Abstract
Ventricular septal defects (VSD) are the most common congenital heart diseases in children. Among them, perimembranous VSD (pm-VSD) have a higher risk of complications, including aortic valve prolapse and aortic regurgitation (AR). The aim of our study was to assess echocardiographic criteria associated with AR during follow-up of pm-VSD. Forty children with restrictive pm-VSD, followed-up in our unit and who underwent a workable echocardiographic evaluation between 2015 and 2019 were included and retrospectively analyzed. The propensity score was used to match 15 patients with AR to 15 patients without AR. Median age was 2.2 year [1.4–5.7]. Median weight was 14 kg [9.9–20.3]. Aortic annulus z-score, Valsalva sinus z-score, sinotubular junction z-score, valve prolapse and commissure commitment were significantly different between the two groups (p = 0.047, p = 0.001, p = 0.010, p = 0.007, p < 0.001 respectively). Aortic root dilatation, aortic valve prolapse and commissure commitment to a perimembranous VSD are associated to aortic regurgitation.
Similar content being viewed by others
Introduction
Ventricular septal defects (VSD) are the most common congenital heart diseases in children, representing 37% of congenital malformation1.Although the indication for closure of hemodynamically significant VSDs is no matter of debate2,the jury is still out regarding small, restrictive VSDs without left ventricular dilation.
In doubly committed juxta-arterial defects and perimembranous defects, the anatomical position of the defect frequently affects the mobility and function of the aortic leaflets, resulting in aortic incompetence3, which is generally mild at first but progressive in nature4. Perimembranous VSD (pm-VSD), is deemed to be less associated with AR. In this type of VSD, the mechanism of AR seems to implicate several factors.
The aim of this study was to assess echocardiographic criteria associated with aortic regurgitation in restrictive pm-VSD.
Methods
Study design
Between January 2015 and January 2019, a total of 158 children with pm-VSD were admitted to our department for medical or surgical treatment.
All patients < 18 year old with perimembranous VSD and for who a workable and recent (less than three months) echocardiographic evaluation was available, were considered for inclusion. Patients with non-perimembranous VSD and patients with associated congenital heart anomalies other than atrial septal defect were excluded.
This study complies with the Declaration of Helsinki. La Timone Children’s Hospital Clinical Investigation Committee (Direction de Recherche en Santé) and the local ethic committee (Commission d'Accès aux Données de Santé du CHU) approved the study protocol (PADS22-135). Local ethical approval was given with waiver of informed consent for retrospective, anonymized data. Sixty five patients were eligible and thirty children were included after propensity score matching (Fig. 1: flow chart).
The cohort was divided into 2 groups:
-
AR group: patient with aortic valve regurgitation (n = 15)
-
Non-AR group: patient without AR (n = 15)
Clinical data
Demographic, clinical and TTE data were collected from each patient’s medical record. The clinical parameters collected were those recorded on the day of the echocardiography.
Echocardiographic data
All the echocardiographic studies were analyzed by the same observer (FS).
Images analyzing the VSD in 2D, color Doppler and continuous Doppler were reviewed. The size of the pm- VSD and the maximum flow velocity through the defect were recorded.
Parasternal long axis, short axis and four chamber views of the aortic valve were analyzed. Data collection included diameter of the aortic valve annulus, the Valsalva sinus and the ascending aorta (Fig. 2). Respective z-scores (ZS) were calculated according to the Detroit Formula5. The ratio between the VSD diameter measured in parasternal short axis (mm) and the aortic annulus diameter measured in parasternal long axis (mm) was calculated. The anatomy of the aortic valve (AV) was described and each leaflet size was measured.The cusp imbalance index was defined as: [width of right (R) or non- (N) coronary cusp/width of left coronary cusp (L)]6.
The coaptation height (CH) and the effective height (EH) were measured (Fig. 2). The aortic coaptation height (ACH = CH/AV ratio) and the aortic effective height (AEH = EH/AV ratio) indices were calculated as follow: ACH index% = (CH/AV) × 100, AEH index % = (EH/AV) × 1007.
