Abstract
Polycyclic aromatic hydrocarbons (PAHs) are often formed when organic substances do not burn completely. This study evaluates the non-carcinogenic and cumulative risks associated with PAHs levels by testing blood and urine samples in kitchen workers and residents near restaurants in Shiraz, Iran. Metabolites of PAH in the urine samples as well as clinical parameters in the blood samples were measured. The non-carcinogenic and cumulative risk assessments from exposure of the study groups to PAH metabolites were also evaluated. The highest average concentrations of PAH metabolites were related to kitchen workers (2126.7 ng/g creatinine (ng/g cr)). The metabolites of 1-Hydroxypyrene (1-OHP) and 9-Phenanthrene (9-OHPhe) had the highest and lowest mean concentrations, respectively. A direct correlation was observed between the levels of PAH metabolites with malondialdehyde (MDA) and total antioxidation capacity (TAC) levels (p < 0.05). Hazard Index (HIi) was obtained less than one (HIi < 1), indicating low-risk negative health impacts on the target groups. Nevertheless, conducting more studies to determine the health status of these people is quite evident.
Similar content being viewed by others
Introduction
The incomplete combustion of organic waste results in a unique type of environmental pollutant known as PAHs1,2. These contaminants stay in the ecosystem and build up in the food supply3. People may be exposed to these pollutants from different sources and routes1. The most common routes to get exposure to PAHs are inhalation, smoking, food, soil, air, and skin contact4.
Endocrine system disruption is caused by PAHs, which are toxic, cancer-causing, and mutagenic substances. The International Agency for Research on Cancer (IARC) has classified benzo[a]pyrene (BaP) as a carcinogen to humans (group 1)4. The US Environmental Protection Agency (USEPA) has likewise classified them as priority pollutants. PAHs can generate reactive oxygen species (ROS). Furthermore, a strong positive association between exposure to PAHs and indices of oxidative stress has been reported in several studies5,6. Free radicals at the cell membrane cause oxidative stress, which damages the cell membrane and results in cell malfunction. In actuality, PAH metabolites damage membranes and raise levels of aldehydes such as MDA7.
When food is fried, stir-fried, or grilled in cooking oil at high temperatures, cooking oil fumes (COFs) like polycyclic aromatic hydrocarbons (PAHs) are produced and released into the environment8. The inhaling, eating, and even skin absorption of unprotected restaurant staff exposes them to dust and gaseous emissions containing PAHs. Restaurant employees and those who live in or close to that region are at risk of health problems due to such PAHs emissions. Since several PAH metabolites are known to cause cancer, individuals' health may suffer due to these contaminants9,10. The presence of PAH metabolites as biomarkers, such as 1-hydroxynaphthalene (1-OHNap), 2-hydroxynaphthalene (2-OHNap), 2-hydroxyfluorine (2-OHFlu), and 9-OHPhe in the urine is one sign that restaurant workers have been exposed to COF1,5,11,12,13.
Too far, many studies have been carried out to assess PAHs concentrations in various places, including bus terminals, workplaces, and dining establishments14,15,16,17,18. According to certain studies, those who work in restaurants are more likely to develop certain malignancies10,17. For example, research on the concentration of PAH metabolites in the urine of Indian kitchen employees revealed that it was more significant in the workers than in the control group17. In another study, researchers found PAHs and benzo[a]pyrene concentrations at Chinese restaurants were 20.99 ± 13.67 and 1.82 ± 2.24 μg/m3, respectively. Furthermore, they stated that based on an incremental lifetime cancer risk (ILCR) of less than 10–6, the maximum allowable exhaust stack exposure duration for Chinese citizens was 5–19 h/month−119. Most of the information currently accessible on the environmental harm caused by PAHs emissions derives from China and India. No data on PAHs exposure related to kitchen employees have been published from Shiraz, Iran, which has many restaurants. In this study, we investigate PAH metabolites in kitchen employees and persons residing near restaurants in Shiraz, Iran, as well as controls (who were not exposed to restaurant emissions). In this regard, metabolites of PAH (1-OHNap, 2-OHNap, 2-OHFlu, 1-OHP, 9-OHPhe, MDA, and TAC) were measured in urine samples. Also, clinical parameters such as White Blood Cells (WBC), Red Blood Cells (RBC), C-Reactive Protein (CRP), etc., were measured in the blood samples of the studied groups. Finally, non-carcinogenic and cumulative risk assessment studies were conducted.
