Abstract
Atmospheric particulate matter (PM2.5) can damage human health. Biogenic organic compounds emitted from trees may increase the concentration of PM2.5 via formation of secondary aerosols. Therefore, the role of biogenic emissions in PM2.5 formation and the sources of PM2.5 need to be investigated. Dual carbon isotope and levoglucosan analyses are powerful tools to track the sources of total carbon (TC) in PM2.5. We collected a total of 47 PM2.5 samples from 2019 to 2020 inside a pine forest and in urban areas in South Korea. The average δ13C and Δ14C of TC in PM2.5 at the Taehwa Research Forest (TRF) were − 25.7 and − 380.7‰, respectively, which were not significantly different from those collected at Seoul National University (SNU) in urban areas. Contribution of fossil fuel, C3-, and C4- plants to carbonaceous component of PM2.5 were 52, 27, and 21% at SNU, whereas those were 46, 35, and 19% at TRF, respectively. The biomass burning tracer, levoglucosan, was most abundant in winter and correlated with the contribution of C4 plants derived carbon. Results indicate that biogenic aerosols emitted from trees is less likely to be an important source of PM2.5 and that trees can act as a bio-filter to reduce PM2.5.
Similar content being viewed by others
Introduction
Pollution due to fine particulate matter with a diameter of 2.5 μm or less (PM2.5) is a significant challenge, which affects human health and ecosystem, visibility, and climate change1,2. The mean annual concentrations of PM2.5 ranged from 30.6 to 37.2 μg m−3 between 2013 and 2017 in Seoul, the capital of South Korea3, which still exceed the regulatory standard of 15 μg m−3 specified by the Korean government. Higher concentrations of PM2.5 are frequently observed4,5.
Trees can remove atmospheric PM2.5 by directly adsorbing PM2.5 on the surface of leaves and branches, and by absorbing some of the PM2.5 through the stomata6,7. The mean annual PM2.5 levels removed by trees in the urban areas are estimated at 0.27 and 0.15 g m−2 in the conterminous United States and in 86 Canadian cities, respectively6,8. In addition, plants alter the local microclimate conditions by reducing air temperature and increasing relative humidity via canopy transpiration, thus increasing the deposition of PM onto leaves9. Therefore, this process can reduce the concentration of PM2.5 in urban areas6,9,10. For example, in central Sydney, Australia, the PM2.5 concentrations were lower in urban areas with a relatively higher density of green space10. Therefore, urban forest expansion has been regarded as one of the government policies to reduce PM2.5 concentrations in many countries11. South Korea is no exception, and the Korea Forest Service has proposed an increase in urban green space to 15 m2 per capita from the current 9.9 m2 per capita by 202712.
However, trees indirectly increase PM2.5 concentrations. Trees emit biogenic volatile organic compounds (BVOCs) such as isoprene and terpenes as well as alcohols, carbonyls, and acids13. The oxidation products of these BVOCs can form and grow biogenic secondary organic aerosols (SOAs)14. SOAs constitute up to 85% of organic carbon and ~ 35% of PM2.515. SOAs may contribute to the formation of carbonaceous materials in fine particles in the presence of elevated levels of anthropogenic emissions of NOx and oxidants (OH radicals and ozone). For example, the formation of biogenic SOAs can be enhanced by 60–200% due to the emission of NOx and oxidants16. The mass concentration of submicron particles is also increased by 25–200% at polluted sites downwind of Manaus, Brazil17. Furthermore, studies using radiocarbon (14C) reported that a high biogenic fraction can contribute to the formation of carbonaceous component of PM2.5 in cities, accounting for up to 80% of the aerosol carbon18,19,20.
These findings bring a question on how much biogenic emissions contribute to the formation of PM2.5 in the atmosphere and whether the sources of PM2.5 in forests are different from urban areas. 14C is a powerful tracer of the carbon cycle, which separates fossil-fuel-derived carbon from recently photosynthesized carbon. However, the 14C analysis cannot distinguish if the sources of carbon in PM2.5 are generated from biogenic emissions or biomass burning because both are derived from recently photosynthesized carbon21,22. Thus, a biomarker is needed to further identify the sources of biogenic PM2.5. Levoglucosan has been widely used to trace the pyrolytic emissions of PM2.5 released by biomass burning23,24,25,26. In addition to radiocarbon and levoglucosan analyses, the stable carbon isotope ratio (δ13C) can provide further information on the sources of carbon, i.e., C3 and C4 plants, because of their distinct isotopic fractionation27,28. C3 plants (e.g., most trees) incorporate CO2 into the three-carbon compound, 3-phosphoglyceric acid, during the first stage of photosynthesis, whereas C4 plants (e.g., maize and sorghum) initially fix CO2 in the mesophyll cell as a four-carbon compound, oxaloacetate, which results in distinctive δ13C29,30.
The objectives of this study are (1) to investigate the temporal variation in the concentrations of total carbon (TC) including organic and elemental carbon in PM2.5, (2) to compare carbon isotope ratios (δ13C and Δ14C) between a pine forest and urban area, and (3) to track the sources of TC in PM2.5 by analyzing δ13C, Δ14C, and levoglucosan concentrations.
Methods and materials
Sampling sites and collection of PM2.5
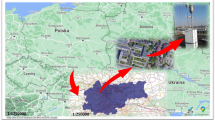
Samples were collected from four sites (Fig. 1a,b) as follows:
-
1.
