Abstract
In vitro maturation of porcine oocytes is characterized by asynchronous cytoplasmic and nuclear maturation, leading to less competent oocytes supporting embryo development. The purpose of this study was to evaluate the combined effect of rolipram and cilostamide as cyclic Adenine monophosphate (cAMP) modulators to find the maximum cAMP levels that temporarily arrest meiosis. We determined the optimal time to maintain functional gap junction communication during pre-in vitro maturation to be four hours. Oocyte competence was evaluated by the level of glutathione, reactive oxygen species, meiotic progression, and gene expression. We evaluated embryonic developmental competence after parthenogenetic activation and somatic cell nuclear transfer. The combined treatment group showed significantly higher glutathione and lower reactive oxygen species levels and a higher maturation rate than the control and single treatment groups. Cleavage and blastocyst formation rates in parthenogenetic activation and somatic cell nuclear transfer embryos were higher in two-phase in vitro maturation than in the other groups. The relative levels of BMP15and GDF9 expression were increased in two-phase in vitro maturation. Somatic cell nuclear transfer blastocysts from two-phase in vitro maturation oocytes showed a lower level of expression of apoptotic genes than the control, indicating better pre-implantation developmental competence. The combination of rolipram and cilostamide resulted in optimal synchrony of cytoplasmic and nuclear maturation in porcine in vitro matured oocytes and there by enhanced the developmental competence of pre-implantation embryos.
Similar content being viewed by others
Introduction
Porcine in vitro production (IVP) is a tool used to create suitable animal models to study human diseases or to develop bio-organs as donors for xenotransplantation due to the relatively high physiological similarity between humans and porcine1. The IVP of porcine embryos is usually performed by somatic cell nuclear transfer (SCNT), an assisted reproductive technology for producing cloned pigs. Nevertheless, the production efficiency of cloned animals, particularly pigs, is low2,3 and is affected by the type of donor cells, quality of oocytes, and type of recipient4,5. Moreover, the nuclear remodeling and reprogramming regimes used during the in vitro maturation (IVM) of oocytes affect the efficiency of the in vitro production (IVP) of embryos6,7,8. Overall, the quality of IVM-derived oocytes is the most important factor in determining the success rate of SCNT and the production of cloned porcine embryos, as the subsequent production is dependent on the developmental competence of pre-implantation embryos9,10,11. Additionally, the remodeling and reprogramming of donor nuclei are affected by the cytoplasmic factors of the recipient, where the level and content of cytoplasmic maturation are determining factors in the efficient production of cloned porcine embryos12,13.
As the oocyte progresses from the preantral to antral stages, it gradually develops the ability to resume meiosis. The resumption of meiosis begins with the dissolution of the nuclear envelope of the oocyte, known as “germinal vesicle breakdown” (GVBD) in the prophase of MI14. After GVBD and the completion of MI, the oocyte moves to meiosis II without an obvious S-phase and halts at metaphase II (MII) until fertilization. Several studies have investigated the mechanism behind meiotic arrest and have found that cyclic adenosine monophosphate (cAMP) as a cellular second messenger, plays a vital role in maintaining oocyte meiotic arrest15,16. Particularly in IVM-derived-porcine oocytes, nuclear maturation resumes following oocyte retrieval from the ovarian follicle due to an abrupt reduction in the level of cAMP and cGMP17,18,19,20. However, even after enhancing the developmental competence of oocytes through IVM, the quality of in vitro mature oocytes remains low compared with their in vivo counterparts21,22. Despite having undergone nuclear maturation, oocytes with incomplete cytoplasmic maturation were reported to inefficiently support the normal development of SCNT-derived embryos23,24. However, during IVM, the abrupt start of meiosis following retrieval of oocytes from the follicles leads to early or premature nuclear maturation that is manifested by the early extrusion of the first polar body, compared with that of in vivo oocytes25,26,27,28. Unstable meiotic progression results in the reduction of the time required for sufficient cytoplasmic maturation29. Furthermore, the gap junction communication (GJC) is responsible for maintaining meiotic arrest by attaining the required level of cAMP, which depends on the stage of nuclear maturation30,31,32,33. Therefore, the developmental competence of IVM-derived oocytes can be better achieved through synchronized cytoplasmic and nuclear maturation34,35.
This two-phase approach of IVM has shown improved developmental competence of the oocytes in different animal species, including bovine36, mice37,38, and sheep39. This improvement is due to the synchronized nuclear and cytoplasmic maturation achieved by delaying nuclear maturation, which ultimately leads to improved embryonic developmental following in vitro fertilization (IVF) and SCNT. Moreover, cAMP, acting as a second messenger, is known to play a critical role in the maintenance of meiotic arrest in mammalian oocytes40,41,42. Additionally, cAMP is synthesized in oocytes and follicular cells, including granulosa and cumulus cells. This protein enters the oocytes through the GJC and plays an active role in maintaining the meiotic arrest of the oocytes43,44,45,46.
To enhance the developmental competence of IVM oocytes, synchronized cytoplasmic and nuclear maturation is needed. This can be achieved by temporarily arresting the meiotic resumption of the nucleus47,48. The GJC is required to maintain a high level of cAMP, which inhibits the maturation-promoting factor and mitogen-activated protein kinase, thereby arresting meiosis49,50. In oocytes, cAMP is regulated by phosphodiesterase (PDE) and adenyl cyclase, which regulates the degradation and synthesis of cAMP51,52,53. In pigs and other animals, the developmental competence of IVM oocytes has been enhanced by regulating cAMP54,55,56,57. Similarly, cAMP has been reported to regulate meiotic arrest in bovine58 and mouse oocytes59.
Studies in pigs have demonstrated that using a single PDE inhibitor during IVM causes transient meiotic arrest in the oocytes60,61,62,63. For instance, a PDE3A inhibitor milrinone improved the quality of porcine embryos by synchronizing the nuclear and cytoplasmic maturation of oocytes with a low level of cytoplasmic development63. Furthermore, cilostamide-treated porcine oocytes showed improved developmental competence reflected by the higher quality of blastocysts from parthenogenetic activation (PA) and SCNT64. Similarly, cilostamide inhibited the activity of PDE3A in rodents and macaque oocytes65,66. Forskolin, another PDE3A inhibitor, successfully regulated cAMP in mouse67,68, rat69,70, dog71, and pig72 oocytes.
Despite progressive efforts, the in vitro competence of oocytes remains lower than that of their in vivo counterparts. On the other hand, rolipram as PDE4 inhibitor has showed a delaying effect on the spontaneous meiotic maturation during in vitro maturation through extended gap junctional communication between CCs and the oocyte, and subsequently improved cytoplasmic maturation and developmental potential of oocyte73. Therefore, IVM oocytes cannot adequately support the development of IVF and SCNT embryos.
We hypothesized that IVM conditions lack certain characteristics to mimic the in vivo conditions, particularly the synchronicity of nuclear and cytoplasmic maturation. Therefore, we aimed to investigate the effects of combined IVM treatment using PDE-3 and PDE-4 inhibitors, known to achieve the optimal cAMP level.
