Abstract
Olive anthracnose, a critical olive fruit disease that adversely impacts oil quality, is caused by Colletotrichum species. A dominant Colletotrichum species and several secondary species have been identified in each olive-growing region. This study surveys the interspecific competition between C. godetiae, dominant in Spain, and C. nymphaeae, prevalent in Portugal, to shed light on the cause of this disparity. When Petri-dishes of Potato Dextrose Agar (PDA) and diluted PDA were co-inoculated with spore mixes produced by both species, C. godetiae displaced C. nymphaeae, even if the percentage of spores in the initial spore mix inoculation was just 5 and 95%, respectively. The C. godetiae and C. nymphaeae species showed similar fruit virulence in separate inoculations in both cultivars, the Portuguese cv. Galega Vulgar and the Spanish cv. Hojiblanca, and no cultivar specialization was observed. However, when olive fruits were co-inoculated, the C. godetiae species showed a higher competitive ability and partially displaced the C. nymphaeae species. Furthermore, both Colletotrichum species showed a similar leaf survival rate. Lastly, C. godetiae was more resistant to metallic copper than C. nymphaeae. The work developed here allows a deeper understanding of the competition between C. godetiae and C. nymphaeae, which could lead to developing strategies for more efficient disease risk assessment.
Similar content being viewed by others
Introduction
The olive tree is the most widely grown woody crop, covering 12 million hectares worldwide, primarily in Mediterranean regions1. European olive production accounts for 65% of the world market, with Spain being the primary producer, followed by Italy, Greece, and Portugal2. As a result, the olive industry exerts an enormous social and economic impact on Mediterranean countries.
The olive tree can be affected by several diseases and pests. The most important fruit disease of the olive tree is anthracnose, caused by Colletotrichum genus fungal species, which share importance with damages caused by the olive fly pest (Bactrocera oleae Rossi) since both can lead to significant yield losses3 and, also, impact oil quality4,5,6. In Spain, an average of 2.6% of the crop losses country-wide are attributed to anthracnose4.
Olive anthracnose presents two differentiated syndromes (i.e., a combination of symptoms or symptoms and pathogen signs): fruit rot and branch dieback. Affected fruits show necrotic lesions at an early ripening stage, which progress until total drupe rot4,5. On affected fruits, the fungus develops asexual fruiting bodies (acervuli) that produce a mass of conidia in a pinkish-orange gelatinous matrix6,7. As humidity drops and temperature increases, the affected olive fruits mummify5,7. The Colletotrichum species then colonize the peduncle of affected fruits, which fall to the ground. However, some mummified fruits may remain in the tree canopy until the next fall-winter7. In olives affected by the second syndrome, apical leaves show necrotic margins, which may progress until their complete drying, causing shoot and main branch dieback4,7,8. This second syndrome has been associated with pathogen toxins produced in affected fruits and mobilized to the shoots7,9. Since acervuli development on infected leaves is uncommon, olive canopy mummified fruits are the primary inoculum source3,5,6. However, some authors suggest that infected leaves are also an inoculum reservoir10. Because Colletotrichum species are specialized in infecting olive fruits, species that could infect and remain on the leaves would have an essential competitive advantage10.
Anthracnose is widespread and can be found in most olive-growing regions, including non-Mediterranean areas. Eighteen Colletotrichum species have been identified as olive anthracnose causal agents around the world3,4,5. These species are clustered into three complexes: C. acutatum J.H. Simmonds, C. gloeosporioides (Penz.) Penz. & Sacc., and C. boninense Moriwaki, Toy. Sato & Tsukib4,11. Notably, a dominant Colletotrichum species is usually described in each region, while other species of this genus are secondary or rare6,10,11. Similarly, every traditional olive-growing country has its own national cultivars, which can be classified according to their importance and dissemination from major cultivars (prevailing in more olive districts) to local cultivars (single olives in a specific district)12. Thus, Colletrotrichum species and traditional olive cultivars combine in pairs characteristic of the different endemic regions. For example, 'Galega Vulgar' is a representative cultivar in Portugal, being susceptible to the fungus4,15. In Portugal, the anthracnose epidemic outbreaks are caused chiefly by the C. nymphaeae species4,11,14. On the same peninsula, in the anthracnose endemic areas of the Andalusia region (in southern Spain), the highly susceptible 'Hojiblanca', 'Lechin de Sevilla', and 'Picudo' are major cultivars3. In Andalusia, C. godetiae is the dominant species in olive orchards, just as in Greece, Montenegro, and Italy5,11,14,25.
Several authors have described the coexistence between an abundant Colletotrichum species accompanied by several secondary species of this genus in olive orchards around the world11,15,16. However, the potential mechanisms determining why certain Colletotrichum species are dominant over others remain unknown. This unbalanced presence of the Colletotrichum species might also be attributed to (1) differences in the capacity of species to produce inoculum in olive leaves, (2) adaptation to the regional climate, (3) a pathogenic specialization in local dominant cultivars, (4) the ability to infect other hosts (e.g., weeds), or (5) a priority effect, i.e., the order of species arrival in the olive orchard can influence the species pattern10,13,14. Also, the selection pressure exerted by the recurrent application of copper-based fungicides (the main fungicides used in olive orchards) could also drive the differences among Colletotrichum species based on their resistance17.