Materials and methods
Location, statistical population, and study inclusion criteria
This cross-sectional research was done on kitchen employees and those living near restaurants in Shiraz, Iran. The sample size was determined using data from prior research to discover the biggest difference between the two exposure groups (type I and type II) and the control group. The statistical population of the study included 144 people. These included 57 people working in restaurant kitchens, 57 people living near restaurants, and 30 people as a control group (people who do not work or live near the restaurant). We also matched people from various groups based on frequency and age. Inclusion criteria for the study included living in the city 3 days before the test, not using acetaminophen, adult cold medicine, and nutritional supplements for at least 3 days before the sampling date, and so on.
The steps of collecting questionnaire information and taking blood and urine samples from people
All individuals signed written informed consent forms before sampling. A questionnaire with 55 questions was given to individuals to gather information on their sociodemographic traits, activity level, dietary intake of PAHs, and smoking experience. The questionnaire included age, lifestyle, residence features, dietary patterns, environmental tobacco smoke (ETS), and time spent outside. Information from the individuals was requested after 72 h. Face-to-face interviews were conducted with each participant.
We also performed anthropometric measurements after the interview, including weight, height, and blood pressure. The National Health and Nutrition Examination Survey (NHANES) methodology was used for measuring height and weight20. After a ten-minute break, blood pressure was checked on the right hand using a blood pressure monitor (OMRON, M2 Japan).
The samples (blood and urine) were collected in the morning. Samples were immediately transported to a laboratory at 0–4 °C, where they were maintained at − 20 °C until analysis. An automated biochemistry analyzer (Poch-100i, Sysmex, Kobe, Japan) also evaluated high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), triglycerides (TG), and fasting blood sugar (FBS). The liver function enzymes (sGpt and sGot) were also measured along with TAC, MDA, CRP, and creatinine. Additionally, this study was approved by the Ethics Committee of Shiraz University of Medical Sciences (IR.SUMS.REC.1399.703), and all methods were performed in accordance with the relevant guidelines and regulations21.
Urine sample preparation and analysis
We combined 6 mL of the urine sample with 12 mL of sodium acetate (C2H3NaO2) buffer (0.1 M, pH 5) to determine the PAH metabolites (1-OHNap, 2-OHNap, 2-OHFlu, 1-OHP, and 9-OHPhe). 80 μL of β-glucuronidase/arylsulfatase was added to the mixture and hydrolyzed for five hours in a water bath at 37 °C. To separate the supernatant, the mixture was then shaken for 15 min at 3000 rpm. A Gas Chromatography (GC) vial was filled with the supernatant. In order to quantify the PAH metabolites, 3 mL of this supernatant was injected into the GC–MS apparatus (Agilent, HP5-MS capillary column, 7890, USA)22,23. To adjust the concentrations of PAH metabolites, the creatinine (ng/g cr) was determined according to Jaffe’s spectrophotometric technique at the ultraviolet (500 nm) absorption spectrum4.
Determination of the estimated daily intake (EDIi), hazard quotient (HQi), and hazard index (HIi)
Equation (1) was applied to the calculation of EDIi24.
In Eq. (1), EDIi is equal to the estimated daily intake of PAHs (nanogram per kilogram body weight per day), CU is the molar concentration of PAH metabolites in urine (nmol/L for EDIi), and V24h is the total volume of urine excreted during 24 h for adults (1.6 L per day).The molecular weight of the parent PAHs constituents (Nap, Flu, Phe, and Pyr of 128.17, 166.22, 178.23, and 202.26 g/mol, respectively) are expressed by MW. FUE refers to the amount of urine PAH metabolites excreted as a fraction of oral intake (Nap, Flu, Phe, and Pyr of 1, 0.78, 0.09, and 0.08, respectively), and BW is body weight25.
The HQi was used to evaluate the non-carcinogenic risk (Eq. 2)4,24:
In Eq. (2), HQi and RFDi are the hazard quotient and reference dose for oral exposure (Nap = 20, Flu = 40, Phe = 30, and Pyr = 30 µg/kg-BW day), respectively. Furthermore, the HIi was computed to assess the cumulative risk of exposure to dietary PAHs (Eq. 3)4,24:
Low-risk adverse health impacts on the target population were reflected by HIPAHs < 1.