Results
Optimal concentration of rolipram and effective time for two-phase IVM in porcine oocytes
To evaluate the effects of rolipram and cilostamide in maintaining functional GJC between the oocytes and their surrounding cells during pre-IVM, a Lucifer yellow dye transfer assay was conducted (Fig. 1). We used the reference time of 6 h for pre-IVM incubation based on previous findings63. Before IVM, only 5% of the COCs had closed GJC, whereas 80% had open GJC. Moreover, the percentage of oocytes with open GJC was 8.9 ± 0.8% and 10.5 ± 1.7%, 21.6 ± 3.5%, and 16.8 ± 4.1% in the control group and 25, 50, and 100 μM rolipram groups, respectively. The 50 μM rolipram group demonstrated a significant difference in the percentage of oocytes with open GJC from the other groups (P < 0.05). Similarly, the percentage of oocytes with partially open GJC was 29.3 ± 5.0%, 28.3 ± 2.5%, 40.7 ± 3.9%, and 31.2 ± 7.0% in the corresponding groups. Finally, the respective percentages of oocytes with closed GJC were 61.8 ± 8.5%, 59.1 ± 8.3%, 37.7 ± 13.3%, and 52.0 ± 13.8%, with a significant difference (P < 0.05) between the treated and control groups (Table 1). Therefore, we determined the optimal concentration of rolipram for maintaining functional GJC in porcine oocytes to be 50 μM. We then determined the effective time for two-phase IVM in porcine oocytes using rolipram and cilostamide, inhibiting cAMP in cumulus cells and intraoocytes, respectively.
Images of the open status of functional GJCin porcine oocytes after pre-IVM rolipram–cilostamide treatment. Open functional GJC, almost all layers of cumulus cells are stained with lucifer yellow dye after intraoocyte microinjection (a,a′); partially open functional GJC, only one or two layers of cumulus cells are stained (b,b′); and closed functional GJC (c,c′), in bright light and UV, respectively.
The optimal concentrations of rolipram and cilostamide were determined based on the maintenance of functional GJC during pre-IVM incubation. The percentage of oocytes with open, partially open, and closed GJC for the first 2 h of pre-IVM incubation was 42.6 ± 0.21%, 33.5 ± 0.17%, and 23.9 ± 0.12%, respectively, in the control group versus 53.7 ± 0.27%, 36.4 ± 0.18%, and 9.9 ± 0.05%, respectively, in the rolipram–cilostamide-treated group (P < 0.05). Similarly, the percentage of oocytes with open, partially open, and closed GJC for the first 4 h of pre-IVM incubation was 16.9 ± 0.08, 17.2 ± 0.09, and 66.0 ± 0.33%, respectively, in the control group versus 31.2 ± 0.16, 33.8 ± 0.17, and 35.0 ± 0.18%, respectively, in the rolipram–cilostamide-treated group (P < 0.05) (Fig. 2).
The optimal time for pre-IVM incubation of porcine oocytes with rolipram–cilostamide treatments. Percentage of oocytes with open, partially open, and closed GJC after 2 (A), 4 (B), 6 (C), and 8 (D) h of pre-IVM incubation. A significant difference (P < 0.05) was observed between the control and rolipram–cilostamide-treated groups for open, partially open, and closed GJC at 2 and 4 h but not 6 or 8 h. The percentage of oocytes with open and partially open GJC was less than 10%. As seen in (A,B), after 4 h of pre-IVM incubation, rolipram–cilostamide treatment significantly affected functional GJC status, and this period was determined to be the optimal time for the two-phase IVM of porcine oocytes using rolipram and cilostamide.
Conversely, after 6 h of pre-IVM incubation, the percentage of oocytes with open and partially open GJC were found to be lower as compared with that after4 h of pre-IVM incubation (from 31.2% and 33.8% to 11.1% and 24.6%, respectively) (Fig. 2). Furthermore, after 6 h of pre-IVM, the GJC status of most oocytes changed to closed (64.3% versus 35.0% after 4 h of pre-IVM incubation) (Fig. 2). Accordingly, we determined the effective time for maintaining functional GJC using rolipram and cilostamide in porcine oocytes to be 4 h of pre-IVM incubation. Accounting for sex hormones and the need for the synchronization of cytoplasmic and nuclear maturation, we designed a three-stage IVM for porcine oocytes. First, oocytes were treated with sex hormones and PDE inhibitors (4 h of incubation), followed by incubation for 16 h with sex hormones, and then 20 h of incubation without hormones (Fig. 3).
Effects of two-phase IVM on GSH and ROS level of IVM oocytes
Following IVM, oocytes were denuded, and those with the first polar bodies were used to measure GSH and ROS levels. The GSH levels were significantly higher in the rolipram–cilostamide-treated groups than in the control group, while the ROS levels were found to be significantly lower in the rolipram–cilostamide-treated oocytes than in the control group oocytes (P < 0.05) (Fig. 4).
Epifluorescence photomicrographic images of IVM porcine oocytes. (A) Oocytes were stained with (a,b) Cell Tracker Blue and (a′,b′) 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) to detect intracellular GSH and ROS levels of control (a,a′) and rolipram–cilostamide-treated oocytes (b,b′). (B) Effects of treatments on intracellular levels of IVM porcine oocytes (GSH and ROS levels in each group), bars with *indicate significant differences (P < 0.05). To measure the GSH and ROS levels, at least 40 oocytes were used for each experiment, which was replicated 8 times.
Effect of two-phase IVM on the level of cytoplasmic maturation
The purpose of two-phase IVM is to temporarily inhibit the abrupt resumption of nuclear maturation following retrieval, as cytoplasmic maturation is inadequate to support subsequent embryonic development. The level of cytoplasmic maturation in IVM oocytes was determined by assessing the relative expression level of genes associated with cytoplasmic maturation. The expression level of GDF9 and BMP15 were significantly different between the rolipram–cilostamide-treated group and other groups (Fig. 5).
The relative level of gene expression on quantitative analysis of mRNA transcripts by real-time PCR in control, rolipram, cilostamide, and rolipram–cilostamide-treated porcine oocytes during IVM. At least 80 oocytes were obtained per sample and 8 biological replications were performed. Relative expression of BMP15 (A) and GDF9 (B) to determine the level of cytoplasmic maturation were statistically significantly different among groups. (C) Relative level of CX43 gene expression for level of functional GJC is significantly different between cilostamide and rolipram–cilostamide-treated groups versus the control and rolipram-treated groups (P < 0.05). Values in columns with different superscript letters are significantly different. Control samples were set as arbitrary units, and the target genes were expressed as a fold-change of the corresponding control relative to the house keeping gene (β-actin).