This study seeks to shed light on the unbalanced distribution of Colletotrichum species affecting olive trees, focusing on understanding the interspecific competition between C. godetiae and C. nymphaeae in (1) dual-cultured media of different richness, (2) fruits and leaves of the Portuguese cv. Galega Vulgar and the Spanish cv. Hojiblanca, and (3) olive plants under control conditions.
Through this study, we could elucidate aspects such as the species of the pathogen's cultivar or tissue specialization. Consequently, information extracted from our study provides insights into the Colletotrichum spp. population dynamic in olive groves as well as knowledge to improve olive anthracnose disease risk assessment.
Methods
Fungal isolates
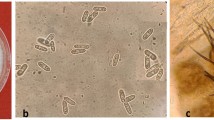
Different C. godetiae and C. nymphaeae strains stored at the mycological collection of the Agronomy Department of the University of Cordoba (Spain) were used during various experiments. Two C. nymphaeae strains, Col-573 and Col-580, were obtained from affected olive groves in Capela and Montesadinha (Portugal). C. godetiae strains Col-556 and Col-703, were obtained from olives that were severely affected by Anthracnose in Beja (Portugal) and Huelva (Spain), respectively. Strains were identified by their internal transcribed spacer 5.8S and β-tubulin regions11. Since both Colletotrichum species show distinctive morphological characteristics on Potato Dextrose Agar (PDA; 25% potato lixiviated, 2% dextrose, 2% agar, and pH 4.518), these were quickly identified and quantified in dual-culture assays. Overall, C. godetiae colonies were dark-grey, while C. nymphaeae colonies were white with cottony mycelium in PDA (Fig. 1A). Our assays assume that the interaction between both Colletotrichum species involves an interspecific (distinct species) competition for the same resource (media, fruits, or leaves)19.
(A) Dark grey colonies of Colletotrichum godetiae (Col-556) white cottony colonies of C. nymphaeae (Col-573) and co-cultured C. godetiae (Col-556) vs C. nymphaeae (Col-573) on Potato Dextrose Agar (PDA) medium; (B) the relative percentage of colonies (Mean and Standard Error values by grouping the three repetitions conducted) of C. godetiae and C. nymphaeae grown on PDA and diluted PDA co-inoculated with five different initial ratios of conidial mixtures. The treatments correspond to different spore ratio of both Colletotrichum species at the beginning of the experiment (generation 1); 95:5; 75:25; 50:50; 25;75; and 5:95 ratios (%) of spores of C. godetiae: C. nymphaeae.
Conidial suspension preparation
For inoculum production, Colletotrichum strains were grown on PDA at 20 °C in darkness for 7 days. Conidial suspensions were individually prepared by scraping pathogen colonies from the medium surface using a scalpel and were transferred to two 250-ml Erlenmeyer flasks containing 100 ml of sterile distilled water. Both resulting suspensions were filtered through a double-layered sterile cheesecloth to remove hyphal fragments. Conidial suspensions were adjusted to a final density of 3 × 105 conidia/ml using a Neubauer chamber.
Competition on media: effects of culture media and temperature
Dual-culture assays were performed to determine the influence of culture media and temperature on the competitive displacement of one species by another23. Three different conidial suspensions (5 × 102 conidia/ml) were prepared: C. godetiae, C. nymphaeae, and a mixture of both species at the same ratio (50:50). The three types of spore suspension were separately cultured by aseptically spreading 100 µl per 90-mm Petri dishes (50 conidia per dish), with 20 ml of PDA or diluted PDA (4% potato lixiviated, 0.75% dextrose, 2% agar, and pH 4.5). Diluted PDA was included as a medium with a carbon concentration below PDA18. Petri dishes were incubated at 15, 20, and 25 °C in darkness. Five replicated dishes per combination of Colletotrichum species-conidial suspension-culture medium-temperature were used in a fully randomized design. After a 4-day-growing period, cultures were examined, and the number of colonies of each species per plate was recorded. Then, the percentage of each species relative to the total number of colonies per plate was calculated.
Competition on media: effect of the initial inoculum
By mixing conidial suspensions of both Col-556 and Col-573 strains, previously prepared as described above, suspensions were prepared to contain percentages of conidia at the following ratios: 100:0, 95:5, 75:25, 50:50, 25:75, 5:95, and 0:100 of C. godetiae:C. nymphaeae. Mixed-conidial suspensions were adjusted to a final density of 5 × 102 conidia.ml.
Competition assays between the Colletotrichum species were conducted in PDA and diluted PDA. Five replicated Petri dishes were used for each conidial suspension and culture medium combination. All Petri dishes were aseptically spread with 100 µl of a conidial suspension and incubated in darkness at 20 °C for 5 days. After incubation, colonies of C. godetiae (Col-556 strain) and C. nymphaeae (Col-573 strain) were recorded as previously described, and results were expressed as relative percentages. Petri dishes with the Colletotrichum colonies were washed using 1 ml of sterile water to obtain a new conidial inoculum for the following cycle. The resulting suspension was adjusted to 5 × 102 conidia/ml using sterile distilled water and transferred to a new set of five Petri dishes. The experiment was completed after five cycles (i.e., conidial transference to medium—incubation—conidia washing and quantification) and was replicated thrice. An additional replicate was conducted using strains Col-703 and Col-580 (C. godetiae and C. nymphaeae, respectively) to contrast the strains' behavior.