Statistical analysis
SPSS 21 software was used to perform all analyses. The Shapiro–Wilk normality analysis was used to check the data distribution. The Mann–Whitney U analysis was used to compare the measured variables' values between various groups. These exposure categories included living near restaurants, workshops, or factories, being near busy roads, car repairs, bus stops, passive smoking, the type of heating appliance used inside the home, and kitchen hoods. The significant correlation between the two variables was also determined using the Spearman correlation. Multivariate linear regression analysis was used to evaluate the relationship between PAH metabolites levels and independent factors that may affect their concentration in urine.
Ethical approval
The Shiraz Medical University's ethics committee in Shiraz, Iran, approved this work (ethics code: IR.SUMS.REC.1399.703).
Consent to participate
I consent to participate in the research project and the following has been explained to me: (1) the research may not be of direct benefit to me; (2) my participation is completely voluntary; (3) my right to withdraw from the study at any time without any implications to me; (4) the risks including any possible inconvenience, discomfort or harm as a consequence of my participation in the research project; (5) the steps that have been taken to minimize any possible risks; (6) public liability insurance arrangements; (7) what I am expected and required to do; (8) whom I should contact for any complaints with the research or the conduct of the research; (9) I am able to request a copy of the research findings and reports; (10) security and confidentiality of my personal information.
Results and discussion
Characteristics of the participants
Table 1 presents the demographics of the participants (control group (n = 30), kitchen workers (n = 57), and individuals who live close to restaurants (n = 57)). There were 49 (87.5%) males and 8 (12.5%) females among the participants (kitchen workers and people living near restaurants). Among the control group, 25 (83.33%) were male, while 5 (16.67%) were female. The average age of kitchen workers, people living near the restaurant, and the control group was 33.6, 33.9, and 33.41 years, respectively. The participant's body weight index (BMI) ranged from 24.7 to 25.46 kg/m2. According to the findings, 37 participants were exposed to passive smoking for 48 h before sampling. The most common transportation by volunteers was by private car and taxi. More than 70% of each group used a kitchen hood. Over half of the participants lived close to sources of PAHs emissions, including bus terminals, parking lots, and traffic areas.
Distribution of PAH metabolites levels in urine
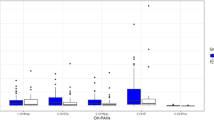
Exposure to PAHs is a significant concern because of its effects on human health. The mean levels of PAH metabolites in the urine samples of the study groups are displayed in Fig. 1 and other details are given in Table S1 (Supplementary data). The highest and lowest mean levels were related to 1-OHP and 9-OHPhe, respectively, in the three study groups. Similar findings were also achieved in research on kitchen workers in India13. Another research found that 1-OHP was more prominent than other PAH metabolites26. Further, the highest mean level of PAH metabolites measured in a study of PAHs biomonitoring adults in a Middle Eastern region was 1-OHP5, which is consistent with our results. 1-OHP is rapidly excreted through the urine, which could explain its high urinary level5.
Kitchen workers, people who live near restaurants, and the control group had a total average concentration of PAH metabolites (ΣOH-PAHs) of 2126.7, 1973.7, and 1687.61 ng/g cr, respectively (Fig. 1). Kitchen workers and those living near restaurants had higher ΣOH-PAHs than the control group, but this difference was only significant between the workers and control groups (p value < 0.05). A study in India confirmed this finding, revealing that kitchen workers had higher ΣOH-PAHs than the control group27. The ΣOH-PAHs in the urine of Shiraz citizens were 1988.1 ng/g cr5, which was lower than that found among the people who worked in restaurant kitchens. This seems logical because these workers are more exposed to PAHs due to being involved in the frying processes. It was also found in another study on school children in Shiraz that the ΣOH-PAHs (1460 ng/g cr) were lower than those in our study16. Differences in age and time spent at work and home might explain the discrepancy between the results.
Based on our findings, a significant difference in 9-OHPhe level was seen between people residing near restaurants and the control group (p = 0.017). According to a study conducted by Siddique, a significant concentration of phenanthrene was formed in all samples after cooking (frying)28, so it could be a logical reason for a higher 9-OHPhe level in samples of people living near restaurants (20.08 ng/g cr) than in the control group (7.86 ng/g cr). This result is similar to that reported by Wang et al.29. Nevertheless, there was no significant difference in other urinary PAH metabolites concentration among the three groups of our study (p > 0.05).