Effect of two-phase IVM on cleavage and blastocyst development in PA embryos
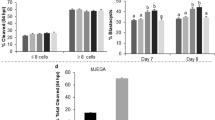
To evaluate the effects of two-phase IVM on the developmental competence of PA embryos, we cultured the IVM oocytes after activation. The cleavage rate was not significantly different among the control, rolipram-, cilostamide-, and rolipram–cilostamide-treated groups, whereas blastocyst formation rates were significantly higher in the rolipram–cilostamide group than in other groups (Table 2). The cleavage rate in the control, rolipram-, cilostamide-, and rolipram–cilostamide-treated groups was 71.5%, 72.6%, 72.9%, and 76.0%, respectively. Therefore, the rolipram–cilostamide-treated group showed a significantly higher cleavage rate than the control group, while no significant difference was observed between the rolipram–cilostamide-treated group and the other treatment groups. Moreover, the blastocyst development rate was significantly different between the rolipram–cilostamide-treated group and the other groups (27.2%, 28.1%, 30.0%, and 35.5% in the control, rolipram-, cilostamide-, and rolipram–cilostamide-treated groups, respectively). Finally, the blastocyst development rate did not differ significantly between the rolipram group and the control or cilostamide groups.
Effect of two-phase IVM on cleavage and blastocyst development rate in SCNT embryos
To evaluate the effects of two-phase IVM on the developmental competence of SCNT-derived embryos, the cleavage and blastocyst development rates were determined after culturing reconstructed oocytes for 2 and 7 days, respectively, in a PZM-5 medium. The cleavage rate was significantly different between the control and rolipram–cilostamide-treated groups (69.1% versus 77.0%, respectively). Similarly, the blastocyst development rate was significantly higher in the rolipram–cilostamide-treated group than in the control group (27.3%versus 19.5%, respectively; P = 0.000; Table 3).
Pre-implantation developmental competence of two-phase blastocysts
We evaluated the pre-implantation developmental competence of embryos derived from the two-phase IVM oocytes using a differential staining technique to determine the total cell count, ICM, and TE ratio. The results showed a significant difference between the rolipram–cilostamide-treated and control groups in terms of total cell count, ICM, and TE ratio, respectively. Specifically, the ICM (10.7 ± 1.31 versus 6.8 ± 0.78) and TE ratio (25.2 ± 0.83 versus 26.8 ± 1.63) were significantly higher in the rolipram–cilostamide-treated group compared to the control group (Fig. 6). Moreover, the ICM to TE ratio was significantly higher in the embryos derived from the rolipram–cilostamide-treated oocytes than in the control group oocytes (0.269 versus 0.399, respectively).
Effect of combined two-phase IVM with rolipram and cilostamide on the embryonic development of transgenic cloned porcine embryos. Representative images of blastocysts developed from oocytes matured in control (A) and rolipram–cilostamide (B) during IVM. At least 68 blastocysts were stained from each group. (C) Inner cell mass to TE ratio in differential staining. The inner cell mass to TE ratio in blastocysts from two-phase IVM differed significantly from that from the control (0.399 vs 0.269, respectively, P < 0.05). The nuclei of ICM and TE cells were stained with Hoechst (blue) and PI (red) dye, respectively. The data are from at least three independent experiments, and the values represent the mean ± SEM.
Relative level of gene expression
The effect of two-phase IVM was evaluated according to the relative level of mRNA expression for nuclear modeling and reprogramming in SCNT blastocysts (Fig. 7). Notably, the expression of the pro-apoptotic gene (caspase 3) was found to be statistically significantly different between control and two-phase treated groups (P < 0.05). Moreover, there is a tendency to reduce the expression of pro-apoptotic gene BAX while the expressions of BCL2, Oct4, and SOX2 tended to be increased in the two-phase treated groups (P < 0.1).
Relative quantitative analysis of mRNA transcript expression by real-time PCR in control and two-phase IVM porcine oocyte blastocysts. At least 5 blastocysts were obtained per sample and 8 biological replications were performed. Control samples were set as arbitrary units, and the target genes were expressed as the fold-change of the corresponding control relative to the house keeping gene (β-actin). *Indicates a significant difference at P < 0.05.
Discussion
During the IVM of mammalian oocytes, cAMP modulators demonstrate significant improvement in terms of oocyte developmental competence to support subsequent embryonic development. Specifically, cAMP modulators, including milrinone, cilostamide, and 3-isobutyl-1-methylxanthine, have positive effects on the developmental competence of oocytes. Improving oocyte competence enhances later embryonic development to the blastocyst stage74. To do so, the optimal level of cAMP must be achieved to regulate adenyl cyclase activators and/or PDE inhibitors that cause temporary meiotic arrest without permanently impairing meiotic progression. Previous reports have demonstrated that cytoplasmic maturation can be synchronized with nuclear maturation using PDE inhibitors either during IVM or pre-IVM75,76,77.
Several studies have reported that cAMP modulators enhance the developmental competence of porcine oocytes after IVF and SCNT. The use of cAMP modulators during IVM results in the proper cytoplasmic maturation of oocytes to support the subsequent embryonic development78,79,80. Another study reported that cAMP modulators, including cilostamide and forskolin, effectively cause meiotic arrest in the oocytes following pre-IVM for 24 h32. However, cAMP modulators may negatively regulate the meiosis of oocytes, particularly when higher concentrations are used or oocytes are exposed for a longer duration81. Similar findings were also reported during the IVM of bovine oocytes58. However, our previous study showed that the use of higher concentrations of milrinone during IVM of porcine oocytes had no negative effects63. In the current study, we observed that higher concentrations of PDE inhibitors have non-beneficial effects, which could be due to permanent or prolonged meiotic arrest, which negatively affects IVM.
The current study demonstrated that pre-IVM treatment of rolipram (using its optimal concentration) significantly affected the developmental competence of IVM oocytes. Rolipram affects the functional GJC required for attaining higher levels of cAMP to induce temporary meiotic arrest in porcine oocytes. Similarly, rolipram treatment induced meiotic arrest in rat oocytes82 but failed to induce meiotic arrest in bovine oocytes83.
Various studies have reported a higher level of cytoplasmic maturation during the two-phase approach compared to the single-phase PDE inhibitor approach. The non-specific PDE inhibitor IBMX inhibited both cumulus cell and oocyte PDEs, whereas the PDE3-specific inhibitor cilostamide inhibited only intraoocyte oocyte PDEs84. In contrast, PDE4 inhibitors was reported to affect only cAMP levels in cumulus cells48,80. In the current study, we proposed using a combination of PDE3 and PDE4 inhibitors to attain optimal synchronization of cytoplasmic and nuclear maturation. This two-phase IVM procedure significantly affected the GJC and subsequent developmental competence of porcine oocytes. Specifically, the two-phase procedure effectively maintained the GJC and the level of cytoplasmic maturation in the porcine oocytes compared to the single-phase PDE-treated groups and the control group. These findings are consistent with previous reports of improved competence of bovine oocytes in the presence of invasive adenyl cyclase85,86.