A Korean pine (Pinus koraiensis) forest located at the Taehwa Research Forest (TRF) (37°30.5ʹN, 127°31.6ʹE, Fig. 1c), where pines were planted in 1960s31. TRF is a part of Seoul National University Forests, and is located in the suburban area32.
-
2.
The rooftop of a building located in the Seoul National University (SNU) campus (37°45.8ʹN, 126°95.0ʹE, Fig. 1d) in the southern part of Seoul (~ 10 million population), the capital of South Korea.
-
3.
A pine forest at the Hongneung Urban Forest (HUF) near Mt. Cheonjang (37°59.6ʹN, 127°04.5ʹE, Fig. 1e) in Seoul, which is a fragmented forest surrounded by urban residential area. Dominant species include Pinus densiflora and P. koraiensis.
-
4.
A green space with pine trees located at Cheongnyangni Traffic Island (CRI) (37°58.0ʹN, 127°04.5ʹE, Fig. 1f) in Seoul, which is a small triangle area (0.06 ha) between roads.
(a) The location of study sites in South Korea, (b) PM2.5 sampling points for high-volume air sampler (yellow circles) and the national PM2.5 monitoring sites (blue triangles). The high-volume air sampler was set at (c) the Taehwa Research Forest (TRF), (d) the Seoul National University (SNU), (e) the Hongneung urban forest (HUF) and (f) Cheongnyangni traffic island (CRI). The map of East Asia in (a) was created by MeteoInfo 3.5.5 (http://www.meteothink.org) and the satellite imagery in (b) was created on the Vworld platform (http://map.vworld.kr) provided by the Korean Ministry of Land, Infrastructure and Transport.
According to the Food and Agriculture Organization of the United Nations33, forest is defined as “land spanning more than 0.5 hectares with trees higher than 5 m and a canopy cover of more than 10 percent, or trees able to reach these thresholds in situ”. In that sense, TRF is a pine forest in rural area. HUF is also a pine forest, but located in urban area. CRI is not a forest but a simple green space with pine trees near busy traffic roads in urban area. SNU is in the urban area. The population size and density are often used to define a place as ‘urban’. Seoul is one of the largest metropolitan areas (~ 10 million population) in the world.
A high-volume air sampler (HVAS; HV-1700RW, Sibata, Tokyo, Japan) was used to collect the PM2.5 on quartz filters at a flow rate of 1000 L min−123,28,34,35. An HVAS was placed on the rooftop of a building at SNU, and the other HVAS was installed inside the forests or green space. The high-volume air samplers were automatically calibrated by a built-in flowmeter, temperature sensor, and atmospheric pressure sensor. No plant parts were sampled in this study. Between 2019 and 2020, a total of 47 samples were collected with a time resolution of 24–72 h (usually 24 h). To collect PM2.5 in the atmosphere, the quartz filters were pre-baked at 400 °C for 4 h prior to use for removing the organic contaminants. Quartz filters are widely used to measure carbonaceous aerosols23,28,34,35 because their composition is carbon-free, and can remain stable at temperatures up to 900 °C for carbon isotope analysis. However, the quartz filters were not used to measure the mass of PM2.5 because the filter size is too large (203 mm by 254 mm) to be precisely weighed on an analytical balance. Instead, beta attenuation mass monitors (BAM) are widely used to measure the concentration of PM2.5 indirectly. Both methods complement each other, BAM for relatively simple estimation of PM2.5 concentration and HVAS for isotopic analysis. Similar patterns were observed between PM2.5 by BAM and TC in PM2.5 by HVAS (Fig. 3a–c), but they were not used for cross-calibration.
Most of the samples were collected at TRF (n = 21) and SNU (n = 18) to compare the sources of carbonaceous component of PM2.5 between forest and urban areas (Table 1). A total of 11 samples were simultaneously collected at both TRF and SNU. The additional samples were collected inside the green space or forests at CRI (n = 6) and HUF (n = 2), respectively, in the urban areas during fall and winter (Table 1). After sampling, filters were wrapped in pre-baked aluminum foil and stored at − 20 °C. Field blank filters were also prepared by placing the filter in the sampler for 24 to 72 h without gas flow, and then stored as described above. The mass of carbon on the field blank filters was negligible compared with that of collected PM2.5.
PM2.5 monitoring data in South Korea
The AirKorea website (http://www.airkorea.or.kr) provides nationwide PM2.5 data collected from outdoor monitoring sites via β-ray absorption. The method is used to estimate the concentration of PM2.5 based on the relationship between attenuation of beta radiation and PM2.5 deposited on a glass fiber filter tape within an instrument. We downloaded the daily PM2.5 concentration data of the three national monitoring sites (Fig. 1b) from the AirKorea website. One of the monitoring sites for both HUF and CRI was located next to the road, and the monitoring sites for TRF and SNU, respectively, were located at the rooftop of each Community Center. The monitoring site for TRF was located outside the forest, 6.6 km apart from TRF in the rural area, whereas the other monitoring sites were located within 2.4 km of our sampling sites in the urban area. All three national monitoring sites are located outside forests, providing information on PM2.5 concentration.
Based on the daily PM2.5 data reported by the AirKorea, the sampling dates for HVAS were chosen when the concentration of PM2.5 was higher than ~ 15 μg m−3, which is the air quality guideline of PM2.5. The HVAS was also used in order to collect enough amount of carbon for isotopic analysis which was > ~ 1 mg-C.
