Abstract
This cross-sectional study was designed to assess alterations of choroidal and retinal microvasculature in patients with Heart Failure with Reduced Ejection Fraction (HFrEF) and compare them with a normal age and sex-matched population. Fifty-two eyes of 26 patients with HFrEF (left ventricular ejection fraction [LVEF] < 40%) and 64 eyes of 32 healthy individuals were considered as the patient and the control groups, respectively. We found no statistically significant differences in age-adjusted mean central macular thickness (CMT), superficial or deep retinal capillary plexus vascular densities, and choriocapillaris flow (CC flow) density between the HFrEF group and the normal controls, with the exception of the parafoveal mean superficial capillary plexus vascular density (P = 0.023), which remained statistically significant after adjusting for age (P = 0.034). The patients with HFrEF had a significantly lower subfoveal choroidal thickness (SFCT) than the normal subjects (264 ± 82 vs 313 ± 72; P = 0.009), and the difference was still statistically significant after age adjustment (P = 0.026). Although choroidal vascularity index (CVI) was lower in the HFrEF group than in the control group, the difference was not statistically significant before and after age adjustment (73.45 ± 6.67 vs 75.77 ± 5.92; P = 0.118 and P = 0.096, respectively). In conclusion, in patients with HFrEF, we observed a reduction in parafoveal retinal VD in the superficial capillary plexus, as well as SFCT, but no significant change in CVI, CMT, or CC flow density.
Similar content being viewed by others
Introduction
Heart failure with reduced ejection fraction (HFrEF) is a complicated clinical condition associated with high morbidity and mortality, with a 1-year death rate of 7.2% and a 1-year hospitalization rate of 31.9% in chronic HF patients. In hospitalized patients with acute HF, these rates rise to 17.4% and 43.9%, respectively1.
HFrEF is also accompanied by a systemic response attempting to compensate for the insufficiency2. Multiple investigations have demonstrated a possible association between HFrEF and other peripheral vessels in the human body, including the cerebral, renal, and ocular vasculatures3.
The retina has the highest oxygen consumption per volume in the body, and it is supplied with blood from 2 sources: the inner retina receives capillaries from the central retinal artery through the superficial and deep capillary plexuses, while the outer retina, which is mainly non-vascular, is supplied by the choroid4,5.
The choroid, a richly vascularized structure with the highest blood flow, provides most of the intraocular blood flow and supplies the energy demands of the outer retina and the retinal pigment epithelium6.
The development of enhanced depth imaging optical coherence tomography (EDI-OCT) and optical coherence tomography angiography (OCTA) has enabled sufficient, noninvasive, and repeatable in-depth analyses of retinal and choroidal structures as well as blood flow in comparison with previous imaging techniques such as ultrasonography and fluorescein/indocyanine green angiography7,8.
Accumulating evidence supports the notion that the retinal and choroidal microvasculature can be indicators or potential biomarkers of systemic cardiovascular diseases and the future direction of their severity, as well as a therapeutic index and an emerging endpoint of target organ damage9.
In this cross-sectional study, we aimed to evaluate alterations in central macular and choroidal thickness (CT), the choroidal vascularity index (CVI), and chorioretinal microvascular density using OCTA in patients with HFrEF and then compare them with a normal age-matched population.
Methods
Subjects
This cross-sectional study recruited patients with a diagnosis of HFrEF (left ventricular ejection fraction [LVEF] < 40%) who were referred from the Rajaie Cardiovascular Medical and Research Center to the Farabi Eye Hospital between November 2021 and March 2022. All patients were managed with guideline-directed medical therapy, and all patients had been visited regularly to uptitrate the guideline directed medical therapy to the dose they could tolerate and none was in acute decompensated HF10.
This study was approved by the institutional review board of Iran University of Medical Sciences, Tehran, Iran. The study protocol adhered to the tenets of the Declaration of Helsinki, and all participants gave written informed consent before entering into the study.
An age and sex-matched control group was selected from individuals who underwent routine ocular examinations at our clinic. They all had unremarkable ocular examinations and no history of ocular or cardiac diseases.
Exclusion criteria were the presence of acute HF or any acute cardiac condition (e.g., recent myocardial infarction, recent hospitalization within 4 weeks, any recent dysrhythmia with hospitalization, and a history of pulmonary embolism), a history of retinal vascular diseases including diabetic retinopathy, retinal artery or vein occlusion or insufficiency, myopic retinopathy, retinal dystrophy, glaucoma, uveitis, age-related macular degeneration, any retinal surgery or laser photocoagulation, systemic or ocular diseases that prevented ocular examinations, and severe media opacity, which might affect the image quality. Additionally, patients who had recently used a vasoconstrictive medication or had any associated systemic disorders that may affect the eye (e.g., diabetes, systemic corticosteroid use, and poorly controlled hypertension which defined as systolic blood pressure ≥ 140 mmHg and/or a diastolic blood pressure ≥ 90 mmHg) were also excluded from both the patient and control groups. Patients were included if they had a refractive error between − 6 and + 6 diopter and a best-corrected visual acuity of 20/25 or better.
Clinical parameters analyzed in the patients included LVEF measured in transthoracic echocardiography or cardiac MRI and systolic and diastolic blood pressures. In the HFrEF group, cardiomyopathy was categorized as ischemic and dilated.
The patients underwent thorough ophthalmic examinations, including slit-lamp biomicroscopy and dilated indirect ophthalmoscopy. A masked optometrist measured the best-corrected visual acuity using a Snellen chart, and the results were then converted into the logarithm of the minimum angle of resolution.
Image acquisition and processing
En face OCT-A and EDI-OCT images were obtained from macula using the RTVue XR 100 Avanti instrument (Optovue, Inc, Fremont, CA, USA) for all the patients.
For OCT-A acquisition, a 6 × 6 mm fovea-centered image was obtained from each eye. A quality score of more than 5/10 (according to the built-in RTVue software quality assessment report) was set as the minimum quality requirement, and imaging was repeated until this goal was achieved. Eyes with low image quality or different artifacts, including defocusing, movement, shadow, and decentration, preventing the accurate measurement of vascular density (VD) were excluded. With the aid of the integral module in the Angio Analytics software (version 2017.1.0.151), the different retinal layers were segmented automatically. All automated segmentations of retinal layers in OCTA images were rechecked and segmentation errors were manually corrected by two retina experts (EKP and HRE). The central macular thickness (CMT), densities of the superficial and deep capillary plexuses, and also choriocapillaris flow density (CC flow) were calculated using the built-in software. Retinal and choriocapillaris VD was calculated as a percentage of the area occupied by flowing blood vessels in the selected area of each layer.
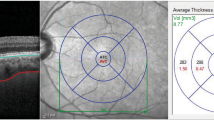
The foveal region was defined as a circle with a 1 mm diameter surrounded by the parafoveal region, defined as a ring with an internal diameter of 1 mm and an external diameter of 3 mm, and the perifoveal region, defined as a ring with an internal diameter of 3 mm and an external diameter of 6 mm (Fig. 1A,B). Measurements are taken in the superficial capillary plexus comprised whole superficial VD, foveal superficial VD, parafoveal superficial VD, and perifoveal superficial VD. Measurements obtained in the deep capillary plexus consisted of whole deep VD, foveal deep VD, parafoveal deep VD, and perifoveal deep VD.
The images depict the density maps of (A) the superficial capillary plexus (SCP) and (B) the deep capillary plexus (DCP). The blue circles over the images are 1-, 3-, and 6-mm circles that represent the foveal, parafoveal, and perifoveal regions, respectively. The tables beside each region show the density measurement in the different sectors of SCP and DCP.
For the measurement of CMT, SFCT, and CVI on EDI-OCT acquisition, the patients were positioned appropriately, and fovea-centered OCT B-scans at 8 mm × 12 mm raster patterns were captured. As the choroidal structures exhibit diurnal variations, all EDI-OCT scans were performed between 8:00 AM and 11:30 AM11.
Only complete, well-centered scans with at least a 6-signal strength value and no motion or blinking artifacts were accepted. After each assessment, 2 masked, independent reviewers assessed the best image on a computer screen. When the 2 graders determined that both the inner and outer borders of the choroid were distinguishable, the image was captured and used for analysis.
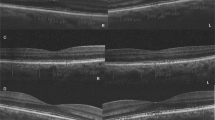
With the use of the caliper function of the AngioVue software the SFCT was measured as previously stated in other research: the vertical distance between the hyper-reflective line of the Bruch membrane and the inner surface of the sclera (Fig. 2A). Multiple studies have demonstrated high degrees of intersystem, interobserver, and intervisit repeatability for SFCT readings beneath the fovea. Every value was measured twice, and the mean was then determined12,13.
(A) A fovea-centered enhanced depth imaging optical coherence tomography image of a patient was used for subfoveal choroidal thickness measurement. With the aid of the caliper function of the AngioVue software, the subfoveal choroidal thickness was measured as the vertical distance between the hyper-reflective line of the Bruch membrane and the inner surface of the sclera. (B) The choroidal vascularity index was measured using the FIJI software. The light pixels in the selected choroidal region were classified as choroidal stroma or interstitial area, whereas the dark pixels were classified as the luminance area. The ratio of the luminance area to the total choroidal area is referred to here as the choroidal vascularity index.
CVI was measured using the method described by Agrawal et al.14 in FIJI (an extended version of the ImageJ software, version 1.51 h; National Institutes of Health, Bethesda, Maryland; accessible at http://imagej.nih.gov/Fiji/). Further, the total choroidal area, the luminance area, and the stromal area were determined (Supplement 1). The ratio of the luminance area to the total choroidal area is referred to here as CVI (Fig. 2B).
All manual segmentations, including the delineation of the sclerochoroidal junction and the measurement of CT, were conducted by a skilled grader (ZM) and rechecked by another independent grader (EKH). In the case of any dispute, the outlines were segmented by consensus.
The inter-rater reliability of the CMT, SFCT, and CVI measurements was evaluated using the absolute agreement model of the interclass correlation coefficient on 20 EDI-OCT images, initially segmented by the 2 independent graders15.
Statistical analysis
Quantitative data are presented as the mean ± the standard deviation and qualitative data as counts and percentages. The parameters were compared between the 2 groups using the t test and the χ2 test, whenever appropriate. In addition, the generalized estimating equation was employed to consider the probable correlation of the measurements between the 2 eyes of a subject16,17,18. Another generalized estimating equation analysis was applied to adjust for the possible confounders. All the statistical analyses were performed using the SPSS software (IBM Corp, released 2019; IBM SPSS Statistics for Windows, version 25.0. Armonk, NY: IBM Corp). A P value of less than 0.05 was considered statistically significant.
Results
The current study included 52 eyes of 26 patients with HFrEF as the patient group and 64 eyes of 32 healthy individuals as the control group. The demographic and clinical characteristics of both groups are presented in Table 1. There were no statistical differences between the 2 groups in terms of their mean age, sex, best-corrected visual acuity, spherical refraction, and systolic and diastolic pressure at the time of examination. The mean LVEF in the HFrEF group and the control group was 25 ± 13 and 53 ± 11%, respectively (P = 0.021). None of the patients in the control group had symptoms relevant to a diagnosis of HF with preserved EF.
The whole image age-adjusted mean retinal superficial and deep vascular plexus densities in the HFrEF patients was lower than that of the normal control group although was not statistically significant (47.6 ± 3.6 vs 49.7 ± 3.6; P = 0.162 and 47.9 ± 6 vs 49.4 ± 6.3; P = 0.374, respectively). Also, foveal and perifoveal age-adjusted mean superficial and deep capillary plexus densities did not show statistically significant differences between the HFrEF group and the normal controls (P = 0.650, P = 0.220, P = 0.147, P = 0.361, respectively).
Parafoveal mean superficial capillary plexus densities showed statistically significant differences between the HFrEF group and the normal controls (47.7 ± 7.6 vs 51.1 ± 5.1; P = 0.023), and the difference remained statistically significant after adjusting for age (P = 0.034). But, parafoveal age-adjusted mean deep capillary plexus densities did not show statistically significant differences between the HFrEF group and the normal controls (52.4 ± 4.6 vs 53.4 ± 5.7; P = 0.499). The mean superficial and deep retinal vessel densities of both groups are shown in Table 2 and Fig. 3.
Optical coherence tomography angiography (OCTA) maps of the superficial (A), deep retinal capillary plexus (B), and choriocapillaris flow (C) in a 30-year-old healthy participant included in the current investigation. Corresponding cross-sectional macular optical coherence tomography (OCT) was utilized for the measurement of subfoveal choroidal thickness (SFCT) (D) and choroidal vascular index (CVI) (E). OCTA maps of the superficial (F), deep retinal capillary plexus (G), and choriocapillaris flow (H) in a 41-year-old patient with Heart Failure with Reduced Ejection Fraction (HFrEF) included in the current study. The patient's corresponding cross-sectional macular optical coherence tomography (OCT) is used to calculate subfoveal choroidal thickness (SFCT) (H) and choroidal vascular index (CVI) (J).
The inter-class correlation coefficients for SFCT and CVI measurements were 0.98 (95% CI 0.97–0.99) and 0.96 (95% CI 0.91–0.98), respectively. The mean central macular thickness (CMT), Choriocapillaris flow density (CC flow), SFCT, and CVI of both groups are shown in Table 3. Age and sex-adjusted central macular thickness (CMT) was not statistically significant between the patients with HFrEF and normal subjects (256 ± 23 vs 253 ± 16; P = 0.592). Also, age and sex-adjusted Choriocapillaris flow (CC flow) was not statistically significant between the patients with HFrEF and normal subjects (0.65 ± 0.05 vs 0.65 ± 0.04; P = 0.660). The patients with HFrEF had a significantly lower SFCT than the normal subjects (264 ± 82 vs 313 ± 72; P = 0.009), and the difference was still statistically significant after age adjustment (P = 0.026). Although CVI was lower in the HFrEF group than in the control group, the difference was not statistically significant before and after age adjustment (73.45 ± 6.67 vs 75.77 ± 5.92; P = 0.118 and P = 0.096, respectively) (Fig. 3).
In the patient group, SFCT was not correlated with LVEF, systolic blood pressure, and diastolic blood pressure (P = 0.845, P = 0.930, and P = 0.123, respectively). In the patient group also, CVI was not associated with LVEF, systolic blood pressure, and diastolic blood pressure (P = 0.430, P = 0.413, and P = 0.776, respectively). Also in the normal group, we could not find any correlation between SFCT or CVI and cardiovascular factors like LVEF, systolic blood pressure, and diastolic blood pressure. The analyses also showed no statistical differences in relation to the type of cardiomyopathy in the HFrEF group (P = 0.063). Subgroup analysis based on the type of cardiomyopathy (ischemic or dilated) revealed no statistically significant differences between the two groups in terms of microvascular and structural characteristics of the retina and choroid (P > 0.05 for all).
Discussion
Although numerous clinical markers and risk factors have been identified, novel clinical markers and approaches are required to assess patient risk stratification with HFrEF. Relying solely on retinal vasculature as a biomarker can complicate the evaluation of patients with HF. On the other hand, the ability to examine the choroidal layers with swept source or EDI-OCT offers new research opportunities for patients with HFrEF and the ischemic type of HFrEF, which is the leading cause of LV dysfunction in adults worldwide19,20,21,22.
SFCT is commonly used in research trials. Still, it reflects only the whole choroidal structure and may be affected by various factors such as age, axial length, intraocular pressure, and systolic blood pressure23,24,25. It also fails to distinguish vascular from stromal components26. Therefore, since 2014, the focus of investigations has been switched to finding a method for choroidal structure analysis with a better distinction between the lumen and the stroma27. The term “CVI” was introduced as the ratio of the luminance area to the total choroidal area and was reported to be around 65% in healthy eyes14. It has been proved that CVI is a more informative, reliable, and steady index of proportional change in the choroidal vasculature as it is relatively resistant to changes in other physiological parameters14,28.
Similar to other organs, the choroid could be susceptible to any chronic cardiac processes. Extreme hypertensive retinopathy is associated with hypertensive choroidopathy and choroidal thickening. Hypercholesterolemia is linked to choroidal thickening, whereas smoking, ocular ischemia syndrome, and systemic hypertension are linked to a thinner choroid. Alternatively, data addressing the association between CT and carotid artery stenosis and diabetes mellitus are conflicting9,29,30,31,32,33.
Comparing 56 eyes of 56 patients with chronic HF with 56 eyes of 56 individuals of the same sex, Altinkaynak et al.34 discovered that the mean SFCT value was statistically considerably lower in patients with chronic HF, and it was positively correlated with EF. They hypothesized that a low cardiac output might lead to vasoconstriction development in peripheral vessels like orbital and choroidal arteries to maintain critical organs’ blood supply, causing choroidal ischemia and retinal pigment epithelium atrophy. Rakusiewicz et al.21 discovered that children with congestive HF due to dilated cardiomyopathy had a thinner CT at all measured locations. They found that chronic HF impacted CT and that this parameter might be useful for monitoring the clinical progression of dilated cardiomyopathy in children. Nonetheless, another study by Alur et al.35 found neither a significant difference in SFCT between patients and controls nor a correlation between SFCT and EF. Although there is no clear explanation for the divergent results, they may be explained by the wide range of EF, dissimilar treatments received by each study’s patients, and the impact of diuretics and angiotensin-converting enzyme inhibitors on SFCT29.
HFrEF is accompanied by peripheral vasoconstriction, which ensures appropriate perfusion and oxygenation to the heart, brain, and other vital tissues3,36. It is suggested that this compensatory vasoconstriction affects the choroidal arteries and, thus, lowers SFCT34. When choroidal vasoconstriction persists, the ensuing chronic ischemia may result in the atrophy of the retinal pigmented epithelium, which exacerbates SFCT reduction2,29,32,33. Consequently, as shown in the current study, HFrEF can be represented in choroidal thinning, and HF can be reflected in the thinning of the choroid.
Regardless of SFCT abnormalities in individuals with HFrEF, it is unclear which component of the choroid (the stroma or the lumen of the choroidal vessels) is most affected by HFrEF. To our knowledge, the present study is the first to evaluate CVI in the setting of HFrEF. We demonstrated that CVI was decreased in the HFrEF group, but the association was not statistically significant before and after age adjustment (P = 0.118 and P = 0.098, respectively), suggesting that diminishing CT in HFrEF can be associated with the shrinkage of both components of the choroid (i.e., the stroma and the lumen).
Any defect or disruption in the choroid or its blood flow may result in degenerative changes and neovascularization25. In contrast to the retinal vasculature, which utilizes autoregulation to maintain relatively constant blood flow despite variations in ocular perfusion pressure, the regulation of blood flow in the choroid is complex37. It is now well established that the choroid is not a passive vascular bed in which a decrease in perfusion pressure leads to a linear decrease in blood flow6,37,38. The choroid has neural control and is supplied by sympathetic and parasympathetic nerve fibers, which may help the choroid to compensate for blood pressure alterations through vasoconstriction or dilation39,40. There is also a compensatory myogenic mechanism in the choroid: smaller resistance arteries can change smooth muscle contraction and tone to maintain vessel wall stretch in the desired range40. It seems that the choroid has a weak but significant ability to autoregulate its blood flow in response to changes in ocular perfusion pressure41.
Previous studies have reported that patients with HF have reduced retinal vessel density compared with the normal population42. It has been hypothesized that the mentioned peripheral vasoconstriction in response to a low cardiac output is the pathophysiological mechanism that triggers this finding42,43,44. Therefore, retinal vessel density as measured by OCTA may provide an insight into the global microperfusion and hemodynamic state of patients with chronic HF22,45. In the current study, except for the parafoveal mean retinal superficial capillary plexus vascular density, we found no statistically significant differences in age-adjusted mean central macular thickness (CMT), superficial or deep retinal capillary plexus vascular densities and choriocapillaris flow density (CC flow) between the HFrEF group and the normal controls. Prior to the current investigation, very few OCTA evaluations of choroidal or retinal superficial and deep capillary plexus densities in patients with heart failure have been conducted. Rakusiewicz et al. found that children with congestive HF due to dilated cardiomyopathy had reduced superficial and deep capillary plexus densities at all assessed locations. They discovered that chronic HF affected retinal vascular density and those retinal vascular parameters may be relevant for monitoring the clinical course of pediatric dilated cardiomyopathy22. Although there is no clear explanation for the discrepancies between our results and those of other studies, they may be due to the wide range of EF and age of patients, the different treatments received by the patients in each study, and the effect of diuretics and angiotensin-converting enzyme inhibitors on retinal vascular density22,46. Since the blood supply to the retina is physiologically kept constant by specific autoregulation mechanisms, such a decrease in parafoveal superficial VD in patients with chronic HF can also be justified by insufficiency to autoregulate the retinal blood supply in these patients22.
The main advantage of our study is its use of a noninvasive technology that enables the reproducible and quantitative evaluation of abnormalities in the retinal and choroidal microvasculature in a homogeneous group of patients with HFrEF. This study does come, however, with several limitations. Firstly, the relatively small sample size might affect the results. Secondly, the cross-sectional design of the current study precluded longitudinal change assessment in the retinal and choroidal vasculatures. Thirdly, the study was conducted in a tertiary center, which may have led to the recruitment of more chronic or complicated patients and, thus, selection bias. Fourthly, the duration of chronic HF was not specified, which might have affected the results. Finally, the influence of individually started HF treatment on the obtained results concerning retinal VD and the choroidal vasculature should not be ruled out.
We believe that our findings demonstrate a new multidisciplinary pathway in the diagnosis and management of HFrEF, which is a syndrome affecting literally every organ in the human body. Accordingly, we advocate retinal and choroidal vasculature examination as a promising endpoint in future studies.
In conclusion, we demonstrated a reduction in parafoveal superficial retinal VD as well as SFCT in patients with HFrEF, although CMT and CVI were not changed significantly. Further long-term observations of larger numbers of patients will determine whether these biomarkers may be useful in clinical practice in patients with HFrEF.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Murphy, S. P., Ibrahim, N. E. & Januzzi, J. L. J. Heart failure with reduced ejection fraction: A review. JAMA 324, 488–504 (2020).
Figueroa, M. S. & Peters, J. I. Congestive heart failure: Diagnosis, pathophysiology, therapy, and implications for respiratory care. Respir. Care 51, 403–412 (2006).
Rabina, G. et al. Carotid artery endarterectomy effect on choroidal thickness: One-year follow-up. J. Ophthalmol. 20, 18 (2018).
Osborne, N. N. et al. Retinal ischemia: Mechanisms of damage and potential therapeutic strategies. Prog. Retin. Eye Res. 23, 91–147 (2004).
Flammer, J. et al. The eye and the heart. Eur. Heart J. 34, 1270–1278 (2013).
Nickla, D. L. & Wallman, J. The multifunctional choroid. Prog. Retin. Eye Res. 29, 144–168. https://doi.org/10.1016/j.preteyeres.2009.12.002 (2010).
Iovino, C. et al. Choroidal vascularity index: An in-depth analysis of this novel optical coherence tomography parameter. J. Clin. Med. 9, 595 (2020).
Spaide, R. F., Fujimoto, J. G., Waheed, N. K., Sadda, S. R. & Staurenghi, G. Optical coherence tomography angiography. Prog. Retin. Eye Res. 64, 1–55 (2018).
Alur, İ. Evaluation of retinal vessel caliber, choroidal thickness, and ocular perfusion pressure in patients with low cardiac ejection fraction. Cardiovasc. Surg. Interventions 6, 1–6 (2019).
Heidenreich, P. A. et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 145, e895–e1032 (2022).
Singh, S. R. et al. Diurnal variation in subfoveal and peripapillary choroidal vascularity index in healthy eyes. Indian J. Ophthalmol. 67, 25 (2019).
Mrejen, S. & Spaide, R. F. Optical coherence tomography: Imaging of the choroid and beyond. Surv. Ophthalmol. 58, 387–429 (2013).
Shao, L. et al. Reproducibility of subfoveal choroidal thickness measurements with enhanced depth imaging by spectral-domain optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 54, 230–233. https://doi.org/10.1167/iovs.12-10351 (2013).
Agrawal, R. et al. Choroidal vascularity index as a measure of vascular status of the choroid: Measurements in healthy eyes from a population-based study. Sci. Rep. 6, 21090 (2016).
Faghihi, H. et al. Choroidal features in flat irregular pigment epithelial detachment associated with Chronic central serous chorioretinopathy: Avascular versus vascularized. PLoS One 16, e0257763 (2021).
Fan, Q., Teo, Y. Y. & Saw, S. M. Application of advanced statistics in ophthalmology. Invest. Ophthalmol. Vis. Sci. 52, 6059–6065 (2011).
Murdoch, I. E., Morris, S. S. & Cousens, S. N. People and eyes: Statistical approaches in ophthalmology. Br. J. Ophthalmol. 82, 971–973 (1998).
Cheng, C. Y., Liu, J. H., Chiang, S. C., Chen, S. J. & Hsu, W. M. Statistics in ophthalmic research: Two eyes, one eye or the mean?. Zhonghua Yi Xue Za Zhi (Taipei) 63, 885–892 (2000).
Seo, W. W., Yoo, H. S., Kim, Y. D., Park, S. P. & Kim, Y. K. Choroidal vascularity index of patients with coronary artery disease. Sci. Rep. 12, 1–8 (2022).
Kwon, D. H., Kim, Y. C. & Kang, K. T. Clinical significance of choroidal thickness in eyes with ocular ischemic syndrome. Korean J. Ophthalmol. 36, 66–73 (2022).
Rakusiewicz, K., Kanigowska, K., Hautz, W. & Ziółkowska, L. Choroidal thickness changes in children with chronic heart failure due to dilated cardiomyopathy. Int. Ophthalmol. 41, 2167–2177 (2021).
Rakusiewicz, K., Kanigowska, K., Hautz, W. & Ziółkowska, L. The impact of chronic heart failure on retinal vessel density assessed by optical coherence tomography angiography in children with dilated cardiomyopathy. J. Clin. Med. 10, 25 (2021).
Sezer, T., Altınışık, M., Koytak, İA. & Özdemir, M. H. The choroid and optical coherence tomography. Turk. J. Ophthalmol. 46, 30–37 (2016).
Agrawal, R. et al. Exploring choroidal angioarchitecture in health and disease using choroidal vascularity index. Prog. Retin. Eye Res. 77, 100829 (2020).
Tan, K.-A. et al. State of science: Choroidal thickness and systemic health. Surv. Ophthalmol. 61, 566–581 (2016).
Laviers, H. & Zambarakji, H. Enhanced depth imaging-OCT of the choroid: A review of the current literature. Gr. Arch. Clin. Exp. Ophthalmol. 252, 1871–1883 (2014).
Sonoda, S. et al. Luminal and stromal areas of choroid determined by binarization method of optical coherence tomographic images. Am. J. Ophthalmol. 159, 1123-1131.e1 (2015).
Breher, K., Terry, L., Bower, T. & Wahl, S. Choroidal biomarkers: A repeatability and topographical comparison of choroidal thickness and choroidal vascularity index in healthy eyes. Transl. Vis. Sci. Technol. 9, 8 (2020).
Yeung, S. C., You, Y., Howe, K. L. & Yan, P. Choroidal thickness in patients with cardiovascular disease: A review. Surv. Ophthalmol. 65, 473–486 (2020).
AkcaBayar, S. et al. Structural analysis of the retina and choroid before and after carotid artery surgery. Curr. Eye Res. 45, 496–503 (2020).
Wang, J. et al. Retinal and choroidal vascular changes in coronary heart disease: An optical coherence tomography angiography study. Biomed. Opt. Express. 10, 1532 (2019).
Ahmad, M., Kaszubski, P. A., Cobbs, L., Reynolds, H. & Smith, R. T. Choroidal thickness in patients with coronary artery disease. PLoS One 12, 1–12 (2017).
Hondur, A. M., Ertop, M., Topal, S., Sezenoz, B. & Tezel, T. H. Optical coherence tomography and angiography of choroidal vascular changes in congestive heart failure (2020).
Altinkaynak, H. et al. Subfoveal choroidal thickness in patients with chronic heart failure analyzed by spectral-domain optical coherence tomography. Curr. Eye Res. 39, 1123–1128 (2014).
Alur, İ. Evaluation of retinal vessel caliber, choroidal thickness, and ocular perfusion pressure in patients with low cardiac ejection fraction. Cardiovasc. Surg. Interv. 6, 1–6 (2019).
Zelis, R., Sinoway, L. I., Musch, T. I., Davis, D. & Just, H. Regional blood flow in congestive heart failure: Concept of compensatory mechanisms with short and long time constants. Am. J. Cardiol. 62, 2–8 (1988).
Schmidl, D. et al. Factors associated with choroidal blood flow regulation in healthy young subjects. Invest. Ophthalmol. Vis. Sci. 57, 5705–5713 (2016).
Akahori, T., Iwase, T., Yamamoto, K., Ra, E. & Terasaki, H. Changes in choroidal blood flow and morphology in response to increase in intraocular pressure. Invest. Ophthalmol. Vis. Sci. 58, 5076–5085 (2017).
Reiner, A., Fitzgerald, M. E. C., Del Mar, N. & Li, C. Neural control of choroidal blood flow. Prog. Retin. Eye Res. 64, 96–130 (2018).
Spaide, R. F. Choroidal blood flow: Review and potential explanation for the choroidal venous anatomy including the vortex vein system. Retina 40, 1851–1864 (2020).
Polska, E. et al. Regulation of choroidal blood flow during combined changes in intraocular pressure and arterial blood pressure. Invest. Ophthalmol. Vis. Sci. 48, 3768–3774 (2007).
Nägele, M. P. et al. Retinal microvascular dysfunction in heart failure. Eur. Heart J. 39, 47–56 (2018).
McGeechan, K. et al. Meta-analysis: Retinal vessel caliber and risk for coronary heart disease. Ann. Intern. Med. 151, 404–413 (2009).
Li, C. et al. Retinal microvasculature impairment in patients with congenital heart disease investigated by optical coherence tomography angiography. Clin. Exp. Ophthalmol. 48, 1219–1228 (2020).
Alnawaiseh, M. et al. Ocular perfusion in patients with reduced left ventricular ejection fraction measured by optical coherence tomography angiography. Gr. Arch. Clin. Exp. Ophthalmol. 259, 3605–3611 (2021).
Arnould, L. et al. The EYE-MI pilot study: A prospective acute coronary syndrome cohort evaluated with retinal optical coherence tomography angiography. Invest. Ophthalmol. Vis. Sci. 59, 4299–4306 (2018).
Author information
Authors and Affiliations
Contributions
E.K., Z.M., N.M., H.F., A.F. and E.K.P. performed concept, methodology, and drafted the manuscript. N.N., M.M.B., P.S., M.M., S.S. and H.R.E. edited and revised the manuscript. Z.M., N.M., H.F., A.F. and H.R.E. analyzed statistics. All authors contributed to the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khalilipur, E., Mahdizad, Z., Molazadeh, N. et al. Microvascular and structural analysis of the retina and choroid in heart failure patients with reduced ejection fraction. Sci Rep 13, 5467 (2023). https://doi.org/10.1038/s41598-023-32751-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-32751-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.