Two-phase IVM approaches intend to improve the developmental competence of IVM oocytes through the spontaneous regulation of nuclear maturation and oxidative stress84. In the current study, our two-phase IVM approach enabled synchronized cytoplasmic and nuclear maturation, which is evident by the significantly higher relative expression of the genes associated with cytoplasmic maturation, including BMP15 and GDF9. Similar findings were reported in other studies where the cytoplasmic maturation of porcine oocytes was confirmed through BMP15 and GDF9 upregulation87,88. The findings of the current study are also consistent with the study by Funahashi and his team, who reported that the two-phase maturation of the germinal vesicle of porcine oocytes during IVM is responsible for the subsequent developmental competence89. Furthermore, the higher level of GSH and lower level of ROS after two-phase IVM were consistent with a previous study by Park et al.32 and contributed to improving the developmental competence of the oocytes.
Our results demonstrated that two-phase IVM yielded better oocyte competence. Moreover, the two-phase IVM approach resulted in a significantly higher blastocyst formation of PA embryos than that of other groups. Several studies have reported similar findings in sheep39, rats90, bovine91, and pigs71. This improved developmental competence of PA embryos was likely due to the synchronized maturation of the nucleus and cytoplasm to support the subsequent embryonic development. Similar findings were reported in mice92 and other mammals93. Moreover, the relative reductions in the expression of BAX94 and Caspase-395 in two-phase as compared to the control group were a good indication of the development competence of embryos from two-phase treated group (Fig. 7). The relative level of the expression of anti-apoptotic genes94 and nuclear reprograming related gene OCT4 and SOX294,96 were well recognized genes as indicator of developmental competence of in vitro produced porcine embryos.
In the cloned embryos derived from oocytes subjected to two-phase IVM, both the cleavage rate and blastocyst formation rate were significantly higher than those derived from the control oocytes. Another study showed similar findings using cilostamide, which significantly improved the formation of porcine SCNT blastocysts71. In bovine embryos, the two-phase IVM approach led to significant variation in the level of cytoplasmic maturation and subsequent blastocyst formation73. The results of the current study revealed that meiotic progression could be reversibly attenuated through the combination of PDE3 and PDE4 inhibitors to synchronize nuclear and cytoplasmic maturation in porcine oocytes.
Finally, the evaluation of the blastocysts for pre-implantation development by differential staining and tunnel assay also showed that two-phase IVM significantly improved the developmental competence in porcine blastocysts, consistent with the 2016 findings by Park et al.32,63. Our findings also support the hypothesis that a higher total cell count and ICM are indicators of the developmental competence of blastocysts in various species of mammals97,98. In conclusion, we found that two-phase IVM of porcine oocytes can be attained through the combination of the PDE3 and PDE4 inhibitors (50 µM rolipram and 20 µM cilostamide) for 4 h pre-IVM. This two-phase IVM played a significant role in synchronizing the cytoplasmic and nuclear maturation, which is pivotal in nuclear remodeling and reprogramming, particularly in SCNT. We suggest further experiments be conducted for the wide-scale application of two-phase IVM in porcine oocytes.
Conclusions
Rolipram and cilostamide application during pre IVM as phosphodiesterase inhibitor 4 and 3, respectively, had improved the functioned GJC in porcine oocyte as indicated by enhancing the in vitro matured oocyte competence in terms of cytoplasm maturation and supported the subsequent embryonic development. We also showed that the combined treatment of rolipram and cilostamide attained the relatively better preimplantation development competence of multigene modified cloned porcine embryos.
Materials and methods
Culture media
Sigma-Aldrich (St. Louis, MO, USA) is source of all chemicals and reagents used unless stated. Cilostamide and rolipram stock solutions were prepared in dimethyl sulfoxide and aliquots were prepared during pre-IVM. Cilostamide was used at a concentration of 20 µM99 based on previous studies, and we determined the optimal concentration and time of rolipram for pre-IVM as there were no previous study. The medium used for IVM tissue culture medium-199 (Invitrogen, Carlsbad, CA, USA), which were supplemented with 10% porcine follicular fluid (v/v), 0.6 mM cysteine, 0.91 mM pyruvate, 75 mg/mL kanamycin, and 1 mg/mL insulin. The in vitro culture (IVC) medium used for embryonic in vitro culture was porcine zygote medium-5 (PZM5; IFP, Yamagata, Japan).
Oocyte collection and in vitro maturation
Oocyte IVM was performed following our laboratory protocols as previously described100. Ovaries from gilts. Oocytes with sizes of 3–8 mm in diameter were aspirated and aspirates were washed as mentioned in our previous study101.
Measurement of GJC
The functional GJC was evaluated through the intraoocyte microinjection of Lucifer yellow, whose dissemination level to cumulus cells was evaluated102. The classification of the functional state of JGC were open, partially open, or closed103.
Intraoocyte GSH and ROS levels measurement
Glutathione (GSH) and reactive oxygen species (ROS) levels were measured as described previously104,105,106. 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA; Invitrogen), on the bases of the intensity of fluorescence to measure the ROS level. The GSH level determination was performed using Cell Tracker™ Blue (CMF2HC). About ten to thirteen oocytes from each treatment group (control, rolipram treated, cilostamide treated and combination of rolipram and cilostamide treated) were incubated in TLH-PVA supplemented with 10 mM H2DCFDA and 10 mM CMF2HC for 30 min in the dark. Then, the oocytes were washed Dulbecco’s Phosphate Buffered Saline (dPBS) (Invitrogen) containing 0.1% (w/v) PVA. The GSH and ROS levels were evaluated in different groups using epifluorescence microscope (Leica DM IRB; Leica Microsystems) and fluorescence intensity evaluation is performed using ImageJ software (version 1.41; National Institutes of Health, Bethesda, MD, USA)101.
Preparation of donor cells
Cells with genes modified for human immune reactivity, i.e., alpha-1,3-galactosyltransferase (GGTA1), cytidine monophosphate-N-acetylneuraminic acid hydroxylase (CMAH), and alpha 1,3-galactosyltransferase 2 (A3GALT2) triple knockout (GGTA1 + CMAH + iGb3S TKO) porcine cells were used as donor cells107. The cells were seeded in a four-well plate and cultured for at least 2 days in dPBS (Invitrogen) with 15% (v/v) fetal bovine serum and 75 mg/mL kanamycin. Cells followed until a complete monolayer formed and passage 3 or 4 were used as donor cells. A single-cell suspension was prepared by trypsinizing cultured cells and resuspending in TLH containing 0.4% (w/v) bovine serum albumin (BSA) (TLH–BSA) before nuclear transfer.