Clinical parameters levels in the studied subjects
Descriptive statistics for the clinical parameters of the participants are presented in Table 2. In this study, no significant difference was found between the parameters measured in the different groups (p > 0.05). However, the results of the study by Singh et al.13 in India report that continuous exposure to heat in kitchens can impair the kidney function of kitchen workers. Furthermore, in a study of residents of Shiraz, a significant difference in red blood cell (RBC) count and TG level was found between male and female participants5. This result was not observed in our study; it is probably due to the fact that in the current study, most of the participants were male.
Measure of the concentrations of MDA (µm/mM cr) and TAC (mM/mM cr) in urine samples, and CRP (mg/L) in blood samples
Figure 2 shows a measurement of mean concentrations of MDA (µm/mM cr) and TAC (mM/mM cr) in urine samples and CRP (mg/L) in blood samples from study groups.
According to the test results, no significant difference was observed between the three groups in the concentrations of MDA, TAC, and CRP (p value > 0.05). The mean mass concentration of MDA was (0.00082, 0.00075, and 0.00082), TAC (1.21, 1.4, and 1.01), and CRP levels (3.67, 3.65, and 3.65) in A, B, and C, respectively (Fig. 2). Pan et al.8 found in their study that kitchen staff (369 µmol/mol cr) had significantly higher urinary MDA levels than service staff (267.2 µmol/mol cr), which contradicts the current study.
Spearman's rank correlation coefficient was used to examine the association between measured concentrations in the study groups. A significant and positive linear relationship between the concentration of red blood cells in blood samples and TAC (CC = 0.33, p = 0.00), CRP (CC = 0.19, p = 0.02), and MDA (CC = 0.17, p = 0.04) was observed. In other words, with increasing RBC concentration, the concentration of TAC, CRP and, MDA increases linearly and this correlation is higher in TAC due to the higher correlation coefficient. Furthermore, RBCs in one study had a direct linear proportional relationship with MDA30, which is consistent with our findings.
Moreover, there was a significant negative linear correlation between TAC concentrations and MDA (CC = − 0.22, p = 0.01). This means that as TAC concentration rises, MDA concentration falls linearly and vice versa. In a biomonitoring study, MDA and TAC levels were significantly higher and lower, respectively, in men with idiopathic infertility than in fertile men31 which, like the present study, showed a relationship inverse between the TAC and MDA parameters.
The correlations between PAH metabolites (ng/g cr) and MDA, TAC, and CRP levels
Table 3 shows the correlations between PAH metabolites (ng/g cr) and MDA (mM/mM cr), TAC (mM/mM cr), and CRP (mg/L) in the groups studied.
The results indicated that there was a significant correlation between TAC levels and all measured metabolites of PAH, except for 9-OHPhe in the A and C groups, as well as 1-OHP in the B and C groups. The TAC assay was been applied to determine the antioxidant capacity of some heterogeneous compounds with antioxidant activity in body fluids and thus can also help in assessing the overall antioxidant status. Other research has found that increased exposure to PAH compounds increases oxidative stress and decreases TAC, increasing lung cancer susceptibility32.
Furthermore, according to the results shown in Table 3, it can be seen that as the amount of PAH metabolites measured in the urine samples increased, the TAC concentration decreased. Previous research has also indicated that accelerating oxidative stress and reducing TAC affect the prevalence of lung cancer33,34.
Exposure to PAHs has been identified as one of many causes of adverse health effects. It has been suggested that this phenomenon could be due to oxidative damage. For example, some studies on PAHs have been conducted in vitro and in vivo. Therefore, MDA is a commonly used biomarker to assess oxidative stress. Based on our results, there was a significant correlation between MDA levels and all measured PAH metabolites except the control group. The results showed that MDA concentrations increased significantly with increasing concentrations of urinary PAH metabolites. Researchers found that urinary MDA levels were positively associated with hydroxy-PAH levels in a rural population from the North China Plain (p < 0.05)35. Epidemiological studies have also reported an association between exposure to PAHs and urinary concentrations of MDA35. For example, Bae et al.36 reported that urinary MDA concentrations increased significantly with increasing urinary 1-hydroxy pyrene concentrations. A similar result was discovered in this study. Cooking oil smoke is one of the main sources of PAHs. Kitchen workers are highly exposed because they do not wear respiratory protection8. Therefore, it can cause oxidative DNA damage and lipid peroxidation12,37. Urinary levels of 1-OHP and MDA often represent occupational exposure to PAHs and oxidative stress among kitchen workers and their neighbors38,39.