Dual carbon isotope analysis
The PM2.5 samples were acidified with 10% HCl to remove inorganic carbon, and then dried at 50 °C36,37,38. Each dried filter was transferred to a pre-burned quartz tube containing CuO as an oxidant and silver wire. The quartz tube was evacuated, flame-sealed, and heated at 850 °C for 4 h to oxidize total carbon (TC) including organic and elemental carbon. The resulting CO2 in the quartz tube was sent to the national ocean sciences accelerator mass spectrometry facility (https://www2.whoi.edu/site/nosams/) to measure dual carbon isotope ratios (δ13C and Δ14C) of TC in PM2.5 via accelerator mass spectrometry. The IAEA-C8 oxalic acid was routinely used as a reference standard material, and its δ13C and 14C activity was measured within the recommended range (https://nucleus.iaea.org/sites/ReferenceMaterials/Pages/IAEA-C-8.aspx). To our knowledge, this is the first study reporting δ13C and Δ14C of PM2.5 over an entire year in South Korea.
Source apportionment of TC in PM2.5 using IsoSource and Bayesian statistics
First, the sources of TC in PM2.5 were separated into fossil fuel and non-fossil fuel sources based on radiocarbon results38. The contribution of each non-fossil fuel source (i.e., C3 and C4 plants) was further quantified by incorporating the results of stable carbon isotope analysis using IsoSource and Bayesian mixing models. Three endmembers were used: fossil fuel, C3 plants, and C4 plants (Fig. 2). The δ13C of the three endmembers was set to − 29.0 ± 1.3‰ for fossil fuel, − 26.7 ± 1.8‰ for C3 plants, and − 16.4 ± 1.4‰ for C4 plants27,39. The Δ14C of carbon derived from fossil fuel was set to − 1000‰, while the Δ14C values of C3 and C4 plants were set to 30‰36, similar to the Δ14C-CO2 in the troposphere because plants absorb atmospheric CO2 during photosynthesis. Both the δ13C and Δ14C results were incorporated to draw quantitative results of carbon sources. The dual isotopic mixing model is commonly used in ecological studies to determine the proportions of various sources in a mixture28,39,40.
IsoSource, a popular mixing model, was used to quantify TC in PM2.5 sources into fossil fuels, C3 plants, and C4 plants (https://www.epa.gov/eco-research/stable-isotope-mixing-models-estimating-source-proportions)41. All possible combinations of source proportions were calculated with IsoSource using a 0.1‰ tolerance. The radiocarbon results were used as additional constraints to further reduce the range of contributions from the fossil fuel sources. The IsoSource results were used as prior information via Bayesian inferences using Markov Chain Monte Carlo (MCMC) method (Figs. S1 and S2 in Supplementary Information). In this MCMC model, the sources were also separated into fossil fuels, C3 plants, and C4 plants. To estimate seasonal source contributions to TC in PM2.5, we combined all the data points from each season in the MCMC calculation. Annual source apportionment was conducted by combining all the data points at each site. MCMC was implemented using the simmr-0.4.5 (https://cran.r-project.org/web/packages/simmr/index.html) in R-4.1.142.
Levoglucosan analysis
The dual carbon isotope analysis does not distinguish if the sources of carbon in PM2.5 are generated from biogenic emissions or biomass burning. In order to investigate the possibility of the biomass burning as a source of carbon in PM2.5, levoglucosan analysis was employed to complement carbon isotope analysis23,24,25,26. The levoglucosan concentration, which is a biomarker of biomass burning, was analyzed23,24,25,26. The samples were sonicated with dichloromethane: methanol (3:1, v/v) at 20 °C for 1 h. The extract was concentrated using nitrogen gas in TurbovapII (Zymark Co., USA) and filtered using a 0.45-μm PTFE syringe filter (Pall Corporation, USA). After filtration, the extract was reconcentrated to a final volume of 1 mL using Turbovap II and Reacti-Therm (Thermo Scientific, TS-18822, USA) and stored in a freezer until analysis.
Derivatization via silylation was conducted to analyze polar compounds. A 50 μL aliquot of the final extract volume was completely dried by gently blowing nitrogen gas, followed by reaction with 50 μL of N, O-bis-(trimethylsilyl) trifluoroacetamide (BSTFA) combined with 1% trimethylchlorosilane (TMCS) (Sigma Aldrich, USA) and 50 μL of pyridine (HPLC grade, Sigma Aldrich, USA) at 75 ℃ for 90 min. After derivatization, the concentration of levoglucosan was determined using a GC/MS (7890B/5977B, Agilent, USA) operating at an ionization energy of 70 eV in EI mode.
Statistical analysis
Differences in PM2.5 concentrations and carbon isotope signatures among sites and seasons were analyzed via one-way analysis of variance (ANOVA), followed by Tukey’s HSD (honest significant difference) test. Type II error (i.e., false negative) can occur when the sample size is too small. The number of samples per season ranged from 2 to 7 in this study, making the statistical analysis on them prone to type II error. Although we fully recognize the limitations of the analysis due to the relatively small number of samples per season, we still have attempted to provide seasonal information of TC in PM2.5 as Korea is located in the Asian monsoon climate, where the highest PM2.5 concentrations were reported in winter and decreased to a minimum in summer3. A linear regression analysis was used to assess the relationship between levoglucosan concentration and fractional contribution of carbon derived from C3 or C4 plants to PM2.5. The analyses were conducted using R-4.1.142.
Results
δ13C and Δ14C of TC in PM2.5
Both δ13C and Δ14C values of TRF were not significantly different from those of SNU on the same dates (n = 11) (Fig. 2 and Table 1). In contrast, the Δ14C values of CRI were significantly lower than those of TRF on the same collection dates in fall and winter (n = 5 each), with the mean Δ14C values of CRI and TRF at − 444.3‰ and − 380.7‰, respectively (Table 1). The δ13C and Δ14C values of TC in PM2.5 varied temporally (Table 1) Enriched δ13C and Δ14C values were observed in winter and spring, while, in summer, δ13C and Δ14C were significantly depleted than other seasons (Fig. S3 in Supplementary Information).
Concentration of TC and PM2.5
A total of 47 PM2.5 samples were collected during the study period (Table 1). The mean concentrations of TC in PM2.5 were 5.0, 7.0, 8.2, and 9.7 μg C m−3 at TRF, HUF, CRI, and SNU, respectively (Table 1). The mean concentration of TC in PM2.5 at SNU was about two-fold higher than at TRF (p < 0.05) (Table 1). The seasonal variation of TC in PM2.5 was relatively small in summer (Jun.–Aug.) and larger in winter (Dec.–Feb.) (Fig. S4 in Supplementary Information) and the concentration of TC in PM2.5 in winter was approximately 1.5-fold higher than in summer (p < 0.05).
The mean annual concentrations of PM2.5 observed by the β-ray absorption method were 22, 20, 22 μg m−3, respectively, at the national PM2.5 monitoring sites located near TRF, HUF&CRI, and SNU in 2020, which were about 0.3–6.6 km away from our sampling sites (Fig. 3a). The concentration of PM2.5 was not significantly different among monitoring sites (p > 0.1) regardless of seasons (Fig. 3a). The contribution of TC to PM2.5 near monitoring sites ranged from 7 to 45% at TRF, from 16 to 86% at HUF and CRI, and from 8 to 69% at SNU (Table 1). The contribution of TC to PM2.5 was the highest in spring at TRF and SNU (Table 1; Fig. 3).
(a) The concentrations of PM2.5 (μg m−3) at the national PM2.5 monitoring sites near SNU, HUF&CRI (one site; section "PM2.5 monitoring data in South Korea"), and TRF. (b) The concentration (μg C m−3) of TC in PM2.5 at TRF. (c) The concentrations of TC in PM2.5 at SNU, HUF, and CRI. The green, brown, and grey bars in (b) and (c) represent carbon derived from C3 plants, C4 plants, and fossil fuel, respectively. (d) The concentration (ng m−3) of levoglucosan.
Source apportionments
The contribution of the three sources (fossil fuel, C4 plants, and C3 plants) to TC in PM2.5 was calculated. The contribution of carbon derived from fossil fuel ranged from 21 to 73% (Fig. 3b,c), with an average of 46%, 46%, 51%, and 51% at TRF, HUF, CRI, and SNU, respectively, based on the MCMC-Bayesian model (Table 2). The relative contribution of carbon from fossil fuel to TC in PM2.5 was higher in summer than in winter at TRF and SNU (Table 2), which was expected from the depleted δ13C and Δ14C levels in summer (Fig. S3c in Supplementary Information).
The mean contributions of carbon from C4 plants to TC in PM2.5 were 19%, 17%, 21%, and 21% at TRF, HUF, CRI, and SNU, respectively (Table 2). The largest contribution of C4 plants to TC in PM2.5 was 42% and 55% for TRF and SNU, respectively, observed on April 10, 2020. A relatively lower contribution of C4 plants was observed in summer (10% at TRF and 15% at SNU) compared with the other seasons (Table 2; Fig. 3b,c).
The mean contributions of carbon from C3 plants to TC in PM2.5 were 35%, 38%, 31%, and 27% at TRF, HUF, CRI, and SNU, respectively (Table 2). The highest contribution of C3 plants to TC in PM2.5 was observed in spring (49% at TRF and 32% at SNU), while in winter, the carbon contributions were 37, 34, 28, and 25% at TRF, HUF, CRI, and SNU, respectively (Table 2; Fig. 3b,c).
The concentration of TC in PM2.5 from each source was calculated by multiplying the concentration of TC with the individual contribution. The mean concentration of TC in PM2.5 derived from C3 plants was 2.1 μg C m−3 (range: 0.7–4.2 μg C m−3) at TRF, and 2.6 μg C m−3 (range 0.5–6.5 μg C m−3) at SNU. Although the concentration of TC in PM2.5 was about two-fold higher in SNU than in TRF (Table 1), the concentration of TC in PM2.5 derived from C3 plants was similar in the urban area and the pine forest (Table 2; Fig. 3b,c). In contrast, the mean concentration of TC in PM2.5 derived from fossil fuel was 2.1 (range: 1.1–4.2 μg C m−3) at TRF and 4.3 (range: 2.0–8.4 μg C m−3) at SNU. The concentration of TC in PM2.5 derived from C4 plants ranged from 0.2 to 2.8 with an average of 0.8 μg C m−3 at TRF, and ranged from 0.1 to 9.1 μg C m−3 with an average of 2.7 μg C m−3 at SNU (Table 2; Fig. 3b,c).
Levoglucosan concentration
The mean concentrations of levoglucosan were 39.8, 8.3, 34.6, and 57.5 ng m−3 in spring, summer, fall, and winter, respectively, at TRF (Fig. 3d), whereas those at SNU were 30.4, 6.2, 12.6, and 70.4 ng m−3, respectively (Fig. 3d). There was a positive correlation between the concentration of levoglucosan and fractional contribution of C4 plants (Fig. 4a).
Discussion
Many studies have demonstrated that PM2.5 can be removed by trees6,8,9,10, which leads to urban forest expansion. However, PM2.5 can be indirectly produced by BVOCs emitted by plants14,43,44. PM2.5 can also be directly produced by fossil fuel or biomass burning. These contrasting views question of their relative importance in the formation of PM2.5. Although some studies on TC in PM2.5 have been conducted in East Asia, typically in urban area, they used only δ13C (not including Δ14C), different kind of dual isotopes (δ13C and δ15N), or only during a limited season not over an entire year34,35. In contrast, this is the first study, to our knowledge, which investigated changes of δ13C and Δ14C of PM2.5 over an entire year inside the forest and the urban areas in South Korea.
A review of PM2.5 sources based on 14C analysis demonstrated that the proportion of carbon sources except fossil fuels (i.e., total carbon minus carbon derived from fossil fuel) generally exceeded 30% of TC in PM collected even in urban locations45. If BVOCs derived from trees strongly contribute to the formation of PM2.5, the PM2.5 collected inside a forest would yield higher Δ14C than in urban areas. In other words, as the portion of fossil carbon increases, Δ14C will decrease. TC in PM2.5 collected in the forest (TRF) and urban areas (SNU) had depleted Δ14C, indicating the relative contribution of fossil carbon is constantly high (Table 2 and Fig. 2). The similar sources of PM2.5 in the forest and urban areas (Table 2 and Fig. 2) have been shown even during summer when the emission of BVOCs was the highest46,47, or during spring when the concentrations of pollen were the highest48 (Fig. S3 in Supplementary Information). This suggests that biogenic emission of plants could be a less important source of PM2.5 even during summer. The Δ14C values of PM2.5 were also similar between TRF and HUF. CRI is located next to major traffic roads (Fig. 1), where direct input of PM2.5 by vehicles could lower Δ14C. These results imply that trees can produce biogenic PM2.5, but most of PM2.5 within the forests or urban green spaces are likely to be transported from the outside.
Despite similar sources, the TC concentration in PM2.5 at TRF was two-fold lower than at SNU (Table 1). Considering the similar concentrations of PM2.5 at the monitoring sites which are located outside of forest (Fig. 3a), the lower concentrations of TC at TRF suggest that pine forests may reduce PM2.5 transported from the outside. The ratio of fossil carbon to the total mass of PM2.5 can be roughly calculated by multiplying the proportion of TC in PM2.5 by the proportion of fossil carbon in TC. Since ~ 30% of PM2.5 was carbonaceous material in general (“TC in PM2.5 (%)” in Table 1)19,45, and ~ 50% of the carbonaceous material was derived from fossil fuel (Table 2), ~ 15% of PM2.5 (i.e., 0.3 × 0.5 = 0.15) is derived from fossil carbon. Although 15% appears small, the majority of the PM2.5 is still attributable to the other aerosols, such as sulfur oxides and nitrogen oxides, derived from fossil fuel burning27,28.
The remaining portions, ~ 50% of TC in PM2.5 were attributed to the non-fossil fuels. A previous study conducted at TRF during the growing season (Aug.–Oct., 2014) showed approximately 76% of TC was from non-fossil sources35. We further attributed the non-fossil sources of TC in PM2.5 to C3 plants- (~ 30%) and C4 plants-derived carbon (~ 20%) (Table 2), which was calculated over entire seasons including winter when the concentration of PM2.5 was the largest. The range of δ13C of marine aerosols could be in those between C3 plants and C4 plants40,49. Both C4 plants and marine aerosols could be selected as an end member28,40. However, we have selected C4 plants instead of marine aerosols as an endmember because of the variation of the concentration of levoglucosan (Fig. 3d). Levoglucosan is a tracer of biomass burning and thus cannot be an important component of marine aerosols50. The concentration of levoglucosan demonstrated clear seasonal variation, with the largest value up to 75.91 ng m−3 observed during winter, followed by fall and spring, and the lowest during summer (Fig. 3d). This seasonal variation has been observed in major cities in the East Asia26,51,52,53,54.
The concentration of levoglucosan was positively correlated with fractional contribution of C4 plants (up to R2 = 0.78, p < 0.05 in Fig. 4a), but not correlated with fractional contribution of C3 plants (Fig. 4b). This indicates that the higher concentration of levoglucosan, especially in winter was likely due to the burning of C4 plants (e.g., corn residues). The biomass burning of C4 plants has been widely considered as one of the sources of TC in PM2.5 because of the enriched δ13C and the high concentration of levoglucosan during winter26,34,55. The enriched Δ14C and δ13C as well as the highest concentration of levoglucosan were also reported during winter in China26,27,28.
Recent studies have demonstrated that the burning of corn residues accounted for up to 80% of the total agricultural waste burning in China56,57,58. China was the second-largest producer of corn with 23% of the global corn yield in the marketing year (Sep., 2019–Aug. 2020), following the U.S. very closely (https://www.fas.usda.gov/data/world-agricultural-production). In contrast, corn production in South Korea is substantially lower than in China. It has been also reported that the burning of corn residues was ~ 10% of the total agricultural waste burning in several rural areas of South Korea59, although agricultural waste burning over the entire South Korea is rarely reported. Since northwesterly winds around Korea are predominant during winter as the Siberian High (i.e., Siberian Anticyclone) develops (60; Fig. S5 in Supplementary Information), TC derived from C4 biomass burning could be not only generated within South Korea but also transported from North Korea and/or China61,62.
Su et al.63 quantified the proportion of nine sources of PM2.5 by using a multivariate factor analysis in the western urban area of Shenzhen, China in the fall (Sep.– Nov., 2019). Among them, the contribution of biomass burning to the total concentration of PM2.5 was only 11%61. The results of dual carbon isotope and levoglucosan analyses in this study showed about 20% of the carbonaceous component of PM2.5 might be derived from the burning of C4 plants. Considering TC accounted for ~ 30% of PM2.5 [Table 119,45, the carbon derived from biomass burning of C4 plants could contribute at least ~ 6% (i.e., 0.2 (above 20%) × 0.3 (above 30%) = 0.06) of the total PM2.5, while inorganic materials can be attributed to another fraction of PM2.564.
The carbon derived from C3 plants was estimated at only ~ 9% of PM2.5 in TRF and SNU, which can be attributed to biogenic emissions by trees or biomass burning of C3 plants. The concentrations of TC derived from C3 plants were similar at TRF and SNU, whereas the concentrations of TC derived from fossil-carbon and C4 plants in the urban areas were about two-fold the levels at TRF (Table 2; Fig. 3b,c). Thus, biogenic emission of pines is not likely to be an important source of PM2.5 in the forest, suggesting that trees act as a bio-filter of PM2.5 in urban areas.
Conclusions
We analyzed dual carbon isotope ratios and levoglucosan concentrations in a pine forest and in urban areas to provide source apportionment of TC in PM2.5. The δ13C and Δ14C of TC in PM2.5 indicated similar sources of carbon in PM2.5 in the forest and urban areas, although the total concentration of TC in PM2.5 was approximately two-fold lower in the forest than in urban areas. TC in PM2.5 was predominantly affected by human activities such as fossil-fuel combustions and C4 biomass burning rather than emissions from C3 plants. These results suggest that strategies to reduce atmospheric PM2.5 should focus on fossil fuel combustion and biomass burning instead of biogenic emissions from trees.
Data availability
The data used to support the findings of this study are included within the article and its supplementary information files.
References
Wang, Y., Zhang, R. & Saravanan, R. Asian pollution climatically modulates mid-latitude cyclones following hierarchical modelling and observational analysis. Nat. Commun. 5, 3098. https://doi.org/10.1038/ncomms4098 (2014).
Xing, Y. F., Xu, Y. H., Shi, M. H. & Lian, Y. X. The impact of PM2.5 on the human respiratory system. Thorac. Dis. 8(1), E69. https://doi.org/10.3978/j.issn.2072-1439.2016.01.19 (2016).
Kim, Y., Yi, S. M. & Heo, J. Fifteen-year trends in carbon species and PM2.5 in Seoul South Korea (2003–2017). Chemosphere 261, 127750. https://doi.org/10.1016/j.chemosphere.2020.127750 (2020).
Hong, C. et al. Impacts of climate change on future air quality and human health in China. Proc. Natl. Acad. Sci. U.S.A. 116(35), 17193–17200 (2019).
Seo, J. et al. Effects of meteorology and emissions on urban air quality: a quantitative statistical approach to long-term records (1999–2016) in Seoul, South Korea. Atmos. Chem. Phys. 18(21), 16121–16137 (2018).
Nowak, D. J., Hirabayashi, S., Bodine, A. & Greenfield, E. Tree and forest effects on air quality and human health in the United States. Environ. Pollut. 193, 119–129 (2014).
Nguyen, T., Yu, X., Zhang, Z., Liu, M. & Liu, X. Relationship between types of urban forest and PM2.5 capture at three growth stages of leaves. J. Environ. Sci. 27, 33–41 (2015).
Nowak, D. J., Hirabayashi, S., Doyle, M., McGovern, M. & Pasher, J. Air pollution removal by urban forests in Canada and its effect on air quality and human health. Urban For. Urban Green. 29, 40–48 (2018).
Zhu, C. et al. Effect of spatial heterogeneity of plant communities on air PM10 and PM25 in an urban forest park in Wuhan, China. Urban For. Urban Green. 46, 126487. https://doi.org/10.1016/j.ufug.2019.126487 (2019).
Irga, P. J., Burchett, M. D. & Torpy, F. R. Does urban forestry have a quantitative effect on ambient air quality in an urban environment?. Atmos. Environ. 120, 173–181 (2015).
Heidt, V. & Neef, M. Ecology, Planning, and Management of Urban Forests (Springer, 2008).
Korea Forest Service. Second Master Plan (2018–2027) for urban forests (Korea Forest Service, 2019) (in Korean).
Park, J. H. et al. Active atmosphere-ecosystem exchange of the vast majority of detected volatile organic compounds. Science 341(6146), 643–647 (2013).
Ehn, M. et al. A large source of low-volatility secondary organic aerosol. Nature 506(7489), 476–479 (2014).
Keywood, M., Guyes, H., Selleck, P. & Gillett, R. Quantification of secondary organic aerosol in an Australian urban location. Environ. Chem. 8(2), 115–126 (2011).
Shrivastava, M. et al. Urban pollution greatly enhances formation of natural aerosols over the Amazon rainforest. Nat. Commun. 10(1), 1–12 (2019).
de Sá, S. S. et al. Urban influence on the concentration and composition of submicron particulate matter in central Amazonia. Atmos. Chem. Phys. 18(16), 12185–12206 (2018).
Lemire, K. R., Allen, D. T., Klouda, G. A. & Lewis, C. W. Fine particulate matter source attribution for Southeast Texas using 14C/13C ratios. J. Geophys. Res. Atmos. 107(D22), ACH-3 (2002).
Szidat, S. et al. Radiocarbon (14C)-deduced biogenic and anthropogenic contributions to organic carbon (OC) of urban aerosols from Zürich, Switzerland. Atmos. Environ. 38(24), 4035–4044 (2004).
Heal, M. R. et al. Application of 14C analyses to source apportionment of carbonaceous PM2.5 in the UK. Atmos. Environ. 45(14), 2341–2348 (2011).
Genberg, J. et al. Source apportionment of carbonaceous aerosol in southern Sweden. Atmos. Chem. Phys. 11(22), 11387–11400 (2011).
Liu, D. et al. The use of levoglucosan and radiocarbon for source apportionment of PM2.5 carbonaceous aerosols at a background site in East China. Environ. Sci. Technol. 47(18), 10454–10461 (2013).
Szidat, S. et al. Contributions of fossil fuel, biomass-burning, and biogenic emissions to carbonaceous aerosols in Zurich as traced by 14C. J. Geophys. Res. Atmos. https://doi.org/10.1029/2005JD006590 (2006).
Puxbaum, H. et al. Levoglucosan levels at background sites in Europe for assessing the impact of biomass combustion on the European aerosol background. Geophys. Res. Atmos. https://doi.org/10.1029/2006JD008114 (2007).
Salma, I. et al. Source apportionment of carbonaceous chemical species to fossil fuel combustion biomass burning and biogenic emissions by a coupled radiocarbon–levoglucosan marker method. Atmos. Chem. Phys. 17(22), 13767–13781 (2017).
Zhao, Z. et al. Stable carbon isotopes and levoglucosan for PM2.5 elemental carbon source apportionments in the largest city of Northwest China. Atmos. Environ. 185, 253–261 (2018).
Ni, H. et al. Source apportionment of carbonaceous aerosols in Xi’an, China: insights from a full year of measurements of radiocarbon and the stable isotope 13C. Atmos. Chem. Phys. 18(22), 16363–16383 (2018).
Mo, Y. et al. Dual carbon isotope-based source apportionment and light absorption properties of water-soluble organic carbon in pm25 over China. J. Geophys. Res. Atmos. 126(8), e2020JD033920. https://doi.org/10.1029/2020JD033920 (2021).
Monson, R. K., Edwards, G. E. & Ku, M. S. C3–C4 intermediate photosynthesis in plants. Bioscience 34(9), 563–574 (1984).
O’Leary, M. H. Carbon isotope fractionation in plants. Phytochemistry 20(4), 553–567 (1981).
Lee, H. et al. The resilience of the carbon cycles of temperate coniferous and broadleaved forests to drought. For. Ecol. Manag. 491, 119178. https://doi.org/10.1016/j.foreco.2021.119178 (2021).
Kim, S. Y. et al. Impact of biogenic volatile organic compounds on ozone production at the Taehwa Research Forest near Seoul, South Korea. Atmos. Environ. 70, 447–453 (2013).
Food and Agriculture Organization of the United Nations. Global Forest Resources Assessment 2020: Terms and Definitions. (Food and Agriculture Organization of the United Nations, 2018).
Kundu, S. & Kawamura, K. Seasonal variations of stable carbon isotopic composition of bulk aerosol carbon from Gosan site, Jeju Island in the East China Sea. Atmos. Environ. 94, 316–322 (2014).
Lim, S. et al. Source signatures from combined isotopic analyses of PM2.5 carbonaceous and nitrogen aerosols at the peri-urban Taehwa Research Forest, South Korea in summer and fall. Sci. Total Environ. 655, 1505–1514 (2019).
Wang, X., Ge, T., Xu, C., Xue, Y. & Luo, C. Carbon isotopic (14C and 13C) characterization of fossil-fuel derived dissolved organic carbon in wet precipitation in Shandong Province China. J. Atmos. Chem. 73(2), 207–221 (2016).
Wei, X. et al. 14C as a tool for evaluating riverine POC sources and erosion of the Zhujiang (Pearl River) drainage basin South China. Nucl. Instrum. Methods Phys. Res. B. 268(7–8), 1094–1097 (2010).
Zhang, Y. L. et al. Fossil and nonfossil sources of organic and elemental carbon aerosols in the outflow from Northeast China. Environ. Sci. Technol. 50(12), 6284–6292 (2016).
Raymond, P. A. The composition and transport of organic carbon in rainfall: Insights from the natural (13C and 14C) isotopes of carbon. Geophys. Res. Lett. 32(14), 2879. https://doi.org/10.1029/2005GL022879 (2005).
Ceburnis, D. et al. Quantification of the carbonaceous matter origin in submicron marine aerosol by 13C and 14C isotope analysis. Atmos. Chem. Phys. 11(16), 8593–8606 (2011).
Phillips, D. L. & Gregg, J. W. Source partitioning using stable isotopes: Coping with too many sources. Oecologia 136(2), 261–269 (2003).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2021). https://www.R-project.org/.
McFiggans, G. et al. Secondary organic aerosol reduced by mixture of atmospheric vapours. Nature 565(7741), 587–593 (2019).
Watanabe, A. C., Stropoli, S. J. & Elrod, M. J. Assessing the potential mechanisms of isomerization reactions of isoprene epoxydiols on secondary organic aerosol. Environ. Sci. Technol. 52(15), 8346–8354 (2018).
Heal, M. R. The application of carbon-14 analyses to the source apportionment of atmospheric carbonaceous particulate matter: A review. Anal. Bioanal. Chem. 406(1), 81–98 (2014).
Spracklen, D. V. et al. Aerosol mass spectrometer constraint on the global secondary organic aerosol budget. Atmos. Chem. Phys. 11(23), 12109–12136 (2011).
Son, Y. S., Kim, K. J., Jung, I. H., Lee, S. J. & Kim, J. C. Seasonal variations and emission fluxes of monoterpene emitted from coniferous trees in East Asia: Focused on Pinus rigida and Pinus koraiensis. J. Atmos. Chem. 72(1), 27–41 (2015).
Rahman, A. et al. Influence of atmospheric PM2.5, PM10, O3, CO, NO2, SO2, and meteorological factors on the concentration of airborne pollen in Guangzhou China. Atmos. Environ. 212, 290–304 (2019).
Miyazaki, Y., Kawamura, K., Jung, J., Furutani, H. & Uematsu, M. Latitudinal distributions of organic nitrogen and organic carbon in marine aerosols over the western North Pacific. Atmos. Chem. Phys. 11, 3037–3049 (2011).
Fu, P. Q., Kawamura, K., Chen, J., Charrìère, B. & Sempere, R. Organic molecular composition of marine aerosols over the Arctic Ocean in summer: Contributions of primary emission and secondary aerosol formation. Biogeosciences 10, 653–667 (2013).
Cheng, Y. et al. Biomass burning contribution to Beijing aerosol. Atmos. Chem. Phys. 13, 7765–7781 (2013).
Choi, J. K., Heo, J. B., Ban, S. J., Yi, S. M. & Zoh, K. D. Chemical characteristics of PM2.5 aerosol in Incheon, Korea. Atmos. Environ. 60, 583–592 (2012).
Choi, N. R., Lee, S. P., Lee, J. Y., Jung, C. H. & Kim, Y. P. Speciation and source identification of organic compounds in PM10 over Seoul, South Korea. Chemosphere 144, 1589–1596 (2016).
Kim, H., Kim, J. Y., Jin, H. C., Lee, J. Y. & Lee, S. P. Seasonal variations in the light-absorbing properties of water-soluble and insoluble organic aerosols in Seoul, Korea. Atmos. Environ. 129, 234–242 (2016).
Zhang, T. et al. Identification and estimation of the biomass burning contribution to Beijing aerosol using levoglucosan as a molecular marker. Atmos. Environ. 42(29), 7013–7021 (2008).
Cui, S. et al. Spatial and temporal variations of open straw burning based on fire spots in northeast China from 2013 to 2017. Atmos. Environ. 244, 117962. https://doi.org/10.1016/j.atmosenv.2020.117962 (2020).
Jin, Q., Ma, X., Wang, G., Yang, X. & Guo, F. Dynamics of major air pollutants from crop residue burning in mainland China, 2000–2014. J. Environ. Sci. 70, 190–205 (2018).
Li, C. et al. Light absorption characteristics of carbonaceous aerosols in two remote stations of the southern fringe of the Tibetan Plateau, China. Atmos. Environ. 143, 79–85 (2016).
Kim, D. Y., Choi, M.-A., Han, Y.-H. & Park, S.-K. A study on estimation of air pollutants emission from agricultural waste burning (in Korean with English abstract). J. Korea Soc. Atmos. Environ. 32(2), 167–175 (2016).
Jhun, J. G. & Lee, E. J. A new East Asian winter monsoon index and associated characteristics of the winter monsoon. J. Clim. 17(4), 711–726 (2004).
Heo, J. B., Hopke, P. K. & Yi, S. M. Source apportionment of PM2.5 in Seoul Korea. Atmos. Chem. Phys. 9(14), 4957–4971 (2009).
Park, Y. M. et al. Characterizing isotopic compositions of TC-C NO3−-N and NH4+-N in PM2.5 in South Korea: Impact of China’s winter heating. Environ. Pollut. 233, 735–744 (2018).
Su, C. P. et al. Development and application of a mass closure PM2.5 composition online monitoring system. Atmos. Meas. Tech. 13(10), 5407–5422 (2020).
Xie, Y., Zhao, Y. & Xiao, H. Effects of secondary formation of inorganic ions and low molecular weight organic acids and biomass burning in regional pollution of PM2.5 in Central China. Atmos. Pollut. Res. 13(5), 101412 (2022).
Acknowledgements
We thank Hojin Lee at Seoul National University, the staff at Taehwa Research Forest of Seoul National University, and the researchers at Urban Forests Division, National Institute of Forest Science for their assistance with sampling. This study was carried out with the support of ´R&D Program for Forest Science Technology (Project No. 2019156C10-2121-0101)' provided by Korea Forest Service (Korea Forestry Promotion Institute). This work was also supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2021R1A2C1006783; NRF-2021R1A4A1025553).
Author information
Authors and Affiliations
Contributions
J.Y.C.: Methodology, formal analysis, investigation, writing—original draft preparation, and writing—review & editing, visualization. K.L., S.C.L., E.J.L., K.J.Y, I.R., M.K.: Investigation, and writing—review & editing. J.A., S.M.Y., C.R.P.: Resources, and writing—review & editing. N.H.O.: Conceptualization, supervision, writing—original draft preparation, writing—review & editing, and funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cha, JY., Lee, K., Lee, SC. et al. Fossil and non-fossil sources of the carbonaceous component of PM2.5 in forest and urban areas. Sci Rep 13, 5486 (2023). https://doi.org/10.1038/s41598-023-32721-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-32721-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.