SCNT, PA, and embryo culture
The COCs were cultured and incubated overnight in a pre-treated IVM medium. After 40–42 h of IVM, matured oocytes were denuded in 0.1% (w/v) hyaluronidase in a hormone-free IVM medium. Invitro procedures were performed in calcium-free TLH–BSA with 5 mg/mL cytochalasin B. Before manipulation, oocytes were incubated for at least 30 min in a manipulation medium containing 5 µg/mL Hoechst 33342. Following washing, the oocytes were placed into a droplet of manipulation medium covered with mineral oil. Enucleation was performed by aspirating the first polar body by using a 17-mm beveled glass pipette. Enucleation was confirmed under an epifluorescence microscope (TE300; Nikon, Tokyo, Japan). Then, the oocytes were transferred to another medium for cell injection, and around 20 cells were aspirated into pipette; and one cell was inserted as donner cell108. Then, fusion was performed using a BTX 2001 Electro-cell Manipulator. The activated embryos of both PA and SCNT were treated either 7.5 µg/mL cytochalasin B and 0.4 mg/mL demecolcine, respectively then transferred into 25-µL IVC. Cleavage and blastocyst development were evaluated following two and seven days of culturing, respectively, with the day of SCNT or PA designated as Day 0.
Quantitative real-time PCR (q-PCR)
We extracted total RNA from Day 7 blastocysts RNeasy Micro Kit (QIAGEN, Hilden, Germany), according to the manufacturer’s protocol. The primer sequences listed in Table 4 were used to evaluate the abundance of the transcripts.
Differential staining in SCNT blastocysts
We performed differential staining to determine blastocyst competence using the total cell number. The inner cell mass (ICM) to trophectoderm (TE) ratio was used as indicator of pre-implantation embryos development competence, as described in our previous reports104. Briefly, diluted Triton X-100 in PBS (T solution) at concentration of 0.1% (v/v), and propidium iodide (PI) (25 μg/mL) and Hoechst 33342 solutions (H solution) (5 μg/mL) were used for the staining. The staining followed by placing blastocysts in H solution first (40 min) then in T solution (1 min) at room temperature and then brief period (30–40 s) in PI solution.
Experimental design
For pre-IVM treatment, immature COCs were either untreated (control) or treated with 50 µM rolipram, 20 µM cilostamide, or both. Then, the level of cytoplasm maturation, ROS, and GSH of the oocytes and the status of GJC in the pre-IVM oocytes were assessed. Furthermore, the developmental competence of PA and SCNT embryos was assessed. The duration of pre-IVM in this study was determined according to the GJC and IVM oocyte competence, and the starting reference value was taken from pigs109 and humans99.
In experiment 1, the effects of rolipram–cilostamide treatment on GJC during pre-IVM, intraoocyte ROS and GSH levels, and embryonic development after PA were examined. Subsequently, in experiment 2, the effects of rolipram–cilostamide treatment on cytoplasm maturation status, as an indication of the synchrony of nucleus and cytoplasm maturation, were examined in IVM oocytes. In experiment 3, the effects of rolipram–cilostamide combination treatment on the developmental competence of oocytes were compared with those of rolipram and cilostamide monotreatment. In experiment 4, we assessed the pre-implantation developmental competence of PA and SCNT blastocysts (Fig. 3).
Statistical analysis
At least eight replicates of each experiment were performed, and the values were compared as the mean ± standard error of the mean (SEM). SPSS version 22 (SPSS 22.0; IBM, Armonk, New York, USA) was used for the statistical analyses. Moreover, in case of two groups, independent sample t-test was used, while univariate analysis of variance (ANOVA) with Tukey’s multiple comparison tests were used to determine the significant differences among the four experimental groups. P-values < 0.05 were considered significantly different among the experimental groups.
Data availability
The data sets used during the current study are available from the corresponding author on reasonable request.
Abbreviations
- IVM:
-
In vitro maturation
- PA:
-
Parthenogenic activation
- SCNT:
-
Somatic cell nuclear transfer
- dPBS:
-
Dulbecco’s phosphate buffered saline
- cAMP:
-
Cyclic adenine monophosphate
- BMP15:
-
Bone morphogenetic protein 15
- cGMP:
-
Cyclic guanine monophosphate
- GDF9:
-
Growth development factor 9
- IVP:
-
In vitro production
- GJC:
-
Gap junction communication
- IVF:
-
In vitro fertilization
- PDE:
-
Phosphodiesterase
- IVC:
-
In vitro culture
- GSH:
-
Glutathione
- ROS:
-
Reactive oxygen species
- BSA:
-
Bovine serum albumin
- COC:
-
Cumulus oocyte complexes
- SEM:
-
Standard error of the mean
- PZM:
-
Porcine zygote media
- ICM:
-
Inner cell mass
- TE:
-
Trophectoderm
- mRNA:
-
Messenger ribonucleic acid
References
Lai, L. et al. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science 295(5557), 1089–1092 (2002).
Polejaeva, I. A. et al. Cloned pigs produced by nuclear transfer from adult somatic cells. Nature 407(6800), 86–90 (2000).
Yin, X. J. et al. Production of cloned pigs from adult somatic cells by chemically assisted removal of maternal chromosomes. Biol. Reprod. 67(2), 442–446 (2002).
Boquest, A. C. et al. Production of cloned pigs from cultured fetal fibroblast cells. Biol. Reprod. 66(5), 1283–1287 (2002).
Cho, J. et al. Improved efficiencies in the generation of multigene-modified pigs by recloning and using sows as the recipient. Zygote 30, 1–8 (2021).
Nagashima, H. et al. Development of efficient strategies for the production of genetically modified pigs. Theriogenology 59(1), 95–106 (2003).
Kurome, M. et al. Comparison of electro-fusion and intracytoplasmic nuclear injection methods in pig cloning. Cloning Stem Cells 5(4), 367–378 (2003).
Booth, P., Tan, S., Holm, P. & Callesen, H. Application of the zona-free manipulation technique to porcine somatic nuclear transfer. Cloning Stem Cells 3(4), 191–197 (2001).
Zhao, H. et al. Source and follicular fluid treatment during the in vitro maturation of recipient oocytes affects the development of cloned pig embryo. Cell Reprogram. 22(2), 71–81 (2020).
Santiquet, N. W. et al. A pre-in vitro maturation medium containing cumulus oocyte complex ligand-receptor signaling molecules maintains meiotic arrest, supports the cumulus oocyte complex and improves oocyte developmental competence. Mol. Hum. Reprod. 23(9), 594–606 (2017).
You, J., Lee, J., Hyun, S. H. & Lee, E. L-carnitine treatment during oocyte maturation improves in vitro development of cloned pig embryos by influencing intracellular glutathione synthesis and embryonic gene expression. Theriogenology 78(2), 235–243 (2012).
Ikeda, K. & Takahashi, Y. Effects of maturational age of porcine oocytes on the induction of activation and development in vitro following somatic cell nuclear transfer. J. Vet. Med. Sci. 63(9), 1003–1008 (2001).
Kim, J. et al. Developmental competence of morphologically poor oocytes in relation to follicular size and oocyte diameter in the pig. Mol. Reprod. Dev. 77(4), 330–339 (2010).
Pan, B. & Li, J. The art of oocyte meiotic arrest regulation. Reprod. Biol. Endocrinol. 17(1), 8 (2019).
Solc, P. et al. CDC25A phosphatase controls meiosis I progression in mouse oocytes. Dev. Biol. 317(1), 260–269 (2008).
