu–Zn-SOD activity. The reason for the marked antioxidant property of ZnONPs is that Zn is an important constituent of SOD and this enzyme reduces oxidative stress by efficiently scavenging the superoxide free radicals80. Another mechanism behind the antioxidant activity of ZnONPs is the competition of zinc with iron and copper for attachment on the binding site present on cell membranes and that’s why Zn decreases free radical production81. Moreover, it is also proposed that Zn promotes the production of metallothionein and this protein plays an important role in the detoxification of free radicals82 and also activates the antioxidant proteins and enzymes like glutathione peroxidase (GPx) and CAT83.
This study indicated a significantly high level of pro-inflammatory cytokines in broilers of the control positive group84 showed that in intestinal inflammation, pro-inflammatory cytokines intensely increased because these are attracted to the site of inflammation. Treatment with biogenic ZnONPs diminished the pro-inflammatory cytokines (IL-2 and TNF-α). Our results are in agreement with85, who reported that ZnONPs decreased the mRNA expression of IL-2 and TNF-α in serum. The anti-inflammatory property of Zn may be due to Nrf2/HO-1 signal pathway86,87. Substances that activates the Nrf2 pathway, decrease the overproduction of pro-inflammatory cytokines like IL-2 and TNF-α, and enhances immunoglobulin production88,89,90,91 also stated that an activated Nrf2/HO-1pathway; stops the TLR4-mediated inflammatory responses. It is shown that ZnONPs have anticoccidial property and improved growth performance.
Conclusion
Biogenic ZnONPs supplementation to Eimeria tenella infected broilers showed anticoccidial activity by decreasing the oocysts shedding and improve the growth performance. Moreover, ZnONPs decreased the tissue damage which was indicated by decrease in the level of serum enzymes like alanine transferase and aspartate transferase. ZnONPs showed antioxidant and anti-inflammatory activity by significantly increasing the activity of superoxide dismutase alongwith catalase and decreasing the amount of pro-inflammatory cytokines interleukins-2 as well as tumor necrosis factor-α. So, ZnONPs can be used as an alternative to treat coccidiosis. But the exact mechanism of its anti-coccidial action is still unknown so further research is needed to understand its mechanism of action alongwith comparison between different plant mediated synthesis of ZnONPs.
Data availability
All data generated or analyzed during this study are included in this manuscript.
References
Bobkov, M. & Zbinden, P. Occurrence of veterinary drug residues in poultry and products thereof A review. Chimia (Aarau) 72, 707–712 (2018).
Mohammed, B. & Sunday, O. An overview of the prevalence of avian coccidiosis in poultry production and its economic importance in Nigeria. Vet. Res. 3, 35–45 (2015).
Kadykalo, S. et al. The value of anticoccidials for sustainable global poultry production. Int. J. Antimicrob. Agents 51, 304–310 (2018).
Su, S., Miska, K. B., Fetterer, R. H., Jenkins, M. C. & Wong, E. A. Expression of digestive enzymes and nutrient transporters in Eimeria-challenged broilers. Exp. Parasitol. 150, 13–21 (2015).
Miska, K. B. & Fetterer, R. H. Molecular and cellular biology: The effect of Eimeria maxima infection on the expression of amino acid and sugar transporters aminopeptidase, as well as the di- and tri-peptide transporter PepT1, is not solely due to decreased feed intake. Poult. Sci. 97, 1712–1721 (2018).
Abdelhady, A. Y. M., Abdullah, S. H., ElSafty, S. A., Eldeib, M. & Hashim, M. M. Impact of Phyto-Cocci® powder supplementation on broiler growth performance, eimeria oocyst shedding and gut health. Int. J. Poult. Sci. 19, 66–74 (2020).
Bun, S. D., Guo, Y. M., Guo, F. C., Ji, F. J. & Cao, H. Influence of organic zinc supplementation on the antioxidant status and immune responses of broilers challenged with Eimeria tenella. Poul. Sci. 90(6), 1220–1226 (2011).
Abd El-Hack, M. E. et al. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 164, 2726–2744 (2020).