CRP is an inflammation marker that rises when the body is inflamed. In the present study, there was no significant relationship between PAH metabolites and CRP in any of the three study groups (Table 3). However, the results of one study demonstrated that levels of biomarkers for urinary PAHs are positively correlated with serum CRP levels40. Therefore, in the present study, it is possible that the effects of confounders did not show any association between serum CRP and PAH metabolites in the urine of the participants.
Linear regression analysis
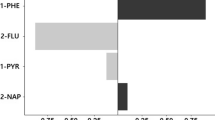
The results of the regression analysis were used to examine the relationship between urine PAH metabolites levels and exposure variables such as subjects' activities, the cooking frequency at home and at work, age of the building, the use of the hood, the weekly consumption of food (meat, fish, grilled fruit and vegetables), passive smoking, body mass index, residence conditions in traffic, and etc. Only secondhand smoke had a significant relationship with the concentration of PAH metabolites in the urine of the studied participants (p < 0.05). 1-OHNap (β = 0.26 and p value = 0.013) and 1-OHP (β = 0.43 and p value = 0.01) were significant according to secondhand smoke in participants (Fig. 3). Therefore, this study considers secondhand tobacco smoke as a possible source of naphthalene and pyrene emissions. Additionally, several other studies have shown that cigarette smokers release significant concentrations of naphthalene into living spaces41,42.
Non-carcinogenic and cumulative risk assessment
Most people believe that cooking and food processing techniques like smoking and drying are the main sources of PAH contamination. Cooking causes a variety of compounds, including PAHs, to be produced in food, depending on a number of factors, such as time, fuel used, distance from the heat source, drainage of fat, and type (grilling, frying, roasting). While the exact mechanisms of how PAHs are created are unknown, it is likely that there are several different ones, including the pyrolysis of meat at high temperatures and the pyrolysis of melted fat when it drips onto a heat source. As a result of eating more grilled and fast food, restaurant employees and people living nearby are exposed to PAH compounds through ingestion. In recent decades, health risk evaluation has been considered a valuable method for evaluating potential environmental risks43. We evaluated the risk of exposure to PAHs in the target subjects by only taking the dietary ingestion route into account because the diet is the primary source of PAHs exposure in non-smokers25.
In the current research, EDIi, HQi, and HIi for 1-OHNap, 2-OHNap, 2-OHFlu, 9-OHPhe, and 1-OHP were estimated to investigate the potential health hazards of PAH metabolites. Table 4 shows the findings associated with EDIi. The results obtained in Table 4 were used to calculate HQi, and HIi based on Eqs. (2) and (3). The results related to HQi and HIi are shown in Fig. 4. Based on the findings, HQi for PAH metabolites (1-OHNap, 2-OHNap, 2-OHFlu, 9-OHPhe, and 1-OHP) was less than one (HQi < 1). Furthermore, HIi was less than one (HIi < 1) in the studied groups, indicating low-risk negative health impacts on the target groups. Similarly, research conducted on Mexican children exposed to high levels of PAHs found that the HQi was less than 1 in those children44. PAHs exposure was not associated with any significant non-cancer health risks in another study conducted in Spain25. As a result of PAHs exposure, Fernandez et al.21 found that Spanish women were not at significant risk of health. According to a study conducted in Mexico, women living in the studied communities had HQi greater than 1, which is associated with increased health risks44.
Strength and limitations
Biomonitoring of restaurant workers and people living near restaurants was conducted for the first time in Shiraz, Iran. We examined the correlation between PAH metabolites and other measured clinical parameters to increase the reliability of the results of this study. Environmental PAHs can enter the body in several ways and cause a variety of side effects. The limitations of our study are mainly related to the small number of subjects in each of the three groups. Therefore, these results should be interpreted with caution so that other studies can support them. In future studies, larger sample sizes should be used for more detailed statistical analysis, especially for CRP, MDA, TAC, and RBC. It is suggested that future research examine how differences in diet and lifestyle affect emission sources.
Conclusion
In this study, the levels of PAH metabolites in the urine and various clinical parameters in the blood of kitchen workers and residents near restaurants were examined. The average level of PAH metabolites in the urine of the kitchen staff was higher than that of the control group and residents of nearby restaurants. The mean concentration of 1-OHP was the highest among PAH metabolites. Additionally, MDA levels in kitchen workers and people living near restaurants were significantly correlated with PAH metabolites. The results of these studies demonstrated the negative linear correlation between TAC concentrations and PAH metabolite concentrations. There were no significant differences in the concentrations of clinical parameters between study groups. However, HIi was observed to be less than 1 in all groups, indicating a low risk of adverse health effects for the target groups. In conclusion, identifying the sources of PAH emissions and reducing their production are key to reducing the harmful effects of PAHs on humans.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to restrictions, e.g., they information that could compromise the privacy of research participants.
References
Cachada, A. et al. Urinary concentrations of monohydroxylated polycyclic aromatic hydrocarbons in adults from the US Population Assessment of Tobacco and Health (PATH) Study Wave 1 (2013–2014). Environ. Int. 123, 201–208. https://doi.org/10.1016/j.envint.2018.11.068 (2019).
Zaj, J., Gomó, E. & Szot, W. Urinary 1-hydroxypyrene in occupationally-exposed and non-exposed individuals in Silesia. Pol. Ann. Agric. Environ. Med. https://doi.org/10.26444/aaem/75940 (2017).
Wang, Y. et al. Multivariate analysis for assessing sources, and potential risks of polycyclic aromatic hydrocarbons in Lisbon urban soils. Minerals 9, 139–156. https://doi.org/10.3390/min9030139 (2019).
Tabatabaei, Z., Hoseini, M., Fararooei, M., Shamsedini, N. & Baghapour, M. A. Biomonitoring of BTEX in primary school children exposed to hookah smoke. Environ. Sci. Pollut. Res. 2, 1–14 (2022).
Shahsavani, S., Fararouei, M., Soveid, M., Hoseini, M. & Dehghani, M. The association between the urinary biomarkers of polycyclic aromatic hydrocarbons and risk of metabolic syndromes and blood cell levels in adults in a Middle Eastern area. J. Environ. Health Sci. Eng. 20, 20 (2021).
Araujo, J. A. & Nel, A. E. Particulate matter and atherosclerosis: Role of particle size, composition and oxidative stress. Part. Fibre Toxicol. 6, 1–19 (2009).
Khademi, H., Khozeimeh, F., Tavangar, A., Amini, S. & Ghalayani, P. The Serum and salivary level of malondialdehyde, vitamins A, E, and C in patient with recurrent aphthous stomatitis. Adv. Biomed. Res. 3, 25 (2014).
Pan, C. H., Chan, C. C., Huang, Y. L. & Wu, K. Y. Urinary 1-hydroxypyrene and malondialdehyde in male workers in Chinese restaurants. Occup. Environ. Med. 65, 732–735. https://doi.org/10.1136/oem.2007.036970 (2008).
Chen, Y. et al. Gaseous and particulate polycyclic aromatic hydrocarbons (PAHs) emissions from commercial restaurants in Hong Kong. J. Environ. Monit. 9, 1402–1409 (2007).
Jørgensen, R. B., Strandberg, B., Sjaastad, A. K., Johansen, A. & Svendsen, K. Simulated restaurant cook exposure to emissions of PAHs, mutagenic aldehydes, and particles from frying bacon. J. Occup. Environ. Hyg. 10, 122–131. https://doi.org/10.1080/15459624.2012.755864 (2015).
Ming-Tsang, W., Lin, P.-C., Pan, C.-H. & Peng, C.-Y. Risk assessment of personal exposure to polycyclic aromatic hydrocarbons and aldehydes in three commercial cooking workplaces. Sci. Rep. 9, 1661 (2019).
Ciarrocca, M. et al. Is urinary 1-hydroxypyrene a valid biomarker for exposure to air pollution in outdoor workers? A meta-analysis. J. Eposure Sci. Environ. Epidemiol. 24, 17–26 (2014).
Singh, A. et al. Heat and PAHs emissions in indoor kitchen air and its impact on kidney dysfunctions among kitchen workers in Lucknow, North India. PLoS One 11, e0148641 (2016).
Mo, Z. et al. Characterization and health risk assessment of PM 2.5-bound polycyclic aromatic hydrocarbons in 5 urban cities of Zhejiang Province, China. Sci. Rep. 9, 25 (2019).
Li, C. T., Lin, Y. C., Lee, W. J. & Tsai, P. J. Emission of polycyclic aromatic hydrocarbons and their carcinogenic potencies from cooking sources to the urban atmosphere. Environ. Health Perspect. 111, 483–487 (2003).
Shahsavani, S., Dehghani, M., Hoseini, M. & Fararouei, M. Biological monitoring of urinary 1-hydroxypyrene by PAHs exposure among primary school students in Shiraz, Iran. Int. Arch. Occup. Environ. Health 90, 179–187 (2017).
Shahsavani, S., Dehghani, M., Hoseini, M. & Fararoei, M. Health risk assessment of atmospheric paticulate-bound polycyclic aromatic hydrocarbons in Shiraz, Iran. J. Air Pollut. Health 1, 153–160 (2016).
Lewné, M., Johannesson, S., Strandberg, B. & Bigert, C. Exposure to particles, polycyclic aromatic hydrocarbons, and nitrogen dioxide in Swedish restaurant kitchen workers. Ann. Work Expos. Health 61, 152–163 (2017).
Chen, J. W., Wang, S. L., Hsieh, D. P. H., Yang, H. H. & Lee, H. L. Carcinogenic potencies of polycyclic aromatic hydrocarbons for back-door neighbors of restaurants with cooking emissions. Sci. Total Environ. 417–418, 68–75 (2012).
Kuczmarski, M. F., Kuczmarski, R. J. & Najjar, M. Effects of age on validity of self-reported height, weight, and body mass index: Findings from the third national health and nutrition examination survey, 1988–1994. J. Am. Diet. Assoc. 101, 28–34. https://doi.org/10.1016/S0002-8223(01)00008-6 (2001).
Association, W. M. Declaration of Helsinki. Ethical principles for medical research involving human subjects. J. Wissenschaft Ethik 14, 233–238 (2009).
García-García, S. et al. Determination of hydroxy polycyclic aromatic hydrocarbons in human urine using automated microextraction by packed sorbent and gas chromatography-mass spectrometry. Int. J. Environ. Res. Public Health 19, 13089. https://doi.org/10.3390/ijerph192013089 (2022).
Feldt, T. et al. High levels of PAH-metabolites in urine of e-waste recycling workers from Agbogbloshie, Ghana. Sci. Total Environ. 466, 369–376. https://doi.org/10.1016/j.scitotenv.2013.06.097 (2014).
Tabatabaei, Z., Shamsedini, N., Baghapour, M. A. & Hoseini, M. Exposure assessment of children living in homes with hookah smoking parents to polycyclic aromatic hydrocarbons: Urinary level, exposure predictors, and risk assessment. Environ. Sci. Pollut. Res. 20, 1–13 (2022).
Fernandez, S. F., Pardo, O., Hernandez, C. S., Garlito, B. & Yusa, V. Children’s exposure to polycyclic aromatic hydrocarbons in the Valencian Region (Spain): Urinary levels, predictors of exposure and risk assessment. Environ. Int. 153, 25 (2021).
Hemat, H., Wittsiepe, J., Wilhelm, M., Müller, J. & Göen, T. High levels of 1-hydroxypyrene and hydroxyphenanthrenes in urine of children and adults from Afghanistan. J. Exposure Sci. Environ. Epidemiol. 22, 46 (2012).
Singh, A. et al. Assessing hazardous risks of indoor airborne polycyclic aromatic hydrocarbons in the kitchen and its association with lung functions and urinary PAH metabolites in kitchen workers. Clin. Chim. Acta https://doi.org/10.1016/j.cca.2015.11.020 (2015).
Siddique, R. et al. Probing the impact of conventional oil frying on the formation of polycyclic aromatic hydrocarbons in rabbit meat. Food Sci. Nutr. 9, 1698–1703 (2021).
Wang, J. et al. elevated oxidative damage in kitchen workers in Chines restaurants. J. Occup. Health 53, 327–333 (2011).
Sawas, A. & Pentyala, S. Evaluation of lipid peroxidation in red blood cells by monitoring the uptake of sucrose and phenol red. J. Appl. Toxicol. Int. J. 24, 223–229 (2004).
Fazeli, F. & Salimi, S. Correlation of seminal plasma total antioxidant capacity and malondialdehyde levels with sperm parameters in men with idiopathic infertility (2016).
Eom, S. Y. et al. Polycyclic aromatic hydrocarbon-induced oxidative stress, antioxidant capacity, and the risk of lung cancer: A pilot nested case-control study. Anticancer Res. 33, 3089–3097 (2013).
Çalışkan-Can, E. et al. Increased levels of 8-hydroxydeoxyguanosine and its relationship with lipid peroxidation and antioxidant vitamins in lung cancer. Clin. Chem. Lab. Med. 46, 107–112 (2008).
Ito, K. et al. Serum antioxidant capacity and oxidative injury to pulmonary DNA in never-smokers with primary lung cancer. Anticancer Res. 32, 1063–1067 (2012).
Yang, Q. et al. Polycyclic aromatic hydrocarbon (PAH) exposure and oxidative stress for a rural population from the North China Plain. Environ. Sci. Pollut. Res. Int. 22, 1760–1769. https://doi.org/10.1007/s11356-014-3284-y (2015).
Bae, S. et al. Exposures to particulate matter and polycyclic aromatic hydrocarbons and oxidative stress in schoolchildren. Environ. Health Perspect. 118, 579–583 (2010).
Lai, C.-H. et al. Exposure to cooking oil fumes and oxidative damages: A longitudinal study in Chinese military cooks. J. Eposure Sci. Environ. Epidemiol. 23, 94–100 (2013).
Ke, Y. et al. Comparative study of oxidative stress biomarkers in urine of cooks exposed to three types of cooking-related particles. Toxicol. Lett. 255, 36–42 (2016).
Pan, C. H., Chan, C. C. & Wu, K. Y. Effects on Chinese restaurant workers of exposure to cooking oil fumes: A cautionary note on urinary 8-hydroxy-2-deoxyguanosine. Cancer Epidemiol. Biomark. Prev. 17, 3351–3359 (2008).
Alshaarawy, O., Zhu, M., Ducatman, A., Conway, B. & Andrew, M. E. Polycyclic aromatic hydrocarbon biomarkers and serum markers of inflammation. A positive association that is more evident in men. Environ. Res. 126, 98–104. https://doi.org/10.1016/j.envres.2013.07.006 (2013).
Batterman, S. et al. Sources, concentrations, and risks of naphthalene in indoor and outdoor air. Indoor Air 22, 266–278 (2012).
Jia, C. & Batterman, S. A critical review of naphthalene sources and exposures relevant to indoor and outdoor air. Int. J. Environ. Res. Public Health 7, 2903–2939 (2010).
Franco, S. S., Nardocci, A. C. & Günther, W. M. R. PAH biomarkers for human health risk assessment: A review of the state-of-the-art. Cad. Saude Publica 24, a569-s580 (2008).
Pérez-Maldonado, I. N., Ochoa-Martínez, Á. C., López-Ramírez, M. L. & Varela-Silva, J. A. Urinary levels of 1-hydroxypyrene and health risk assessment in children living in Mexican communities with a high risk of contamination by polycyclic aromatic hydrocarbons (PAHs). Int. J. Environ. Health Res. https://doi.org/10.1080/09603123.2018.1549727 (2018).
Acknowledgements
This article was extracted from the thesis written by Mrs. Narges Shamsedini, a Ph.D. candidate in Environmental Health Engineering. Hereby, the authors would like to thank the Research Vice-chancellor of Shiraz University of Medical Sciences for financially supporting the research (proposal No. 21268).
Funding
This work was supported by the Research Vice-chancellor of Shiraz University of Medical Sciences [Grant number 21268].
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by N.S., M.N., Z.T., S.R., and S.B.. The first draft of the manuscript was written by N.S. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. Supervision, visualization and investigation were carried out by M.D., M.R.S., A.A., M.H., and M.F. I consent to publication of results from this study on the condition that my identify will not be revealed.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shamsedini, N., Dehghani, M., Samaei, M.R. et al. Non-carcinogenic and cumulative risk assessment of exposure of kitchen workers in restaurants and local residents in the vicinity of polycyclic aromatic hydrocarbons. Sci Rep 13, 6649 (2023). https://doi.org/10.1038/s41598-023-33193-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-33193-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.