Horner, K. et al. Rodent oocytes express an active adenylyl cyclase required for meiotic arrest. Dev. Biol. 258(2), 385–396 (2003).
Somfai, T. et al. Enhancement of lipid metabolism with L-carnitine during in vitro maturation improves nuclear maturation and cleavage ability of follicular porcine oocytes. Reprod. Fertil. Dev. 23(7), 912–920 (2011).
Krisher, R., Brad, A., Herrick, J., Sparman, M. & Swain, J. A comparative analysis of metabolism and viability in porcine oocytes during in vitro maturation. Anim. Reprod. Sci. 98(1–2), 72–96 (2007).
Park, J. E., Kim, M. S., Lee, E. & Lee, S. T. In vitro maturation using an agarose matrix with incorporated extracellular matrix proteins improves porcine oocyte developmental competence by enhancing cytoplasmic maturation. J. Tissue Eng. Regen. Med. 15(10), 807–817 (2021).
Coskun, S. & Lin, Y. C. Mechanism of action of epidermal growth factor-induced porcine oocyte maturation. Mol. Reprod. Dev. 42(3), 311–317 (1995).
de Souza-Fabjan, J. M. G. et al. In vitro production of small ruminant embryos: Late improvements and further research. Theriogenology 81(9), 1149–1162 (2014).
Ferré, L. et al. Recent advances in bovine in vitro embryo production: Reproductive biotechnology history and methods. Animal 14(5), 991–1004 (2020).
Vajta, G., Zhang, Y. & Macháty, Z. Somatic cell nuclear transfer in pigs: Recent achievements and future possibilities. Reprod. Fertil. Dev. 19(2), 403–423 (2007).
Cao, Z. et al. Human exhaled air can efficiently support in vitro maturation of porcine oocytes and subsequent early embryonic development. Anim. Reprod. 15(1), 29 (2018).
Xu, M., Qian, J., Si, L., Qu, X. & Li, J. The effect of epigenetic changes on the extrusion of the first polar body in pig oocytes during in vitro maturation. Cell. Reprogram. 21(3), 129–140 (2019).
Wang, X. et al. Effects of resveratrol on in vitro maturation of porcine oocytes and subsequent early embryonic development following somatic cell nuclear transfer. Reprod. Domest. Anim. 54(9), 1195–1205 (2019).
Edwards, R. G. Maturation in vitro of mouse, sheep, cow, pig, rhesus monkey and human ovarian oocytes. Nature 208(5008), 349–351 (1965).
Saadeldin, I. M. & Cho, J. Current approaches for assisted oocyte maturation in camels. J. Anim. Reprod. Biotechnol. 36(3), 162–167 (2021).
Hulinska, P., Martecikova, S., Jeseta, M. & Machatkova, M. Efficiency of in vitro fertilization is influenced by the meiotic competence of porcine oocytes and time of their maturation. Anim. Reprod. Sci. 124(1–2), 112–117 (2011).
Carabatsos, M. J., Sellitto, C., Goodenough, D. A. & Albertini, D. F. Oocyte–granulosa cell heterologous gap junctions are required for the coordination of nuclear and cytoplasmic meiotic competence. Dev. Biol. 226(2), 167–179 (2000).
Anderson, E. & Albertini, D. F. Gap junctions between the oocyte and companion follicle cells in the mammalian ovary. J. Cell Biol. 71(2), 680–686 (1976).
Park, B. et al. Cilostamide and forskolin treatment during pre-IVM improves preimplantation development of cloned embryos by influencing meiotic progression and gap junction communication in pigs. Theriogenology 86(3), 757–765 (2016).
Zhao, Y. et al. Capacitation IVM improves cumulus function and oocyte quality in minimally stimulated mice. J. Assist. Reprod. Genet. 37(1), 77–88 (2020).
Linher, K., Wu, D. & Li, J. Glial cell line-derived neurotrophic factor: An intraovarian factor that enhances oocyte developmental competence in vitro. Endocrinology 148(9), 4292–4301 (2007).
Wu, D., Cheung, Q.C.-K., Wen, L. & Li, J. A growth-maturation system that enhances the meiotic and developmental competence of porcine oocytes isolated from small follicles. Biol. Reprod. 75(4), 547–554 (2006).
Kim, E. et al. An improved system for generation of diploid cloned porcine embryos using induced pluripotent stem cells synchronized to metaphase. PLoS ONE 11(7), e0160289 (2016).
Kubota, C. et al. Six cloned calves produced from adult fibroblast cells after long-term culture. Proc. Natl. Acad. Sci. 97(3), 990–995 (2000).
Kim, E. et al. Putative embryonic stem cells derived from porcine cloned blastocysts using induced pluripotent stem cells as donors. Theriogenology 85(4), 601–616 (2016).
Rose, R. D., Gilchrist, R. B., Kelly, J. M., Thompson, J. G. & Sutton-McDowall, M. L. Regulation of sheep oocyte maturation using cAMP modulators. Theriogenology 79(1), 142–148 (2013).
Downs, S. M., Daniel, S. A., Bornslaeger, E. A., Hoppe, P. C. & Eppig, J. J. Maintenance of meiotic arrest in mouse oocytes by purines: Modulation of cAMP levels and cAMP phosphodiesterase activity. Gamete Res. 23(3), 323–334 (1989).
Wigglesworth, K. et al. Bidirectional communication between oocytes and ovarian follicular somatic cells is required for meiotic arrest of mammalian oocytes. Proc. Natl. Acad. Sci. 110(39), E3723–E3729 (2013).
Downs, S. M., Hudson, E. R. & Hardie, D. G. A potential role for AMP-activated protein kinase in meiotic induction in mouse oocytes. Dev. Biol. 245(1), 200–212 (2002).
Isobe, N., Fujihara, M. & Terada, T. Cumulus cells suppress meiotic progression in pig oocytes cultured in vitro. Theriogenology 45(8), 1479–1489 (1996).
Shimada, M. & Terada, T. FSH and LH induce progesterone production and progesterone receptor synthesis in cumulus cells: A requirement for meiotic resumption in porcine oocytes. Mol. Hum. Reprod. 8(7), 612–618 (2002).
Zhang, M. et al. Meiotic arrest with roscovitine and follicular fluid improves cytoplasmic maturation of porcine oocytes by promoting chromatin de-condensation and gene transcription. Sci. Rep. 7(1), 1–15 (2017).
Zhu, S. et al. Meiotic block with roscovitine improves competence of porcine oocytes by fine-tuning activities of different cyclin-dependent kinases. J. Cell. Physiol. 235(10), 7530–7540 (2020).
Mehlmann, L. M. Stops and starts in mammalian oocytes: Recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction 130(6), 791–799 (2005).
Pan, B. & Li, J. The art of oocyte meiotic arrest regulation. Reprod. Biol. Endocrinol. 17(1), 1–12 (2019).
Tiwari, M. et al. Role of mitogen activated protein kinase and maturation promoting factor during the achievement of meiotic competency in mammalian oocytes. J. Cell. Biochem. 119(1), 123–129 (2018).