Abd El-Hack, M. E. et al. Prebiotics can restrict Salmonella populations in poultry: A review. Anim. Biotechnol. https://doi.org/10.1080/10495398.2021.1883637 (2021).
Mund, M. D., Khan, U. H., Tahir, U., Mustafa, B. E. & Fayyaz, A. Antimicrobial drug residues in poultry products and implications on public health: A review. Int. J. Food Prop. 20, 1433–1446 (2017).
Abdelnour, S. A. et al. The beneficial impacts of dietary phycocyanin supplementation on growing rabbits under high ambient temperature. Ital. J. Anim. Sci. 19, 1046–1056 (2020).
Khan, R. U. et al. Potential applications of ginger (Zingiber officinale) in poultry diets. Worlds. Poult. Sci. J. 68, 245–252 (2012).
Mohammad, M. H., Ahad, O., Mohammad, N., Fariborz, A. & Omid, N. Prevalence of Eimeria species in scavenging native chickens of Shiraz. Iran. African J. Microbiol. Res. 5, 3296–3299 (2011).
Dakpogan, H. B. & Salifou, S. Coccidiosis prevalence and intensity in litter- based high stocking density layer rearing system of Benin. J. Anim. & Plant Sci. 17, 2522–2526 (2013).
Wang, C. et al. Zinc oxide nanoparticles as a substitute for zinc oxide or colistin sulfate: Effects on growth, serum enzymes, zinc deposition, intestinal morphology and epithelial barrier in weaned piglets. PLoS One 12, (2017).
Gopi, M., Pearlin, B., Kumar, R. D., Shanmathy, M. & Prabakar, G. Role of nanoparticles in animal and poultry nutrition: Modes of action and applications in formulating feed additives and food processing. Int. J. Pharmacol. 13, 724–731 (2017).
Safaei-Ghomi, J., Ghasemzadeh, M. A. & Zahedi, S. ZnO nanoparticles: A highly effective and readily recyclable catalyst for the one-pot synthesis of 1,8-dioxo-decahydroacridine and 1,8-dioxooctahydro-xanthene derivatives. J. Mex. Chem. Soc. 57, (2017).
Hussain, Z. et al. Synthesis, characterization, and pharmacological evaluation of zinc oxide nanoparticles formulation. Toxicol. Ind. Health 34, 753–763 (2018).
Serda, M. et al. Enhanced antibacterial activity of nanocrystalline ZnO due to increased ROS-mediated cell injury. Adv. Funct. Mater. 19, 842–852 (2009).
Al-Zahrani, H., El-Waseif, A. & El-Ghwas, D. Biosynthesis and evaluation of TiO2 and ZnO nanoparticles from in vitro stimulation of Lactobacillus johnsonii. J. Innov. Pharm. Biol. Sci. 5, 16–20 (2018).
Yoon, S. H. & Kim, D. J. Fabrication and characterization of ZnO films for biological sensor application of FPW device. IEEE Int. Symp. Appl. Ferroelectr. https://doi.org/10.1109/ISAF.2006.4349273 (2006).
Ho, K.-M. ZnO nanoparticles applied to bioimaging and drug delivery. Adv. Mater. 25, 5329–5335 (2013).
Sultan, A. et al. Supplementation of zinc oxide nanoparticles has beneficial effects on intestinal morphology in broiler chicken. Pak. Vet. J. 37, 335–339 (2017).
Sharif, H. M. A. et al. Fe3O4 nanoparticles coated with EDTA and Ag nanoparticles for the catalytic reduction of organic dyes from wastewater. ACS Appl. Nano Mater. 2, 5310–5319 (2019).
Aydogan, I. et al. The effect of dietary garlic (Allium sativum), black cumin (nigella sativa) and their combination on performance, intestine morphometry, serum biochemistry and antioxidant status of broiler chickens. Rev. Bras. Cienc. Avic. 22, 1–10 (2020).
Mohi-Eldin, M. M., Haridy, M. A. M., Hussein Khalil, A. M. & Abdelnaeim, K. Immunomodulatory and antiparasitic effects of garlic extract loaded on zinc oxide nanoparticles compared with pure garlic extract on Eimeriastiedae-infected rabbits. Benha Vet. Med. J. 35, 94–105 (2018).