Competition on fruits: effect of the olive cultivar and temperature
Olive fruits of the Spanish and Portuguese cultivars Hojiblanca and Galega Vulgar were collected from 10-year-old olives planted at the World Olive Germplasm Bank (WOGB) in Cordoba (Spain). Both cultivars are vulnerable to the pathogen13. Fruits were harvested at 81–85 phenological growth stage (BBCH scale)20 and stored at 4 °C until use. Ms. Pablo Morello, responsible of the WOGB, gave us permission for the collection of olive material necessary for the present study.
Fruits were first washed using running water, surface disinfested by immersion in a 10% solution of commercial bleach (Cl at 50 g/l) in sterile water for 1 min, rinsed with sterile water, and air-dried in a biological safety cabinet for 1 h. Fruits were then placed in humid chambers consisting of 22 × 16 × 10 cm plastic containers (20 fruits per chamber), with 100% relative humidity40.
Olive fruits were inoculated by spraying a conidial suspension (1 × 105 conidia/ml; 0.4 ml per fruit) of C. godetiae, C. nymphaeae, or a mixture (50:50) of conidia of both species to assess the interspecific competition during fruit infection. Humid chambers with inoculated fruits were incubated at 15, 20, or 25 °C. A fully randomized design was performed using 162 humid chambers (2 cultivars × 3 conidial suspensions × 3 incubation temperatures × 3 repetitions).
Disease severity was evaluated weekly using a 0–5 rating system, where 0 = no visible symptoms, 1 = visible symptoms affecting less than 25% of the fruit surface, 2 = visible symptoms affecting 26–50% of the surface, 3 = visible symptoms affecting 51–75% of the surface, 4 = visible symptoms affecting 76–99% of the surface, and 5 = completely rotted fruit (100%), showing the typical pinkish-orange gelatinous pustules. For each replication, severity values (0–5) were used to calculate the disease severity index (DSI) using the following formula: DSI = [(Σni × i)/N] × 100, where i means severity (0–5), ni stands for the number of fruits with a severity i, and N is the total number of fruits. Data were used to calculate the relative area under the disease progress curve (rAUDPC) through the trapezoidal integration of DSI values over time21.
Following 4 weeks of incubation, 8–10 completely rotted olive fruits (severity = 5) from the three replicated humid chambers were grouped and used as an inoculum source. To this end, affected fruits were transferred to 250-ml Erlenmeyer flasks containing 100 ml of sterile distilled water, which were gently shaken manually for 2 min to wash the inoculum. The resulting conidial suspension was filtered as previously described and adjusted to 1 × 105 conidia/ml for new fruit inoculation, mirroring the nature disease cycle. The experiment was ended after three "disease cycles" and conducted twice. In order to quantify the competitive displacement of one species by another, fruits inoculated with the mixture were transferred to 250-ml flasks at the end of the experiment and washed using 100 ml of sterile water. The conidial suspension (5 × 102 conidia/ml) was then spread onto PDA medium, and the number of colonies of each species was recorded as previously described.
Survival on detached leaves
Three hundred sixty healthy separate leaves of susceptible cultivars Hojiblanca and Galega Vulgar were harvested from adult trees of the WOGB and inoculated to assess the ability of C. godetiae and C. nymphaeae to infect this tissue. To that end, leaves (180 per cultivar) were surface disinfested in a 10% commercial bleach solution (Cl at 50 g/l) in sterile water for 1 min and placed into six humid chambers (20 leaves per chamber). Leaves were subsequently inoculated spraying a conidial (5 × 102 conidia/ml, 0.4 ml per leaf) suspension of C. godetiae: C. nymphaeae at a ratio of 50:50 (spores: spores), as described earlier in the case of fruits inoculation. Again, a completely randomized design was used with 18 humid chambers (2 cultivars × 3 conidial suspensions × 3 repetitions) incubated at 20 °C in darkness. Seven days after inoculation, leaves were again surface disinfested to remove the surface conidia that had not established infection in the leaves. Leaves were later incubated for 5 additional days. After incubation, leaves were cut into 0.5-cm2 sections (five per leaf), placed onto PDA dishes, and incubated under the same conditions. After 6 days of incubation, colonies of each isolate grown over the pieces of leaf were quantified, and the percentage of pieces colonized with each species was calculated. The experiment was conducted twice.
Survival on plant leaves
Three-year-old olive plants of cv. Arbequina, a cultivar that is moderately resistant to Colletotrichum spp.13, were inoculated with conidial suspensions of C. godetiae, C. nymphaeae, or a mix of both species to assess their ability to survive on leaves throughout the season. Plants were purchased from a commercial nursery and washed using running water. All fruits were manually removed. Conidial suspensions (5 × 102 conidia/ml) of C. godetiae: C. nymphaeae at ratios of 100:0, 50:50, and 0:100, were prepared as previously detailed. Plants were inoculated by spraying a conidial suspension using 30 ml per plant. Plants sprayed with sterile water were used as non-inoculated control. Inoculated plants were maintained in a dark growing chamber (20 °C, 100% relative humidity) for 24 h to provide conditions conducive to infection. Plants were then transferred to a greenhouse at 25–30 °C, keeping an inter-treatment spacing of 1 m. Olive plants were subject to drip irrigation as required.