Adhikari, D. & Liu, K. The regulation of maturation promoting factor during prophase I arrest and meiotic entry in mammalian oocytes. Mol. Cell. Endocrinol. 382(1), 480–487 (2014).
Perrin, A. J., Patel, A., Flueck, C., Blackman, M. J. & Baker, D. A. cAMP signalling and its role in host cell invasion by malaria parasites. Curr. Opin. Microbiol. 58, 69–74 (2020).
Gupta, A. et al. Cyclic nucleotides regulate oocyte meiotic maturation and quality in mammals. J. Reprod. Healthcare Med. 1(1), 1 (2020).
Alam, M. H. & Miyano, T. Interaction between growing oocytes and granulosa cells in vitro. Reprod. Med. Biol. 19(1), 13–23 (2020).
Appeltant, R., Beek, J., Vandenberghe, L., Maes, D. & Van Soom, A. Increasing the cAMP concentration during in vitro maturation of pig oocytes improves cumulus maturation and subsequent fertilization in vitro. Theriogenology 83(3), 344–352 (2015).
Guimarães, A., Pereira, S., Leme, L. & Dode, M. Evaluation of the simulated physiological oocyte maturation system for improving bovine in vitro embryo production. Theriogenology 83(1), 52–57 (2015).
Lee, D. et al. Effects of cyclic adenosine monophosphate modulators on maturation and quality of vitrified-warmed germinal vesicle stage mouse oocytes. Reprod. Biol. Endocrinol. 18, 1–8 (2020).
Fair, T. & Lonergan, P. Developments in the use of embryo technologies in dairy cows. In Advances in Breeding of Dairy Cattle (eds van der Werf, J. & Pryce, J.) 531–70 (Burleigh Dodds Science Publishing, 2019).
Mayes, M. A. & Sirard, M.-A. Effect of type 3 and type 4 phosphodiesterase inhibitors on the maintenance of bovine oocytes in meiotic arrest. Biol. Reprod. 66(1), 180–184 (2002).
Kumari, M., Cover, P. O., Poyser, R. H. & Buckingham, J. C. Stimulation of the hypothalamo-pituitary-adrenal axis in the rat by three selective type-4 phosphodiesterase inhibitors: In vitro and in vivo studies. Br. J. Pharmacol. 121(3), 459–468 (1997).
Sasseville, M., Côté, N., Guillemette, C. & Richard, F. J. New insight into the role of phosphodiesterase 3A in porcine oocyte maturation. BMC Dev. Biol. 6(1), 1–18 (2006).
Sela-Abramovich, S., Edry, I., Galiani, D., Nevo, N. & Dekel, N. Disruption of gap junctional communication within the ovarian follicle induces oocyte maturation. Endocrinology 147(5), 2280–2286 (2006).
Sasseville, M., Côté, N., Vigneault, C., Guillemette, C. & Richard, F. J. 3′5′-Cyclic adenosine monophosphate-dependent up-regulation of phosphodiesterase type 3A in porcine cumulus cells. Endocrinology 148(4), 1858–1867 (2007).
Roy, P. K. et al. Enhancing oocyte competence with milrinone as a phosphodiesterase 3A inhibitor to improve the development of porcine cloned embryos. Front. Cell Dev. Biol. 9, 647616 (2021).
Dieci, C. et al. The effect of cilostamide on gap junction communication dynamics, chromatin remodeling, and competence acquisition in pig oocytes following parthenogenetic activation and nuclear transfer. Biol. Reprod. 89(3), 68 (2013).
Wiersma, A. et al. Phosphodiesterase 3 inhibitors suppress oocyte maturation and consequent pregnancy without affecting ovulation and cyclicity in rodents. J. Clin. Investig. 102(3), 532–537 (1998).
Jensen, J. et al. Phosphodiesterase 3 inhibitors selectively block the spontaneous resumption of meiosis by macaque oocytes in vitro. Hum. Reprod. 17(8), 2079–2084 (2002).
Urner, F. & Schorderet-Slatkine, S. Inhibition of denuded mouse oocyte meiotic maturation by tumor-promoting phorbol esters and its reversal by retinoids. Exp. Cell Res. 154(2), 600–605 (1984).
Raza, S. H. A. et al. The role of forskolin as a lipolytic stimulator during in vitro oocyte maturation and the in vitro embryo production of livestock. Reprod. Domest. Anim. 56, 1486 (2021).
Dekel, N., Aberdam, E. & Sherizly, I. Spontaneous maturation in vitro of cumulus-enclosed rat oocytes is inhibited by forskolin. Biol. Reprod. 31(2), 244–250 (1984).
Richard, F. J., Tsafriri, A. & Conti, M. Role of phosphodiesterase type 3A in rat oocyte maturation. Biol. Reprod. 65(5), 1444–1451 (2001).
Thongkittidilok, C. et al. Cilostamide and forskolin maintain gap junction function of incubated dog follicles. Theriogenology 142, 222–228 (2020).
Racowsky, C. Effect of forskolin on maintenance of meiotic arrest and stimulation of cumulus expansion, progesterone and cyclic AMP production by pig oocyte—Cumulus complexes. Reproduction 74(1), 9–21 (1985).
Thomas, R. E., Thompson, J. G., Armstrong, D. T. & Gilchrist, R. B. Effect of specific phosphodiesterase isoenzyme inhibitors during in vitro maturation of bovine oocytes on meiotic and developmental capacity. Biol. Reprod. 71(4), 1142–1149 (2004).
Grupen, C. G., Fung, M. & Armstrong, D. T. Effects of milrinone and butyrolactone-I on porcine oocyte meiotic progression and developmental competence. Reprod. Fertil. Dev. 18(3), 309–317 (2006).
Beek, J. Novel Insights in the Role of Proteases During Porcine Fertilization In Vitro (Ghent University, 2015).
Somfai, T. et al. 299 in vitro development of immature porcine oocytes fertilized in vitro to the blastocyst stage. Reprod. Fertil. Dev. 17(2), 300 (2004).
Van, N. T. T., Van Thuan, N. & Bui, H.-T. Improve the developmental competence of porcine oocytes from small antral follicles by pre-maturation culture method. Theriogenology 149, 139–148 (2020).
Laforest, M. F., Pouliot, É., Guéguen, L. & Richard, F. J. Fundamental significance of specific phosphodiesterases in the control of spontaneous meiotic resumption in porcine oocytes. Mol. Reprod. Dev. Incorpor. Gamete Res. 70(3), 361–372 (2005).
Procházka, R. et al. The role of MAPK3/1 and AKT in the acquisition of high meiotic and developmental competence of porcine oocytes cultured in vitro in FLI medium. Int. J. Mol. Sci. 22(20), 11148 (2021).
Richani, D. & Gilchrist, R. B. Approaches to oocyte meiotic arrest in vitro and impact on oocyte developmental competence. Biol. Reprod. 106, 243 (2021).