Zhang, J. et al. Effects of zinc oxide nanoparticles on growth, intestinal barrier, oxidative status and mineral deposition in 21-day-old broiler chicks. Biol. Trace Elem. Res. 200, 1826–1834 (2022).
Hidayat, C., Sumiati, S., Jayanegara, A. & Wina, E. Supplementation of dietary nano Zn-phytogenic on performance, antioxidant activity, and population of intestinal pathogenic bacteria in broiler chickens. Trop. Anim. Sci. J. 44, 90–99 (2021).
Nandhini, P., Surya, K., Kalaiselvi, V., Ramya, V. & Vidhya, N. Synthesis and characterization of pure and capped zinc oxide nanoparticles using Nigella Sativa. Int. J. Adv. Sci. Eng. 7, 1756–1760 (2020).
Rafique, M. et al. Plant-mediated green synthesis of zinc oxide nanoparticles from Syzygium Cumini for seed germination and wastewater purification. Int. J. Environ. Anal. Chem. 102, 23–38 (2022).
Kumara Swamy, M., Sudipta, K. M., Jayanta, K. & Balasubramanya, S. The green synthesis, characterization, and evaluation of the biological activities of silver nanoparticles synthesized from Leptadenia reticulata leaf extract. Appl. Nanosci. 5, 73–81 (2015).
Melk, M. M. et al. Nano zinc oxide green-synthesized from plumbago auriculata lam. Alcoholic extract. Plants 10, (2021).
Fallis, A. Biotechnology. Guidelines on techniques in coccidiosis research. J. Chem. Inf. Model. 53, 1689–1699 (1995).
Pop, L. et al. Effects of artemisinin in broiler chickens challenged with Eimeria acervulina, E. maxima and E. tenella in battery trials. Vet. Parasitol. 214, 264–271 (2015).
Ali, A. M., Seddiek, S. A. & Khater, H. F. Effect of butyrate, clopidol and their combination on the performance of broilers infected with Eimeria maxima. Br. Poult. Sci. 55, 474–482 (2014).
Kadhim, L. I., Al-Zubaidi, M. T. & AL Saegh, H. A. Influence of dietary supplementation of Nigella sativa on experimental coccidiosis in broiler chickens. ~ 652 ~ J. Entomol. Zool. Stud. 6, (2018).
Lan, L. et al. Anticoccidial evaluation of a traditional Chinese medicine-Brucea javanica-in broilers. Poult. Sci. 95, 811–818 (2016).
Grądzki, Z., Jarosz, Ł, Stępień-Pyśniak, D. & Marek, A. The effect of feed supplementation with Transcarpathian zeolite (clinoptilolite) on the concentrations of acute phase proteins and cytokines in the serum and hepatic tissue of chickens. Poult. Sci. 99, 2424–2437 (2020).
Sokół, R., Gesek, M., Raś-Noryńska, M., Michalczyk, M. & Koziatek, S. Biochemical parameters in Japanese quails Coturnix coturnix japonica infected with coccidia and treated with toltrazuril. Pol. J. Vet. Sci. 18, 79–82 (2015).
Zhang, G. F. et al. Effects of ginger root (Zingiber officinale) processed to different particle sizes on growth performance, antioxidant status, and serum metabolites of broiler chickens. Poult. Sci. 88, 2159–2166 (2009).
Chand, N. et al. Anticoccidial effect of mananoligosacharide against experimentally induced coccidiosis in broiler. Environ. Sci. Pollut. Res. 23, 14414–14421 (2016).
Michels, M. G., Bertolini, L. C. T., Esteves, A. F., Moreira, P. & Franca, S. C. Anticoccidial effects of coumestans from Eclipta alba for sustainable control of Eimeria tenella parasitosis in poultry production. Vet. Parasitol. 177, 55–60 (2011).
Alnassan, A. A. et al. Efficacy of early treatment with toltrazuril in prevention of coccidiosis and necrotic enteritis in chickens. Avian Pathol. 42, 482–490 (2013).
Dkhil, M. A., Al-Quraishy, S. & Wahab, R. Anticoccidial and antioxidant activities of zinc oxide nanoparticles on Eimeria papillata-induced infection in the jejunum. Int. J. Nanomed. 10, 1961–1968 (2015).
Rajiv, P., Rajeshwari, S., & Venckatesh, R. Bio-Fabrication of zinc oxide nanoparticles using leaf extract of Parthenium hysterophorus L. and its size-dependent antifungal activity against plant fungal pathogens. Spectrochim Acta Part A Mol. Biomol. Spectrosc. 112, 384–387 (2013).
Mukherjee, S. et al. Green chemistry approach for the synthesis and stabilization of biocompatible gold nanoparticles and their potential applications in cancer therapy. Nanotechnology 23, (2012).
Bala, N. et al. Green synthesis of zinc oxide nanoparticles using Hibiscus subdariffa leaf extract: Effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv. 5, 4993–5003 (2015).
Naseer, M., Aslam, U., Khalid, B. & Chen, B. Green route to synthesize zinc oxide nanoparticles using leaf extracts of Cassia fistula and Melia azadarach and their antibacterial potential. Sci. Rep. 10, 1–10 (2020).
Thi Tran, Q. M., Thi Nguyen, H. A., Doan, V. D., Tran, Q. H. & Nguyen, V. C. Biosynthesis of zinc oxide nanoparticles using aqueous piper betle leaf extract and its application in surgical sutures. J. Nanomater. 2021, (2021).
Saif, M. S. et al. Phyto-reflexive zinc oxide nano-flowers synthesis: an advanced photocatalytic degradation and infectious therapy. J. Mater. Res. Technol. 13, 2375–2391 (2021).
Abdelkhalek, A. & Al-Askar, A. A. Green synthesized ZnO nanoparticles mediated by Mentha spicata extract induce plant systemic resistance against Tobacco mosaic virus. Appl. Sci. 10, (2020).
Saware, K. & Venkataraman, A. Biosynthesis and characterization of stable silver nanoparticles using ficus religiosa leaf extract: A mechanism perspective. J. Clust. Sci. 25, 1157–1171 (2014).
Jain, S. & Mehata, M. S. Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Sci. Rep. 7, (2017).
Agarwal, H., Venkat Kumar, S. & Rajeshkumar, S. A review on green synthesis of zinc oxide nanoparticles – An eco-friendly approach. Resour. Technol. 3, 406–413 (2017).
Anbuvannan, M., Ramesh, M., Viruthagiri, G., Shanmugam, N. & Kannadasan, N. Anisochilus carnosus leaf extract mediated synthesis of zinc oxide nanoparticles for antibacterial and photocatalytic activities. Mater. Sci. Semicond. Process. 39, 621–628 (2015).
Erci, F., Cakir-Koc, R. & Isildak, I. Green synthesis of silver nanoparticles using Thymbra spicata L. var. spicata (zahter) aqueous leaf extract and evaluation of their morphology-dependent antibacterial and cytotoxic activity. Artif. Cells, Nanomed. Biotechnol. 46, 150–158 (2018).
Alamdari, S. et al. Preparation and characterization of zinc oxide nanoparticles using leaf extract of sambucus ebulus. Appl. Sci. 10, (2020).
Mohammadi-Aloucheh, R., Habibi-Yangjeh, A., Bayrami, A., Latifi-Navid, S. & Asadi, A. Green synthesis of ZnO and ZnO/CuO nanocomposites in Mentha longifolia leaf extract: Characterization and their application as anti-bacterial agents. J. Mater. Sci. Mater. Electron. 29, 13596–13605 (2018).
Gupta, M., Tomar, R. S., Kaushik, S., Mishra, R. K. & Sharma, D. Effective antimicrobial activity of green ZnO nano particles of Catharanthus roseus. Front. Microbiol. 9, (2018).
Rad, S. S., Sani, A. M. & Mohseni, S. Biosynthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from leaf extract of Mentha pulegium (L.) Microb. Pathog. 131, 239–245 (2019).