Furthermore, all plants were irrigated (10 mm) every month with overhead sprinklers to simulate disease inoculum dispersion under natural conditions. A completely randomized design with 5 plants (replicates) per treatment (conidial suspension) was used, and the experiment was repeated twice. Leaf samples were taken at monthly intervals for six months and consisted of 25 leaves randomly collected from the five plants of each treatment. All samples were surface disinfested as previously discussed, to remove the remaining inoculum. Finally, the number of leaves colonized by each isolate was determined.
Resistance to copper
The four Colletotrichum strains used in our studies were evaluated for their resistance to copper since most olive anthracnose fungicides are copper-based4,5. Thus, agar plugs (7-mm diameter) were collected from the edge of actively growing colonies and placed at the center of 9-cm Petri dishes containing PDA medium amended with CuSO4 (100 µl/ml) (Copper sulfate; Merk Lab) or control PDA medium (non-amended medium). Five replicates (Petri dishes) per isolate and treatment were prepared. All dishes, including controls, were incubated at 20 °C in darkness. The diameter of each colony was measured when control colonies reached the dish edge, usually 7 days following incubation. The mycelial growth rate (mm per day) and conidia production rate (conidia per cm2) were determined per isolate and replication. To this end, conidia were suspended and rinsed with 2 ml of sterile water, and mycelia were scraped off its surface using a sterile blade. The conidial concentration in the resulting suspensions was assessed using a Neubauer chamber. Lastly, the effect of Cu on mycelial growth and conidia production was calculated as a percentage of inhibition concerning the control treatment17.
Additionally, Cu absorption of the two Colletotrichum species was determined according to Martins et al.22 with modifications. Briefly, three 9-mm agar plugs of each Colletotrichum isolate were separately placed inside a 100-ml flask containing 50 ml of PDB (Potato Dextrose Broth) amended with CuSO4 (100 µl/ml) or control PDB medium. Flasks were incubated in agitation (130 rpm/min) at 23 °C under light/dark conditions for 7 days. The experiment was replicated thrice. After incubation, mycelium was recovered and dried to constant dry weight at 60 °C on a stove. Dried mycelium was subjected to acidic digestion (6N-HCl) for 24 h in order to dissolve the sample completely. Finally, copper quantification was conducted using Mass Spectrometry and Chromatography (Bruker TQ MS).
All the described procedures were conducted following the European and International guidelines and regulations, including those of the University of (Spain), the European Commission, and Food Agriculture Institution (FAO).
Statistical analysis
The percentage of Colletotrichum colonies forming units on PDA or diluted-PDA mono-cultured or dual-cultured at different temperatures was assessed using a General Linear Model considering ANOVA limitations. Subsequently, treatments were compared using a Tukey test adjusted at P < 0.05. In the competition assay in media, means and 95% confidence intervals (CI95) were calculated, and t values that did not overlap at the CI95 were considered significantly (P < 0.05) different23. In the fruit inoculation assay, rAUDPC data were arcsin-transformed to satisfy normality and homogeneity of variance assumptions. Subsequently, a general ANOVA was applied using the Colletotrichum isolate × incubation-temperature × olive-cultivar × experiment-repetition interaction as the error term. Means were compared according to Tukey's test adjusted at P < 0.05. A Chi-square was performed to compare the spore production of each Colletotrichum species on co-inoculated fruits. Moreover, a Log-linear model was used to review the effect of the cultivar and Colletotrichum (or mix) species on total conidial production by the pathogen on inoculated fruits. In experiments performed with detached leaves, data from each cultivar were analyzed using a Chi-square test. The effect of the Colletotrichum species on the mycelial and spore inhibition (%) due to Cu was studied using a Wilcoxon Signed Rank Test. This test was also performed to compare conidium and hyphae sizes among Colletotrichum species. Data were analyzed using Statistix 10 (version 10; Tallahassee) and SPSS software (version 25.0; SPSS Inc., Chicago).
Results
Fungal isolates
As mentioned above, both Colletotrichum species were unequivocally identified as growing in culture media (PDA and diluted PDA), which allowed us to conduct the different assays in our study. Overall, conidia of Colletotrichum spp. were (8.4)12.5–16.1(18.9) × (3)3.9–5.3(7.4) µm for C. godetiae (Col-556) and (6.6)8.7–20.4(29.2) × (2.4)2.5–7.1(9.3) µm C. nymphaeae (Col-573). Hyphae sizes ranged from (2.1)2.4 to 4.7(6.0) µm for Col-556, and from (1.7)1.9 to 4.2(4.7) µm for Col-573. However, Wilcoxon Rank Sum Test (P > 0.05) yielded no significant differences in fungal structures.
Competition on media: effects of culture media and temperature
Both Colletotrichum species developed colonies differentiated on PDA and diluted PDA. According to the Mixed Effects Model, the Colletotrichum species (P < 0.001) and incubation temperature (P = 0.024) exerted a significant effect on the number of fungal colonies on the media. Conversely, the remaining independent variables (culture media and mono- or co-culturing) and their interactions were not significant (P > 0.05). Thus, the C. nymphaeae formed significantly more colonies than C. godetiae on both media (83.5% vs. 62.9%, respectively). Likewise, both fungal species developed more colonies at 20 °C than 15 °C, irrespective of the culture media used. No significant effect (synergism or antagonism, P = 0.666) of the dual-culture of both species was observed (Table 1).