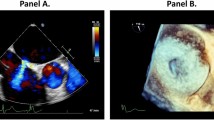
Long axis and 5 chamber views were used to assess cusp (leaflet) prolapse. Commissure commitment was assessed in short axis view (Fig. 3).
Statistical analysis
Propensity score was performed to match pm-VSD with AR patients (AR group) with pm-VSD without AR patients (Non AR group) according to age and weight. Patients were 1:1 matched using the nearest neighbour method without replacement and with a calliper of width equal to 0.25. Table 1 detailed those characteristics before and after matching.
Continuous variables are expressed as mean (with standard deviation) or median (with IQR), where appropriate. Discrete or binary variables are presented as number (percent).
The χ2 test or Fisher’s exact test (if appropriate) were used for categorical variables and Mann Whitney test was used for continuous variables to assessed influencing factors Associations between echocardiographic parameters and AR were determined with univariate binary logistic regression analysis.
All p values were bilateral and significance was pronounced for a p value of 5%. Statistical analysis was performed using SPSS software, version 22.0 (SPSS, Inc., Chicago, IL, USA).
Results
Population characteristics
The median age was 2.2 year [1.4–5.7] and the median weight 14 kg [9.9–20.3]. There was no significant difference concerning demographic parameters between the two groups (Table 2).
Image quality was considered interpretable for all selected patients allowing successful collection of all echocardiographic data. The mean VSD diameter and VSD-to-aortic diameter ratio were respectively 6.1 ± 2.4 mm and 0.43 ± 0.13. The mean VSD peak velocity reached 4.4 ± 0.5 m/s. Median end diastolic LV diameter z score was 1.6 [0.0–2.3], 8 (26.7%) patients had a LV z-score above + 2. In this study, 25 (83.3%) patients presents restrictive VSD.
Review of previous echocardiograms of patients in the AR group shows that: two patients had a stable minimal AR. Three patients had mild to moderate AR of recent worsening and the other patients had mild AR of recent onset. In AR group, 13 (86%) patients were operated on within a year and the procedure was VSD closure only. In non AR group, 8 (53%) were operated. In the last follow up, non-operated patients are stable without AR (or AR worsening).
Univariate analysis
Several echocardiographic parameters differed significantly between the 2 groups: (Table 2).
-
In the AR group, the size of the aortic root, especially the median Valsalva sinus size, was significantly larger than in the non-AR group (3.4 [1.4–3.8] vs 0.45 [0.07–1.3], p = 0.001).
-
In AR group, there was significantly more AVP and commissure commitment (p = 0.007 and p < 0.001 respectively).
-
No significant difference was found for the AEH index, ACH index and cusp imbalance index (p = 0.116, p = 0.115, p = 0.451, respectively).
-
The association of those parameters (aortic root size, AVP and commissure commitment) and AR is shown in Table 3.
Discussion
Complications of subarterial VSDs (corresponding to doubly committed juxta arterial and perimembranous VSDs3), and in particular AVP have been widely studied. The incidence of AVP, reaches 73% in some studies, with progression to AR in 52 to 78% of the patients8,9,10,11,12,13.
Several mechanisms have been are suggested to explain the association of pm-VSD and aortic valve regurgitation. Beside aortic valve prolapse, which remains the main cause, dilatation of the ascending aorta, decrease of the coaptation height and aortic cusp imbalance have been involved.
Dilation of the ascending aorta
Momma et al. showed that dilation of the ascending aorta was often associated with aortic valve distortion14. In our study, dilatation of the ascending aorta was associated with AR.
Coaptation height
Iwashima et al., suggested a decreased coaptation height as a non-invasive marker for the assessment of severity of AVP in the presence of a VSD7.We used, as recommended in their publication7, a ratio to avoid variations in relation with patient’s weight and height difference. We did not find any statistically significant difference between the two groups.
Aortic cusp imbalance
Tomita et al., showed that an imbalance in the width of a cusp could predict a possible progressive worsening of AR6.In the study of Salih and al, concerning 41 consecutive patients, operated for VSD (36 pm-VSD) with prolapsed AV, with or without AR, the presence of cusp imbalance did not impact post-operative AR improvement15.
In our study, this parameter was not significantly different between the two groups.
Prolapse of aortic valve
Several studies have shown that the presence of AVP should indicate regular ultrasound monitoring for AR, which appears in more than half of the cases16,17. AR has a tendency to worsen as soon as it appears and may not disappear in post-operative course. Even minor prolapse can cause AR18 and eccentric AR can reveal AVP of the right coronary cusp that is very likely to progress19. In our population, AVP was found to be associated with AR.
Commissural commitment
Our study is the first to show a significant association between the commissure commitment into the VSD and the AR. Azcarate et al.4 proposed a differentiation of the mechanism of aortic regurgitation according to the position of defect. In infundibular defect (corresponding to Outlet defect with hypoplastic or absent muscular outlet septum3), the mechanism seems to be a lack of support of the leaflet resulting in a gradual prolapse through the defect4. In pm-VSDs, infundibular septum is conserved but the defect is potentially located in the intercommissural zone of the right coronary leaflet and non-coronary leaflet (corresponding to interleaflet triangle) and incriminates both right and non-coronary leaflet20,21. This hypothesis is corroborated by recent publications on the relationship of the septum with the aortic valve in the context of assessment and management of transcatheter implantation of the aortic valve22. Indeed, due to variations in rotation of aortic root, the membranous septum can have variable relationship with its components22,23. Membranous septum can be located below aortic annulus, it can crossed the annulus projecting into interleaflet triangle or it can projected into the noncoronary sinus deep to the aortic leaflet22.
In the case where the membranous septum is located into interleaflet triangle and pm-VSD, aortic incompetence is located in the commissure due to poor coaptation of the two leaflet margins (non-coronary and right coronary leaflet)4. Our findings support this mechanism.
Limitations of the study
Our study has 2 main limitations. : First of all, a technical limitation since echocardiography is a highly operator-dependent and observer-dependent procedure. The operator-dependency has been limited by eliminating all non-workable and incomplete ultrasound studies. The observer-dependency has been limited by proceeding to a methodical rereading of all ultrasound examinations by one single observer.
The second limitation is statistical related to the small size of our population and the retrospective nature of the study. This work, however, provides a basis for a prospective pilot study to further investigate echocardiographic parameters association with AR.
Conclusion
Increased Valsalva sinus size as well as aortic valve prolapse and commissural commitment are clearly implicated and associated with AR in pm-VSD. Our study reinforces the idea that anatomical variables are directly implicated in AR. We hypothesize that commissural commitment to the VSD (Fig. 4) must be considered as a risk factor for aortic regurgitation. Further prospective studies are necessary to validate these preliminary data.
Data availability
Data generated during the current study are available from the corresponding author on reasonable request.
References
Dakkak, W. & Bhimji, S. S. Ventricular septal defect. In StatPearls (StatPearls Publishing, Treasure Island, FL, 2018).
Warnes, C. A. et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: A report of the American College of Cardiology/American Heart Association task force on practice guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease) developed in collaboration with the American society of echocardiography, heart rhythm society, international society for adult congenital heart disease, society for cardiovascular angiography and interventions, and society of thoracic surgeons. J. Am. Coll. Cardiol. 52, e143–e263 (2008).
Lopez, L. et al. Classification of ventricular septal defects for the eleventh iteration of the international classification of diseases—striving for consensus: A report from the international society for nomenclature of paediatric and congenital heart disease. Ann. Thorac. Surg. 106, 1578–1589 (2018).
Azcarate MJM. Ventricular septal defect with aortic regurgitation. An unsolved Problem. Rev Esp Cardiol.2002; 55(9):897–9.6
Pettersen, M. D., Du, W., Skeens, M. E. & Humes, R. A. Regeression equations for calculation of zscores of cardiac structures in a large cohort of healthy infants, children, and adolescents: An echocardiographic study. J. Am. Soc. Echocardiogr. 21, 922–934 (2008).
Tomita, H. et al. Imbalance of cusp width and aortic regurgitation associated with aortic cusp prolapse in ventricular septal defect. Jpn. Circ. J. 65(6), 500–504 (2001).
Iwashima, S. et al. Measurement of aortic valve coaptation and effective height using echocardiography in patients with ventricular septal defects and aortic valve prolapse. Pediatr. Cardiol. 38, 608–616 (2017).
Tohyama, K., Satomi, G. & Momma, K. Aortic valve prolapse and aortic regurgitation associated with subpulmonic ventricular septal defect. Am. J. Cardiol. 79, 1285–1289 (1997).
Eroglu, A. G. et al. Evaluation of ventricular septal defect with special reference to the spontaneous closure rate, subaortic ridge, and aortic valve prolapse II. Pediatr. Cardiol. 38, 915–921 (2017).
Rhodes, L. A. et al. Long follow-up (to 43 years) of ventricular septal defect with audible aortic regurgitation. Am. J. Cardiol. 66(3), 340–345 (1990).
Layangool, T., Kirawittaya, T. & Sangtawesin, C. Aortic valve prolapse in subpulmonic ventricular septal defect. J. Med. Assoc. Thai 86(3), S549–S555 (2003).
Saleeb, S. F. et al. Frequency of development of aortic cuspal prolapse and aortic regurgitation in patients with subaortic ventricular septal defect diagnosed at <1year of age. Am J Cardiol. 99(11), 1588–1592 (2007).
Layangool, T. et al. Natural aortic valve complications of ventricular septal defect: A prospective cohort study. J. Med. Assoc Thai 91(3), S53–S59 (2008).
Momma, K. et al. Natural history of subarterial infundibular ventricular septal defect. Am. Heart J. 108, 1312–1317 (1984).
Salih, H. G., Ismail, S. R., Kabbani, M. S. & Abu-Sulaiman, R. M. Predictors for the outcome of aortic regurgitation after cardiac surgery in patients with ventricular septal defect and aortic cusp prolapse in Saudi patients. Heart Views 17(3), 83–87 (2016).
Atik, S. U. & Eroglu, A. G. Aortic valve prolapse and aortic regurgitation during long term follow up in children with ventricular septal defect. J. Heart Valve Dis. 26(6), 616–623 (2017).
Eroğlu, A. G. et al. Aortic valve prolapse and aortic regurgitation in patients with ventricular septal defect. Pediatr. Cardiol. 24(1), 36–39 (2003).
Mori, K. et al. Echocardiographic evaluation of the development of aortic valve prolapse in supracristal ventricular septal defect. Eur. J. Pediatr. 154(3), 176–181 (1995).
Tomita, H., Yamada, O., Kurosaki, K., Yagihara, T. & Echigo, S. Eccentric aortic regurgitation in patients with right coronary cusp prolapse complicating a ventricular septal defect. Circ. J. 67(8), 672–675 (2003).
Tomita, H. et al. Impact of noncoronary cusp prolapse in addition to right coronary cusp prolapse in patients with a perimembranous ventricular septal defect. Int. J. Cardiol. 65(6), 500–504 (2005).
Tweddell, J. S., Pelech, A. N. & Frommelt, P. C. Ventricular septal defect and aortic valve regurgitation: Pathophysiology and indications for surgery. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card Surg. Annu. 147–152 (2006).
Randhawa, A., Gupta, T., Singh, P., Aggarwal, A. & Sahni, D. Description of the aortic root anatomy in relation to transcatheter aortic valve implantation. Cardiovasc. Pathol. 40, 19–23 (2019).
Tretter, J. T. et al. Variations in rotation of the aortic root and membranous septum with implications for transcatheter valve implantation. Heart 104, 999–1005 (2018).
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
F.S.: analyse of echocardiograms and collect data F.E. wrote the main manuscript text All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El Louali, F., Soler, F., Fouilloux, V. et al. Morphological analysis of ventricular septal defect by echocardiography for prediction of aortic regurgitation in pediatric population. Sci Rep 13, 6697 (2023). https://doi.org/10.1038/s41598-023-32940-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-32940-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.