Sangeetha, S., Archit, R. & SathiaVelu, A. Phytochemical testing, antioxidant activity, HPTLC and FTIR analysis of antidiabetic plants Nigella sativa, Eugenia jambolana, Andrographis paniculata and Gymnema sylvestre. Res. J. Biotechnol. 9, 65–72 (2014).
Jayanthi, P. & Lalitha, P. DPPH Scavenging Assay of The Solvent Extracts and Fractionates of Eichhornia crassipes (Mart.) Solms. J. Pharm. Res. 5, 946–948 (2012).
Anticoccidial Activity of Black Cumin (Nigella Sativa) in Rabbits. Assiut Vet. Med. J. 59, 85–96 (2013).
Vimala, K., Sundarraj, S., Paulpandi, M., Vengatesan, S. & Kannan, S. Green synthesized doxorubicin loaded zinc oxide nanoparticles regulates the Bax and Bcl-2 expression in breast and colon carcinoma. Process Biochem. 49, 160–172 (2014).
Bozkurt, M. et al. Efficacy of in-feed preparations of an anticoccidial, multienzyme, prebiotic, probiotic, and herbal essential oil mixture in healthy and Eimeria spp.-infected broilers. Poult. Sci. 93, 389–399 (2014).
Noack, S., Chapman, H. D. & Selzer, P. M. Anticoccidial drugs of the livestock industry. Parasitol. Res. 118, 2009–2026 (2019).
ElKatcha, M., Soltan, M. & Elbadry, M. Effect of dietary replacement of inorganic zinc by organic or nanoparticles sources on growth performance, immune response and intestinal histopathology of broiler chicken. Alexandria J. Vet. Sci. 55, 129 (2017).
Hafez, A., Hegazi, S. M., Bakr, A. A. & Shishtawy, H. E. Effect of zinc oxide nanoparticles on growth performance and absorptive capacity of the intestinal villi in broiler chickens. Life Sci. J. 14, 67–72 (2017).
Al-Gawad, A. A., Mahdy, O. A., El-Massry, A. A. N. & Al-Aziz, M. S. A. Studies on coccidia of Egyptian balady breed chickens. Life Sci. J. 9, 568–576 (2012).
Györke, A., Pop, L. & Cozma, V. Prevalence and distribution of Eimeria species in broiler chicken farms of different capacities. Parasite 20, (2013).
Aziz Anah, S., Abees Hmood, K. & Aziz Anah, S. Effect of ZnO nanoparticles in treatment of broilers with Eimeria tenella . experimentally infected . 1–11 (2021).
Ghosheh, O. A., Houdi, A. A. & Crooks, P. A. High performance liquid chromatographic analysis of the pharmacologically active quinones and related compounds in the oil of the black seed (Nigella sativa L.). J. Pharm. Biomed. Anal. 19, 757–762 (1999).
El-Maddawy, Z. K. et al. Use of zinc oxide nanoparticles as anticoccidial agents in broiler chickens along with its impact on growth performance, antioxidant status, and hematobiochemical profile. Life 12, (2022).
Mondal, D. K., Chattopadhyay, S., Batabyal, S., Bera, A. K. & Bhattacharya, D. Plasma biochemical indices at various stages of infection with a field isolate of Eimeria tenella in broiler chicken. Vet. World 4, 404–409 (2011).
Wood, E. Biochemistry. A case-oriented approach. Sixth edition By R Montgomery, T W Conway, A A Spector and D Chappell. pp 683. Mosby, St Louis MO. 1996. £24.95 ISBN 0–8151–6483–1. Biochem. Educ. 25, 95 (1997).
Costantini, D. & Møller, A. P. Does immune response cause oxidative stress in birds? A meta-analysis. Comp. Biochem. Physiol. - A Mol. Integr. Physiol. 153, 339–344 (2009).
Kadhim, L. I. Anticoccidial effect of a Negilla sativa seed-based diet on eimeria tenella infection in chickens. Int. J. Poult. Sci. 16, 323–327 (2017).
Zhao, C. Y. et al. Effects of dietary zinc oxide nanoparticles on growth performance and antioxidative status in broilers. Biol. Trace Elem. Res. 160, 361–367 (2014).
Fathi, M., Haydari, M. & Tanha, T. Effects of zinc oxide nanoparticles on antioxidant status, serum enzymes activities, biochemical parameters and performance in broiler chickens. J. Livest. Sci. Technol. 4, 7–13 (2016).
Yuan, J., Xu, Z., Huang, C., Zhou, S. & Guo, Y. Effect of dietary Mintrex-Zn/Mn on performance, gene expression of Zn transfer proteins, activities of Zn/Mn related enzymes and fecal mineral excretion in broiler chickens. Anim. Feed Sci. Technol. 168, 72–79 (2011).
Prasad, A. S. Zinc: An antioxidant and anti-inflammatory agent: Role of zinc in degenerative disorders of aging. J. Trace Elem. Med. Biol. 28, 364–371 (2014).
Parashar, B., Gupta, R. & Khurana, G. A review on process validation as an essential process. Int. J. Res. Pharm. Sci. 4, 226–229 (2013).
Jarosz, M., Olbert, M., Wyszogrodzka, G., Młyniec, K. & Librowski, T. Antioxidant and anti-inflammatory effects of zinc Zinc-dependent NF-κB signaling. Inflammopharmacology 25, 11–24 (2017).
Lee, S. H. et al. Effects of dietary supplementation with phytonutrients on vaccine-stimulated immunity against infection with Eimeria tenella. Vet. Parasitol. 181, 97–105 (2011).
Zhang, J. et al. Efficacy of using zinc oxide nanoparticle as a substitute to antibiotic growth promoter and zinc sulphate for growth performance, antioxidant capacity, immunity and intestinal barrier function in broilers. Ital. J. Anim. Sci. 21, 562–576 (2022).
Reziwan, K., Sun, D., Zhang, B. & Zhao, Z. MicroRNA-1225 activates Keap1-Nrf2-HO-1 signalling to inhibit TNFα-induced osteoclastogenesis by mediating ROS generation. Cell Biochem. Funct. 37, 256–265 (2019).
Barakat, L. A. A., Barakat, N., Zakaria, M. M. & Khirallah, S. M. Protective role of zinc oxide nanoparticles in kidney injury induced by cisplatin in rats. Life Sci. 262, (2020).
Kim, J., Cha, Y. N. & Surh, Y. J. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat. Res. Fundam. Mol. Mech. Mutagen. 690, 12–23 (2010).
Wang, C. et al. Effects of dietary zinc oxide nanoparticles on growth, diarrhea, mineral deposition, intestinal morphology, and barrier of weaned piglets. Biol. Trace Elem. Res. 185, 364–374 (2018).
Rao, J. et al. ATF3-mediated NRF2/HO-1 signaling regulates TLR4 innate immune responses in mouse liver ischemia/reperfusion injury. Am. J. Transplant. 15, 76–87 (2015).
Liu, W. H., Shi, L. S., Chung, M. C., Chang, T. C. & Lee, S. Y. Antcamphin M inhibits TLR4-mediated inflammatory responses by upregulating the Nrf2/HO-1 pathway and suppressing the NLRP3 inflammasome pathway in macrophages. Am. J. Chin. Med. 47, 1611–1626 (2019).
Acknowledgements
Colleagues and lab staff of Department of Pharmacology and Toxicology and Department of Parasitology, University of Veterinary and Animal Sciences, Lahore, Pakistan helped us in the completion of this research and Researchers supporting project number (RSP2023R494), King Saud University, Riyadh, Saudi Arabia.
Funding
Researchers supporting project number (RSP2023R494), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
N.L., A.S., and M.M.A. designed the experiments. N.L., S.M., M.A.H. did the research experiments. A.S., M.O.O., M.A.S. conceptualizes the idea. N.L., A.R.K., W.A., A.A. wrote the initial draft. All authors analyzed the data and reviewed this article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lail, Nu., Sattar, A., Omer, M.O. et al. Biosynthesis and characterization of zinc oxide nanoparticles using Nigella sativa against coccidiosis in commercial poultry. Sci Rep 13, 6568 (2023). https://doi.org/10.1038/s41598-023-33416-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-33416-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.