Gilchrist, R. B. et al. Oocyte maturation and quality: Role of cyclic nucleotides. Reproduction 152(5), R143–R157 (2016).
Gupta, A. & Chaube, S. K. Cilostamide and rolipram prevent spontaneous meiotic resumption from diplotene arrest in rat oocytes cultured in vitro. Eur. J. Pharmacol. 878, 173115 (2020).
Alam, M. H., Lee, J. & Miyano, T. Inhibition of PDE3A sustains meiotic arrest and gap junction of bovine growing oocytes in in vitro growth culture. Theriogenology 118, 110–118 (2018).
Albuz, F. et al. Simulated physiological oocyte maturation (SPOM): A novel in vitro maturation system that substantially improves embryo yield and pregnancy outcomes. Hum. Reprod. 25(12), 2999–3011 (2010).
Aktas, H., Wheeler, M., Rosenkrans, C., First, N. & Leibfried-Rutledge, M. Maintenance of bovine oocytes in prophase of meiosis I by high [cAMP] I. Reproduction 105(2), 227–235 (1995).
Luciano, A. et al. Effect of different levels of intracellular cAMP on the in vitro maturation of cattle oocytes and their subsequent development following in vitro fertilization. Mol. Reprod. Dev. Incorpor. Gamete Res. 54(1), 86–91 (1999).
Yin, Z. et al. Tannin supplementation improves oocyte cytoplasmic maturation and subsequent embryo development in pigs. Antioxidants 10(10), 1594 (2021).
Yang, L. et al. Effect of melatonin on the in vitro maturation of porcine oocytes, development of parthenogenetically activated embryos, and expression of genes related to the oocyte developmental capability. Animals 10(2), 209 (2020).
Funahashi, H., Cantley, T. C. & Day, B. N. Synchronization of meiosis in porcine oocytes by exposure to dibutyryl cyclic adenosine monophosphate improves developmental competence following in vitro fertilization. Biol. Reprod. 57(1), 49–53 (1997).
Feng, P., Catt, K. J. & Knecht, M. Transforming growth factor-β stimulates meiotic maturation of the rat oocyte. Endocrinology 122(1), 181–186 (1988).
Sugimura, S. et al. Effect of pre-in vitro maturation with cAMP modulators on the acquisition of oocyte developmental competence in cattle. J. Reprod. Dev. 64, 233 (2018).
Eppig, J. J., Schultz, R. M., O’Brien, M. & Chesnel, F. Relationship between the developmental programs controlling nuclear and cytoplasmic maturation of mouse oocytes. Dev. Biol. 164(1), 1–9 (1994).
Watson, A. Oocyte cytoplasmic maturation: a key mediator of oocyte and embryo developmental competence. J. Anim. Sci. 85, E1–E3 (2007).
No, J. G. et al. Scriptaid improves the reprogramming of donor cells and enhances canine-porcine interspecies embryo development. Reprod. Biol. 18(1), 18–26 (2018).
Li, X., Wang, Y. K., Song, Z. Q., Du, Z. Q. & Yang, C. X. Dimethyl sulfoxide perturbs cell cycle progression and spindle organization in porcine meiotic oocytes. PLoS ONE 11(6), e0158074 (2016).
Lee, J. H. et al. Development and gene expression of porcine cloned embryos derived from bone marrow stem cells with overexpressing Oct4 and Sox2. Cell Reprogram. 16(6), 428–438 (2014).
Stephens, L. E. et al. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 9(15), 1883–1895 (1995).
Richter, K. S., Harris, D. C., Daneshmand, S. T. & Shapiro, B. S. Quantitative grading of a human blastocyst: Optimal inner cell mass size and shape. Fertil. Steril. 76(6), 1157–1167 (2001).
Shu, Y.-M. et al. Effects of cilostamide and forskolin on the meiotic resumption and embryonic development of immature human oocytes. Hum. Reprod. 23(3), 504–513 (2008).
Roy, P.-K. et al. Modified spirulina maxima pectin nanoparticles improve the developmental competence of in vitro matured porcine oocytes. Animals 11, 9 (2021).
Roy, P. K., Qamar, A. Y., Fang, X., Hassan, B. M. S. & Cho, J. Effects of cobalamin on meiotic resumption and developmental competence of growing porcine oocytes. Theriogenology 154, 24–30 (2020).
Isobe, N., Maeda, T. & Terada, T. Involvement of meiotic resumption in the disruption of gap junctions between cumulus cells attached to pig oocytes. Reproduction 113(2), 167–172 (1998).
Luciano, A. M. et al. Role of intracellular cyclic adenosine 3′, 5′-monophosphate concentration and oocyte-cumulus cells communications on the acquisition of the developmental competence during in vitro maturation of bovine oocyte. Biol. Reprod. 70(2), 465–472 (2004).
Roy, P. K., Fang, X., Hassan, B. M., Shin, S. T. & Cho, J. K. Effects of roscovitine on in vitro development of porcine oocyte using brilliant cresyl blue. J. Embryo Transfer 32(3), 111–122 (2017).
Hassan, B. M., Fang, X., Roy, P. K., Shin, S. T. & Cho, J. K. Effect of alpha lipoic acid as an antioxidant supplement during in vitro maturation medium on bovine embryonic development. J. Embryo Transfer 32(3), 123–130 (2017).
Jeon, Y.-E., Hwangbo, Y., Kim, S.-Y. & Park, C.-K. Alpha-linolenic acid enhances maturation and developmental competence via regulation of glutathione, cAMP and fatty acid accumulation during in vitro maturation of porcine oocytes. J. Anim. Reprod. Biotechnol. 35(4), 357–365 (2020).
Shim, J. et al. Human immune reactivity of GGTA1/CMAH/A3GALT2 triple knockout Yucatan miniature pigs. Transgenic Res. 30(5), 619–634 (2021).
Walker, S. C. et al. A highly efficient method for porcine cloning by nuclear transfer using in vitro-matured oocytes. Cloning Stem Cells 4(2), 105–112 (2002).
Leal, G. R. et al. Role of cAMP modulator supplementations during oocyte in vitro maturation in domestic animals. Anim. Reprod. Sci. 199, 1–14 (2018).
Funding
This work was supported by the Ministry of Science and ICT through the National Research Foundation of Korea (NRF) (Grants # 2021R1A2C2009294 and 2022R1I1A1A01065412) and the Brain Pool program (Grant No.: 2021H1D3A2A02040098).
Author information
Authors and Affiliations
Contributions
Conceptualization: B.M.T. and J.C. Methodology, data curation, and formal analysis: B.M.T., S.B., X.F., C.S., I.M.S. and J.C. Manuscript writing: B.M.T., I.M.S., S.L., J.S., K.C., and J.C. Funding acquisition: I.M.S. and J.C. Supervision and project administration: J.C. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tanga, B.M., Fang, X., Bang, S. et al. The combination of rolipram and cilostamide improved the developmental competence of cloned porcine embryos. Sci Rep 13, 5733 (2023). https://doi.org/10.1038/s41598-023-32677-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-32677-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.