Competition on media: effect of the initial inoculum
Figure 1B represents the percentage of C. godetiae and C. nymphaeae colonies recovered from dual-cultured PDA and diluted PDA. Considering the global data set, including the three repetitions, overall, the strain Col-556 of C. godetiae displaced the strain Col-573 of C. nymphaeae in co-inoculated PDA dishes, gradually prevailing over the population regardless of the initial conidial ratio. In PDA dishes, for example, the percentage of C. godetiae colonies at the last cycle was over 80% in all the treatments, except for mixture C. godetiae: C. nymphaeae 05:95, which was 44.43%. Accordingly, a marked prevalence of C. godetiae was observed in experiments performed on diluted PDA. In fact, the C. godetiae strain was the majority strain on all mixture treatments on completion of the experiment. In the extra replicate that we conducted in diluted PDA using the Col-703 and Col-580 strains of C. godetiae and C. nymphaeae, respectively, we found the same pattern, with the number of colonies of C. godetiae being significantly higher than those of C. nymphaeae at the end of the experiment for any combination used (Fig. 1S).
Competition on fruits: effect of the olive cultivar and temperature.
Disease severity
Disease severity on fruits was separately monitored at every cycle for each combination of cultivar and temperature (Fig. 2A) by grouping the two repetitions conducted since these showed similar patter. Interestingly, the C. godetiae and C. nymphaeae species showed similar (P = 0.501) virulence on the fruits of both olive cultivars. Likewise, our data showed no significant interaction between Colletotrichum species and cultivar (P = 0.527); i.e., no cultivar pathogenic specialization occured. Furthermore, 'Hojiblanca' fruits were more vulnerable (P = 0.032) to the pathogen than 'Galega Vulgar' fruits. Inoculated fruits incubated at 15 °C were less affected (P = 0.045) than those set at 25 °C but like those at 20 °C.
(A) Disease severity (Mean and Standard Deviation values by grouping the two repetitions conducted), evaluated as the relative Area Under the Disease Progress Curve (rAUDPC), of olive fruits of cultivars Hojiblanca and Galega Vulgar inoculated with conidial suspensions of Colletotrichum godetiae, C. nymphaeae, and a mixture of both species (50:50), and incubated at 15, 20 and 25 °C. (B) Colonies (%) of C. godetiae and C. nymphaeae from affected olive fruits of cvs. Hojiblanca and Galega Vulgar co-inoculated with a mix (50:50%) of spores of both species and incubated at 15, 20 and 25 °C. Significant differences between Colletotrichum species according to Chi-Square test at P < 0.001***, P < 0.01**, and P < 0.05*.
Quantification of the inoculum produced by each Colletotrichum species in co-inoculated fruits
Our data showed that the species C. godetiae displaced its competitor at 15 °C on the inoculated fruits of both cultivars, after three simulated cycles of disease. Likewise, C. godetiae was more competitive at 20 °C but only in 'Galega Vulgar' fruits. No significant differences were observed when fruits were incubated at 25 °C (Fig. 2B).
Sporulation of C. godetiae and C. nymphaeae in inoculated fruits
When we evaluated the number of spores produced by the affected olive fruits following the Pearson chi-square of the Log-linear model, the total inoculum per fruit was not affected by cultivar Colletotrichum species or temperature (Fig. 2S).
Survival on detached leaves
When olive leaves cv. Galega Vulgar and Hojiblanca were co-inoculated with both Colletotrichum species; C. godetiae showed a higher capacity than C. nymphaeae to settle in 'Galega Vulgar' leaves (P < 0.01), but these differences were not recorded (P > 0.05) on the cv. Hojiblanca leaves (Fig. 3A).
(A) Relative percentage of colonies of Colletotrichum godetiae and C. nymphaeae recovered from detached leaves of cultivars Galega Vulgar and Hojiblanca co-inoculated with both isolates. Percentage of olive leaves cv. Arbequina, which were co-inoculated (B) or independently inoculated (C) with both Colletotrichum species, the pathogens of which were recovered. *Significance between relative percentages according to the Chi-Square test.
Survival on plant leaves
Overall, Colletotrichum strains of both species were recovered from olive leaves of potted cv. Arbequina plants throughout the experiment (Fig. 3B,C). However, a gradual decline in the percentage of leaves colonized by the Colletotrichum isolates throughout both experiments occurred, i.e., when plants were separately inoculated with both species or co-inoculated. At the end of both experiments (5 months following inoculation), Colletotrichum was isolated from co-inoculated leaves at percentages lower than 10%, but C. godetiae species were not isolated from plant leaves that were inoculated only with this species.
Resistance to copper
Colletotrichum godetiae strains were more copper-tolerant (P < 0.05) than C. nymphaeae strains, according to the percentages of inhibition of mycelial growth (− 0.6 vs. 4.4%) and spore production (− 48.8 vs. 68.8%) when culture media was amended with copper, calculated in relation to an unamended control. In some repetitions (Petri dishes), copper presence induced spore production, mainly on C. godetiae strains. Besides, C. godetiae resistance to copper was because of its ability to prevent the Cu accumulation in the mycelium (avg. 364 µg Cu/g mycelium lower than C. nymphaeae). However, no significant differences (P = 0.173) were detected between both species. In any case, colonies of both species growing in Cu-amended media showed significantly higher Cu content than control colonies, reaching concentrations over 100 times denser (Table 2).
Discussion
The olive tree is one of the most widespread woody crops around the world, and demand for olive oil continues to rise1,12. Olive anthracnose, caused by several Colletotrichum species, is of great concern to olive oil producers because of (1) its negative impact on oil quality3,5, (2) its penchant for developing in high plant density, the greatest exponent which is hedge-shaped olive groves, especially conducive to anthracnose development24 and (3) the Cu compounds use challenges such as the low sensitivity of the pathogen to copper-based fungicides17, and the EU restrictions on the use of plant protection products, to a maximum rate of 4 kg Cu per hectare and year25.
The interaction among phytopathogenic fungal species and antagonistic microorganisms (e.g., biological control agents) has triggered the development of biological control in many crop diseases26, including the olive anthracnose27,28. In contrast, interaction among phytopathogenic agents affecting a given host is much less known29,30. In the case of olive anthracnose, the interaction between pathogenic species is of interest for two main reasons: first, because up to 18 Colletotrichum species have been associated with the diseases; and second, because in most olive-growing regions, a dominant species coexists with several minority ones 6,10,11. For example, C. godetiae is the dominant species in Greece, Italy, and Spain, where it coexists with other species, including C. nymphaeae, which are much less abundant11. The opposite pattern is observed in Portugal, where C. nymphaeae is dominant, while C. godetiae is only occasionally detected4,8,11.
Many ecological reasons might account for the presence of a dominant Colletotrichum species in a given olive-growing region, including (1) a high pathogenic specialization on the major cultivar31, (2) better adaptability to agroclimatic conditions24,31, or (3) a high ability to infect and remain in olive leaves7,10. Therefore, assays have been performed in our study to clarify the differential prevalence of C. godetiae and C. nymphaeae in Spain and Portugal. Fortunately, the morphological features of the colonies in culture media allowed the distinction between both species.
Firstly, competition between C. godetiae and C. nymphaeae in Petri dishes was stimulated in a dual-cultured assay. Overall, C. godetiae displaced its competitor even when a low proportion of spores of this species (5 vs 95%) were initially transferred to two media with different carbon concentrations (PDA and diluted PDA) 32,33. Differences in the capacity to assimilate carbon sources among Colletotrichum fungal species are well-known and have been used in taxonomic proposals34.
Subsequently, we inoculated two major Portuguese and Spanish olive cultivars ('Galega Vulgar' and 'Hojiblanca') with the C. nymphaeae and C. godetiae species to explore a potential cultivar specialization (i.e., significant interaction cultivar × specie). However, against expectations, no cultivar specialization was detected, and both species showed similar virulence. In addition, fruit inoculations with both species did not increase or decrease the severity of anthracnose symptoms, i.e., no synergistic or antagonistic effects occurred35. Even if it was a co-inoculated fruit, C. godetiae competitively displaced C. nymphaeae, but it was a partial displacement (not an exclusion) and cultivar and incubation temperature dependent.
Furthermore, inoculated olive fruits underpinned the difference in susceptibility to both Colletotrichum species of the cv. Galega Vulgar (susceptible) and cv. Hojiblanca (highly susceptible), previously classified under natural infection conditions 11. The absence of Colletotrichum species × cultivar interaction is essential for olive-breeding programs since breeding can be directed to a broad range of pathogen species. For example, Talhinhas et al. showed that the Italian cultivar Frantoio is highly resistant to several Colletotrichum species36. This is even noticeable in new olive genotypes with 'Frantoio' pedigree37.
Different Colletotrichum species can affect different organs in a single host, as happens with the orange tree, in which C. acutatum infects flowers and harvest the syndrome known as post-bloom fruit drop. In turn, C. gloeosporioides causes fruit necrosis during ripening and subsequent orange storage thereof38. In Spain, our field and experiment data point to a specialization by C. godetiae in infecting olive fruit, while leaves play a minimal role as an inoculum source since the pathogen does not develop acervuli in these tissues in the field (acervuli can only be observed in inoculated leaves after a long period of incubation under artificial moisture conditions, J. Moral personal observation)7. Conversely, infected leaves are regarded as a secondary inoculum source in other countries10,39. In any case, the survival capacity of Colletotrichum for settling in olive leaves provides a substantial competitive advantage for the pathogen10. In our study, the ability of C. godetiae species to establish in detached olive leaves was higher than that of C. nymphaeae species. Concerning pathogen survival in olive leaves over time, both fungal species achieved steady infections in 30% of leaves, but this rate declined gradually during the season. This points again toward the relatively low importance of infected leaves as an inoculum source for the next season, particularly when compared with the mummified fruit, in which the pathogen produces millions of spores40. Remarkably, no Colletotrichum acervuli were observed in any inoculated leaves or plants. In any case, no substantial differences between C. godetiae and C. nymphaeae demonstrate the prevalence of one species over the other.
The competitive ability of a given Colletotrichum species is enhanced by its resistance to major fungicides in olive orchards (i.e., copper-based compounds)3,4,5. Here, based on the inhibitions on mycelial growth and spore production due to Cu-amendments, we have demonstrated that C. godetiae strains are less affected by this compound than those of C. nymphaeae. Unlike other studies, in which resistant fungal strains show a higher ability to store Cu in their mycelia22, the C. godetiae strains of our study tended to accumulate less Cu than those of C. nymphaea. We previously described differences in Cu resistance between both species regarding spore germination in the presence of this compound17. While the recurrent use of copper-based fungicides can provide powerful selection pressures, no pesticide applications are available for the olive orchards from which strains were collected. Also, we selected orchards with relatively young plants (6–15 years), excluding Colletotrichum strains from ancient orchards where there is an accumulation process of copper41. In any case, copper-based fungicides present a multi-site activity; therefore, there is a lower risk of pathogens developing Cu resistances42.
In short, we have developed an efficient protocol for simulating interaction cycles between Colletotrichum species for competitive studies. Our results showed that C. godetiae strains tended to shift (no complete exclusion) those of C. nymphaeae on both culture media and olive fruit. Classical coexistence theory argues that species coexistence in a niche cannot occur if species compete for a single limiting resource (reviewed by Mittelbach and McGill43) according to the competitive exclusion principle44. While the coexistence of both Colletotrichum species in each olive orchard has been well-described10,11,14, competitive interactions among these species could mainly occur at the individual tree level, not at the grove level since the anthracnose epidemic in a given tree is primarily driven by self-infection process inside of the tree canopy24. Also, olive fruits cannot be considered a limiting resource for the pathogen since healthy fruits are abundant, even under the most conducive conditions for the disease17. In any case, the coexistence of competitive fungal species is well-known, and it is allowed under different mechanisms despite competition for a single resource45,46. In our pathosystems, for example, a priority effect (i.e., original colonizers are challenging to displace) could account for the coexistence of both fungal especies47,48. Moreover, other characteristics making a Colletotrichum species a better competitor (e.g., the ability to infect weeds) are yet to be studied. Lastly, we do not rule out that the Colletotrichum population affecting olives could change because of agronomical and climatic factors, as suggested15. Further insights into Colletotrichum communities in olive groves could be a powerful tool for identifying population changes and selecting appropriate fungicides.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
IOC. http://www.Internationaloliveoil.Org/. Accessed 5 May 2022.
FAOSTAT. 2018. Crop statistics. FAO, Rome. http://www.fao.org/faostat/en/#data/QC. Accessed 5 May 2022.
Moral, J., Xaviér, C., Roca, L.F., Romero, J., Moreda, W. & Trapero, A. La Antracnosis del olivo y su efecto en la calidad del aceite, Olive Anthracnose and its effect on oil quality. Grasas y Aceites. 65(2), e028 (2014).
Talhinhas, P., Loureiro, A. & Oliveira, H. Olive anthracnose: A yield- and oil quality-degrading disease caused by several species of Colletotrichum that differ in virulence, host preference and geographical distribution. Mol. Plant Pathol. 19, 1797–1807 (2018).
Cacciola, S. O. et al. Olive anthracnose. J. Plant Pathol. 94, 29–44 (2012).
Moreira, V., Mondino, P. & Alaniz, S. Olive anthracnose caused by Colletotrichum in Uruguay: Field symptoms, species diversity and flowers and fruits pathogenicity. Eur. J. Plant Pathol. 160, 663–681 (2021).
Moral, J., De Oliveira, R. & Trapero, A. Elucidation of the disease cycle of olive anthracnose caused by Colletotrichum acutatum. Phytopathology 99, 548–556 (2009).
Moral, J. & Trapero, A. Assessing the susceptibility of olive cultivars to anthracnose caused by Colletotrichum acutatum. Plant Dis. 93, 1028–1036 (2009).
Ballio, A., Bottalico, A., Buonocore, V., Carilli, A., Di Vittorio, V. & Graniti, A. (1969) Mediterranean Phytopathological Union Firenze University Press Production and isolation of aspergillomarasmin B (lycomarasmic acid) from cultures of Colletotrichum gloeosporioides Penz. (Gloeosporium olivarum Aim.) Vindice Di Vittorio and Antonio Gra. Firenze Univ. Press Behalf Mediterr. Phytopathol. Union. 8, 187–196.
Talhinhas, P. et al. Epidemiology, histopathology and aetiology of olive anthracnose caused by Colletotrichum acutatum and C. gloeosporioides in Portugal. Plant Pathol. 60, 483–495 (2011).
Moral, J. et al. Diversity of Colletotrichum species associated with olive anthracnose worldwide. J. Fungi 7, 741 (2021).
Diez, C. M. et al. Olive domestication and diversification in the Mediterranean Basin. New Phytol. 206, 436–447 (2015).
Moral, J. et al. Variability in susceptibility to anthracnose in the world collection of olive cultivars of Cordoba (Spain). Front. Plant Sci. 8, 1892 (2017).
Materatski, P. et al. Diversity of Colletotrichum species associated with olive anthracnose and new perspectives on controlling the disease in Portugal. Agronomy https://doi.org/10.3390/agronomy8120301 (2018).
Schena, L. et al. Quantitative detection of Colletotrichum godetiae and C. acutatum sensu stricto in the phyllosphere and carposphere of olive during four phenological phases. Eur. J. Plant Pathol. 149, 337–347 (2017).
Chattaoui, M. et al. Characterization of a Colletotrichum population causing anthracnose disease on Olive in northern Tunisia. J. Appl. Microbiol. 120, 1368–1381 (2016).
Moral, J. et al. Preliminary selection and evaluation of fungicides and natural compounds to control olive anthracnose caused by Colletotrichum species. Crop Prot. 114, 167–176 (2018).
Sinclair J.B. & Dhingra, O.D. Basic Plant Pathology Methods, Press., C., Ed. (1995).
Begon, M. & Townsend, C. R. Ecology: From Individuals to Ecosystems (Wiley, 2020).
Sanz-Cortés, F. et al. Phenological growth stages of olive trees (Olea europaea). Ann. Appl. Biol. 140, 151–1572002 (2012).
Moral, J., Bouhmidi, K. & Trapero, A. Influence of fruit maturity, cultivar susceptibility, and inoculation method on infection of olive fruit by Colletotrichum acutatum. Plant Dis. 92, 1421–1426 (2008).
Martins, F. et al. Tolerance and bioaccumulation of copper by the entomopathogen Beauveria bassiana (Bals.-Criv.) Vuill. exposed to various copper-based fungicides. Bull Environ. Contam. Toxicol. 89, 53–60 (2012).
Glantz, S. A. Primer of Biostatistics 5th edn. (McGraw-Hill Book Co, 2002).
Moral, J., Jurado-Bello, J., Sánchez, M. I., Oliveira, R. D. & Trapero, A. Effect of temperature, wetness duration, and planting density on olive anthracnose caused by Colletotrichum spp. Phytopathology 102, 974–981 (2012).
Authority European Food Safety. Peer review of the pesticide risk assessment of the active substance copper compounds copper(I), copper(II) variants namely copper hydroxide, copper oxychloride, tribasic copper sulfate, copper(I) oxide. Bordeaux mixture. EFSA J. 16, 1–25 (2018).
Maloy, O. Plant Disease Control: Principles and Practice (Wiley, 1993).
Nigro, F., Antelmi, I., Labarile, R., Sion, V. & Pentimone, I. Biological control of olive anthracnose. Acta Horticul. 199, 439–444 (2018).
Preto, G., Martins, F., Pereira, J. A. & Baptista, P. Fungal community in olive fruits of cultivars with different susceptibilities to anthracnose and selection of isolates to be used as biocontrol agents. Biol. Control 110, 1–9 (2017).
Griffin, A. S., West, S. A. & Buckling, A. Cooperation and competition in pathogenic bacteria. Nature 430, 1024–1027 (2004).
Sommerhalder, R. J., McDonald, B. A., Mascher, F. & Zhan, J. Effect of hosts on competition among clones and evidence of differential selection between pathogenic and saprophytic phases in experimental populations of the wheat pathogen Phaeosphaeria nodorum. BMC Evol. Biol. 11, 188 (2011).
Konno, M., Iwamoto, S. & Seiwa, K. Specialization of a fungal pathogen on host tree species in a cross-inoculation experiment. J. Ecol. 99, 1394–2140 (2011).
Landum, M. C. et al. Antagonistic activity of fungi of Olea europaea L. against Colletotrichum acutatum. Microbiol. Res. 183, 100–108 (2016).
Nuangmek, W., McKenzie, E. H. & Lumyong, S. Endophytic fungi from wild banana (Musa acuminata Colla) works against anthracnose disease caused by Colletotrichum musae. Res. J. Microbiol. 3, 368–374 (2008).
Barnett, H.L. & Hunter, B.B. Illustrated genera of imperfect fungi. in The American Phytopathological Society, 200. Minnesota., Ed. (1998).
Dutt, A., Andrivon, D. & Le May, C. Multi-infections, competitive interactions, and pathogen coexistence. Plant Pathol. 71, 5–22 (2022).
Talhinhas, P., Gonçalves, E., Sreenivasaprasad, S. & Oliveira, H. Virulence diversity of anthracnose pathogens (Colletotrichum acutatum and C. gloeosporioides species complexes) on eight olive cultivars commonly grown in Portugal. Eur. J. Plant Pathol. 142, 73–83 (2015).
Moral, J. et al. Relative susceptibility of new olive cultivars to Spilocaea oleagina, Colletotrichum acutatum, and Pseudocercospora cladosporioides. Plant Dis. 99, 58–64 (2015).
Zulfiqar, M., Brlansky, R. H. & Timmer, L. W. Infection of flower and vegetative tissues of citrus by Colletotrichum acutatum and C. gloeosporioides. Mycologia 88, 121–128 (1996).
Sergeeva, V. & Spooner-hart, R. First report of Colletotrichum acutatum and C. gloeosporioides causing leaf spots of olives (Olea europaea) in Australia. Australas. Plant Dis. Notes 3, 143–144 (2008).
Moral, J. & Trapero, A. Mummified fruit as a source of inoculum and disease dynamics of olive anthracnose caused by Colletotrichum spp. Phytopathology 102, 982–989 (2012).
Mackie, K. A., Müller, T. & Kandeler, E. Remediation of copper in vineyards—A mini review. Environ. Poll. 167, 16–26 (2012).
Brent, K. J. & Hollomon, D. W. Fungicide Resistance in Crop Pathogens: How Can it be Managed? 2nd edn. (GIFAP, 2007).
Mittelbach, G. G. & McGill, B. J. