Abstract
We aimed to analyze the kinetics of T-cell-mediated and B-cell-mediated humoral immune responses against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) before and after booster vaccination, as well as the impacts of the in vitro test results the type of vaccination on the prediction of SARS-CoV-2 infection. A total of 240 healthcare workers vaccinated twice were serially tested using an interferon gamma release assay (IGRA) and a neutralizing antibody (nAb). At the end of the study, we investigated the history of SARS-CoV-2 infection of all the enrolled participants to analyze the effects of the test results and the type of vaccination on SARS-CoV-2 infection. Overall, the positive rates were 52.3% and 80.0% for IGRA and 84.6% and 100% for the nAb test before and after booster vaccination, respectively. However, the positive rates were 52.8% for IGRA and 100% for nAb 3 months after booster vaccination. The in vitro test results and the type of vaccination were not associated with SARS-CoV-2 infection. The antibody response caused by the SARS-CoV-2 vaccination lasted more than 6 months, although the response of the T-cells disappeared rapidly after 3 months. However, these in vitro results and the type of vaccination cannot be used for predicting the risk of SARS-CoV-2 infection.
Similar content being viewed by others
Introduction
As of 19 February 2023, COVID-19 led to 6.9 million deaths globally due to the rapid spread of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)1. With the unprecedented, rapid spread of this novel respiratory disease, there was a global race to discover and deploy a vaccine against COVID-19 to achieve herd immunity2. Due to these efforts, several vaccines had been validated by WHO, which showed various efficacies such as preventing the spread of the virus and decreasing the risk of serious health consequences3.
As of 12 December 2022, approximately 13 billion vaccine doses have been administered worldwide4. With this large scale of vaccination, the public interest in immunity against COVID-19 has been growing. Currently, in vitro methods to evaluate the immune status against pathogens consist of two pivotal axes: the neutralizing antibody (nAb) test for the evaluation of B-cell-mediated humoral response and the interferon-γ (INF-γ) release assay (IGRA) against the SARS-CoV-2 antigen for detecting the T-cell mediated response. The clinical performance of these assays has been globally evaluated, and the IGRA format assay is useful for predicting the severity of infection and clinical prognosis for COVID-19 patients and evaluating T-cell immune response following COVID-19 vaccination5,6,7,8. Similarly, the nAb test showed acceptable performance for the evaluation of B-cell immune status against COVID-19 in clinical settings9,10. However, how the results of these tests vary according to the type of vaccination is still unclear. In addition, there is very little information available regarding the association between the test results and SARS-CoV-2 infection over a long-term follow-up period.
In this study, we enrolled healthcare workers (HCWs), who received two doses of a COVID-19 vaccine and planned to receive booster vaccination, and performed the QuantiFERON-TB SARS-CoV-2 ELISA Kit (QFN-SARS; QIAGEN, MD, USA) and the ichroma™ COVID-19 nAb assay (ichroma nAb; Boditech Med, Gangwon-do, Republic of Korea) for all participants. With these test results, we analyzed the kinetics of the IGRA and nAb test results with respect to the type of the first and second vaccinations and the number of elapsed days after the last vaccination. Additionally, at the end of the study period, we checked the COVID-19 infection history of all the participants and analyzed the impacts of the in vitro test results and the type of vaccination on the prediction of SARS-CoV-2 infection.
Results
A total of 293 specimens from 240 HCWs were tested using QFN-SARS and ichroma nAb assays. These tests were conducted in November 2021 and February 2022. Of all enrolled HCWs, 65 HCWs did not receive the booster vaccination during the study period (booster (−) group), and 175 participants were vaccinated with a booster shot (booster (+) group).
The basic characteristics of the participants are summarized in Table 1. For the HCWs of the booster (−) and booster (+) groups, the median ages (years) were 36 and 43, and the proportions of female HCWs were 66.2% and 70.9%, respectively. The first and second doses were of the following types of vaccines: AstraZeneca COVID-19 Vaccine (AZ), Pfizer-BioNTech COVID-19 vaccine (PF), or Moderna COVID-19 Vaccine (MO). PF was the only vaccine used as the booster shot. There were four combinations of the first and second vaccines for the booster (−) and booster (+) groups: first and second doses of AZ (1AZ–2AZ), first dose of AZ and second dose of PF (1AZ–2PF), first and second doses of PF (1PF–2PF), and first and second doses of MO (1MO–2MO). At the end of the study, we retrospectively checked all participants for COVID-19 infection; 33.8% (booster (−) group) and 41.1% (booster (+) group) of the HCWs had been infected with SARS-CoV-2.
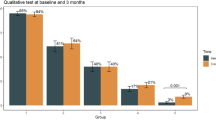
The first blood sampling was performed at a median of 141 days for the booster (−) group and 20 days for the booster (+) group after the last vaccine dose. The positive rates in the first QFN-SARS and ichroma nAb tests were 52.3% and 84.6% for the booster (−) group and 80.0% and 100% for the booster (+) group, respectively (Fig. 1). After 3 months, the second test was performed in some participants of the booster (+) group (n = 53), and the positive rates of QFN-SARS and ichroma nAb were 52.8% and 100%, respectively. In QFN-SARS results, the positive rate was statistically high in the 1st test of booster (+) group (p < 0.001) compared to the booster (−) group and the 2nd test of booster (+) group, and in ichroma nAb results, the positive rate was significantly low in the booster (−) group (p < 0.001) compared to the 1st and 2nd tests of booster (=) group. When considering only the QFN-SARS result of 53 HCWs who had tested both 1st and 2nd tests, the positive rates were 90.6% (48/53) and 52.8% (28/53) for 1st test and 2nd test, respectively (p < 0.001). Additionally, with the first and second test results from all participants, we performed Kaplan–Meier analyses to analyze the kinetics of QFN-SARS and ichroma nAb results depending on the number of days after the last vaccine dose. The proportion of QFN-SARS-positive participants showed a tendency to drop gradually, and approximately half of the booster (+) and booster (−) HCWs were QNF-SARS-negative after 3 and 6 months following the last vaccination, respectively. For ichroma nAb, the nAb-positive participants also showed a declining trend as time elapsed after the last vaccine dose; however, approximately 80% of the booster (−) participants had nAb against SARS-CoV-2 until 6 months following the last dose. The positive rate of nAb was 100% in booster (+) group at the end of the study period. In addition, the time from the initial test to COVID-19 diagnosis was estimated utilizing Kaplan–Meier analysis with the endpoint of time-to-COVID-19-infection (Fig. 2). When comparing the QFN-SARS-positive and -negative groups, the proportion of COVID-19-free HCWs during the study period was significantly higher among the QFN-SARS-negative participants. Similarly, ichroma nAb-negative participants were less likely (but not significantly) to be infected with SARS-CoV-2.
Positive rates of QFN-SARS (a) and ichroma nAb (c) before and after booster shot. Asterisks indicate significant differences (**p < 0.01, ***p < 0.001), and error bars indicate 95% confidence intervals of the positive rates. Kaplan–Meier curves displaying the proportion of QFN-SARS-positive (b) and ichroma nAb-positive (d) HCWs with respect to the days elapsed after the final vaccine dose.
Table 2 shows the results of QFN-SARS and ichroma nAb together with COVID-19 infection history based on the type of SARS-CoV-2 vaccination. In the booster (−) group, the positive rates of QFN-SARS in the 1MO–2MO group and of ichroma nAb in the 1AZ–2PF, and 1MO–2MO groups tend to have a higher positive rates. In the booster (+) group, there was no significant difference in the positive rates of QFN-SARS for the 1st and 2nd tests with respect to the type of vaccination, and all of them were positive for ichroma nAb. In addition, we could not determine any difference in the COVID-19 infection rates according to the vaccine type in the booster (−) and booster (+) groups.
Similarly, we performed Kaplan–Meier analyses to evaluate the protection against SARS-CoV-2 after COVID-19 vaccination (Fig. 3). Comparing the booster (−) and booster (+) groups revealed no difference in the proportion of COVID-19-free HCWs. In addition, there was no association between the type of first and second vaccinations and COVID-19 infection in both the booster (−) and booster (+) groups.
Discussion
Since the rapid spread of COVID-19 globally, there has been growing public interest in the laboratory tests used for the evaluation of the immune status of individuals with respect to SARS-CoV-2 infection11,12. With these global demands, various kits of IGRA-format tests and nAb assays have been introduced in the market for the evaluation of humoral and T-cell-mediated responses5,13,14. Although many studies were conducted to confirm the usefulness of these test results5,6,7,9,15,16, there is still insufficient evidence that these test results are clinically useful13. Therefore, we aimed to determine the changes in these test results before and after COVID-19 booster vaccination, and with respect to the type of COVID-19 vaccination, to confirm whether these test results can be used to predict the risk of SARS-CoV-2 infection over a long-term follow-up period.
Overall, we enrolled a total of 240 HCW participants during the study period, and 65 participants were tested with 1st QFN-SARS and nAb tests before the booster vaccination (the booster (−) group). Although 84.6% of these booster (−) HCWs were positive for the nAb assay (55/65), only about half of them were positive in the QFN-SARS test (52.3%, 34/65). Within 1 month after the vaccination with the booster shot, the positivities of the QFN-IGRA and nAb tests (1st tests of booster (+) group) rose sharply compared to the booster (−) group. Approximately 3 months after the booster shot (2nd tests of the booster (+) group), the positivity of QFN-SARS decreased to 52.8% (28/53), whereas that of nAb remained high (100%, 53/53). Similar trends were observed in the Kaplan–Meier analysis with a linear decreasing trend of the positive rate of QFN-SARS with respect to the number of days after the last vaccination, while that of the nAb assay was over 80% until 6 months after the last vaccination. These results indicate that the antibody response caused by the SARS-CoV-2 vaccination lasts relatively long, although the response of the T-cells disappears quickly.
Recently, Murugesan et al. investigated the long-term accuracy of the SARS-CoV-2 IGRA assay and anti-SARS-CoV-2 IgG antibody assays12. At 0.5 month post infection, 100% and 87.5% of the participants tested positive in the IGRA-SARS and IgG assays; however, the positive rates of both assays decreased to 79.5% and 71.8% at 10 months after COVID-19 infection, respectively. For both assays, the decline in positive rates seemed to be slower than the results of our study; these findings would indicate that the duration of actual infection-induced immunity is longer than that of vaccine-mediated immunity.
With the 1st test results of QFN-SARS and ichroma nAb, we attempted to analyze the relationship between these in vitro test results and SARS-CoV-2 infection. There was no significant difference in SARS-CoV-2 infection between the positive and negative groups of the ichroma nAb assay. However, paradoxical results were observed—the proportion of COVID-19-confirmed cases among the QFN-SARS-positive participants was higher than that among the QFN-SARS-negative participants. During the study period, an epidemic caused by the Omicron variant broke out in the Republic of Korea17,18. Although we could not consider various factors related to SARS-CoV-2 infection, the results of our study showed that the in vitro IGRA and nAb tests against SARS-CoV-2 could not be used as indicators to predict the risk of COVID-19 infection, especially the Omicron variant.
Considering the test results according to the type of vaccination, only the booster (−) group showed significant differences in the positive rates of QFN-SARS and ichroma nAb. We assumed that the distribution of the number of days after the last vaccination was relatively wide in the booster (−) group, which could have affected the positive rates of QFN-SARS and ichroma nAb. In the booster (+) group, the positive rates of QFN-SARS and ichroma nAb did not differ according to the type of vaccination. These results would indicate that the T-cell-mediated immunity and humoral immunity as measured by these in vitro tests would be affected not by the type of vaccination, but by the elapsed time since the last vaccination. The proportion of COVID-19-confirmed participants was not influenced by the type of vaccination in both the booster (−) and booster (+) groups, which indicates that the type of vaccination is not associated with protection against the Omicron variant infection. Therefore, we would recommend that other factors such as close contacts of infected patients, high viral load exposure, comorbidity, or host immune status should be considered when assessing the risk of COVID-19 infection19,20,21, rather than the type of vaccination and the in vitro test results.
Despite the significant results of this study, there are several limitations to our study. First, although there were no deaths due to SARS-CoV-2 infection, we retrospectively collected only the history of COVID-19 infection through a questionnaire, not the symptoms. Therefore, the correlation between the test results and the symptomatic characteristics of COVID-19 could not be analyzed. In addition, there may be hidden infections that have not been diagnosed due to asymptomatic infection. Second, because the study participants were limited to HCWs, we enrolled only relatively young and healthy persons under the age of 60; thus, the results of this study would not be representative of the entire population. In addition, about two-thirds of the participants did not voluntarily dropped out in the second test, possibly resulting in selection bias. Therefore, further large-scale studies should be conducted to overcome the present limitations and provide more informative results regarding the IGRA-SARS and nAb assays.
Our study provides comprehensive QFN-SARS and ichroma nAb test results that reflect T-cell mediated and humoral immunity against SARS-CoV-2 with respect to the number of days after the last vaccination and the type of vaccination. The positive rates of QFN-SARS and ichroma nAb rapidly increased after booster vaccination, and the positivity of the nAb assay remained high until 6 months after the last vaccination. However, the positivity of QFN-SARS showed a rapid decline, and about half of the participants were negative about 3 months after the last vaccination. Our findings also suggest that these in vitro test results and the type of vaccination may not be useful to predict the risk of COVID-19 infection. The results of our study indicate that although the in vitro tests for measuring immunity against SARS-CoV-2 may reflect SARS-CoV-2 vaccination well, their clinical usefulness is still unclear and further large-scale studies should be performed to address this point.
Materials and methods
Study participants
A total of 240 HCWs working in Chung-Ang University Hospital were enrolled in this study. We only enrolled HCWs without any history of COVID-19 infection. All participants were vaccinated twice at the time of enrollment in this study, and only those who wanted booster shots were vaccinated with third vaccination. Based on the booster vaccination, the participants were divided into two groups: booster (−) group and booster (+) group. The first specimens for all participants were sampled at the beginning of this study, and the second blood sampling was done at the end of the study on some subjects of the booster (+) group. At the time of collecting the 1st and 2nd blood specimens, HCWs with a history of COVID-19 infection were excluded. With these specimens, we used the QFN-SARS and the ichroma nAb. At the end of the study in April 2022, we investigated the history of SARS-CoV-2 infection of all the enrolled participants through a questionnaire. The timeline and groups of this study are shown in Supplementary Fig. S1.
This study was conducted from November 2021 to April 2022 at the Chung-Ang University Hospital (Seoul, Republic of Korea), and blood samples were drawn after informed consent was obtained from all the participants.
QuantiFERON-TB SARS-CoV-2 ELISA
The QFN-SARS is a qualitative assay for the detection of IFN-γ released in response to in vitro stimulation by a specific SARS-CoV-2 antigen in human whole blood. For QFN-SARS, 4 ml of whole blood was collected into four QuantiFERON blood collection tubes, which included a Nil tube (negative control), a mitogen tube (positive control), and two S peptide-containing tubes with SARS CoV-2 (Ag1 and Ag2) to stimulate the immune cells. These tubes were gently shaken to ensure adequate mixing of the additive and blood and transferred to a 37 °C incubator. After 16–24 h of incubation, the tubes were centrifuged at 2500×g for 15 min and the plasma separated. Then, levels of IFN-γ were measured for the plasma samples by ELISA. The QFN-SARS results were interpreted according to the manufacturer’s specifications (Supplementary Table S1).
ichroma™ COVID-19 nAb assay
ichroma nAb is a qualitative fluorescence immunoassay for the detection of neutralizing antibodies against SARS-CoV-2. The target antibody of this test is the neutralizing antibody that block the interaction between the receptor binding domain (RBD) of the viral spike glycoprotein with the ACE-2 cell surface receptor. To evaluate the neutralizing antibody status, whole blood samples were drawn, and the serum was separated within 3 h after the collection of whole blood. During the test procedure, the SARS-CoV-2-neutralizing antibody in the sample binds to the fluorescence-labeled SARS-CoV-2 Spike RBD antigen to form the antigen–antibody complex. Then, this complex is loaded onto lateral flow immunoassay kit, where the covalent couple of ACE2 is immobilized, and interferes with the binding of analyte and Fluorescence-labeled (FL) antigen. With the increasing presence of the analytes in the blood specimens, more of the fluorescent antigen is bound, resulting in a lower fluorescence signal. The fluorescence signal was quantified using ichroma™ II (Boditech Med) which is a compact, fluorescence-based immunoassay analyzer, and the test result was reported as ‘Positive’ or ‘Negative’ based on the cut-off index.
Ethical statement
This study was performed in accordance with the relevant guidelines and regulations, and approved by the institutional review board of Chung-Ang University Hospital (IRB No. 2108-007-473). All participants provided written informed consent.
Statistics
All statistical analyses were performed using R version 4.3.3 (http://www.R-project.org/). Median values were calculated with interquartile ranges for quantitative variables. Qualitative variables were expressed as percentages (%), and a two-sided chi square test with post-hoc analysis was used to compare qualitative variables. We used the Kaplan–Meier method to analyze the time-to-event data (the change of test results and SARS-CoV-2 infection). p value < 0.05 was considered statistically significant.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
WHO. COVID-19 Weekly Epidemiological Update, Edition 131, 22 February 2023 (2023).
Francis, A. I., Ghany, S., Gilkes, T. & Umakanthan, S. Review of COVID-19 vaccine subtypes, efficacy and geographical distributions. Postgrad. Med. J. 98, 389–394. https://doi.org/10.1136/postgradmedj-2021-140654 (2022).
Sharif, N., Alzahrani, K. J., Ahmed, S. N. & Dey, S. K. Efficacy, immunogenicity and safety of COVID-19 vaccines: A systematic review and meta-analysis. Front. Immunol. 12, 714170. https://doi.org/10.3389/fimmu.2021.714170 (2021).
WHO. WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/
Murugesan, K. et al. Interferon-gamma release assay for accurate detection of severe acute respiratory syndrome coronavirus 2 T-cell response. Clin. Infect. Dis. 73, e3130–e3132. https://doi.org/10.1093/cid/ciaa1537 (2021).
Echeverria, G. et al. Pre-existing T-cell immunity to SARS-CoV-2 in unexposed healthy controls in Ecuador, as detected with a COVID-19 Interferon-Gamma Release Assay. Int. J. Infect. Dis. 105, 21–25. https://doi.org/10.1016/j.ijid.2021.02.034 (2021).
Stieber, F. et al. Accuracy of interferon gamma release assays for the COVID-19 immunity assessment. J. Virol. Methods 302, 114472. https://doi.org/10.1016/j.jviromet.2022.114472 (2022).
Aiello, A. et al. Accuracy of QuantiFERON SARS-CoV-2 RUO assay and characterization of the CD4(+) and CD8(+) T-cell-SARS-CoV-2 response: Comparison with a homemade IFN-gamma release assay. Int. J. Infect. Dis. https://doi.org/10.1016/j.ijid.2022.07.049 (2022).
Khoury, D. S. et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 27, 1205–1211. https://doi.org/10.1038/s41591-021-01377-8 (2021).
Cheng, Z. J. et al. Clinical application of antibody immunity against SARS-CoV-2: Comprehensive review on immunoassay and immunotherapy. Clin. Rev. Allergy Immunol. https://doi.org/10.1007/s12016-021-08912-y (2022).
Ameratunga, R. et al. Perspective: Diagnostic laboratories should urgently develop T cell assays for SARS-CoV-2 infection. Expert Rev. Clin. Immunol. 17, 421–430 (2021).
Murugesan, K. et al. Long-term accuracy of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) interferon-γ release assay and its application in household investigation. Clin. Infect. Dis. 75, e314–e321 (2022).
Fernández-González, M. et al. Clinical performance of a standardized severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) interferon-γ release assay for simple detection of T-cell responses after infection or vaccination. Clin. Infect. Dis. 75, e338–e346 (2022).
Takei, S. et al. Performance evaluation of the Roche Elecsys® Anti-SARS-CoV-2 immunoassays by comparison with neutralizing antibodies and clinical assessment. PLoS One 17, e0274181 (2022).
Gibson, L. An interferon-gamma release assay for evaluation of cell-mediated immunity in infants with congenital cytomegalovirus infection. Clin. Infect. Dis. 73, 374–375. https://doi.org/10.1093/cid/ciaa700 (2021).
Tormo, N. et al. Commercial Interferon-gamma release assay to assess the immune response to first and second doses of mRNA vaccine in previously COVID-19 infected versus uninfected individuals. Diagn. Microbiol. Infect. Dis. 102, 115573. https://doi.org/10.1016/j.diagmicrobio.2021.115573 (2022).
KCDA. Status and characteristics of the SARS-CoV-2 variant outbreak in the Republic of Korea in January 2022. Public Health Wkly. Rep. 15, 505–510 (2022).
Lee, D. W. et al. Genomic epidemiology of SARS-CoV-2 Omicron variants in the Republic of Korea. Sci. Rep. 12, 22414. https://doi.org/10.1038/s41598-022-26803-w (2022).
Ge, Y. et al. COVID-19 transmission dynamics among close contacts of index patients with COVID-19: A population-based cohort study in Zhejiang province, China. JAMA Intern. Med. 181, 1343–1350 (2021).
Madewell, Z. J., Yang, Y., Longini, I. M., Halloran, M. E. & Dean, N. E. Household secondary attack rates of SARS-CoV-2 by variant and vaccination status: An updated systematic review and meta-analysis. JAMA Netw. Open 5, e229317 (2022).
Meister, T. et al. Clinical characteristics and risk factors for COVID-19 infection and disease severity: A nationwide observational study in Estonia. PLoS One 17, e0270192 (2022).
Acknowledgements
We thank the Diagnostic Immunology department and clinical microbiology department of the Chung-Ang University Hospital for their contribution to this study for a long time.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2020R1A5A1018052). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
M.K.L. and Y.K.L. conceived the presented idea and supervised the findings of this work. T.H.K., Y.C. and O.J.K. performed the calculations and designed the figures. Y.K.L. and S.Y. wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lim, Y.K., Kweon, O.J., Choi, Y. et al. Exploring the vaccine-induced immunity against severe acute respiratory syndrome coronavirus 2 in healthcare workers. Sci Rep 13, 6830 (2023). https://doi.org/10.1038/s41598-023-33397-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-33397-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.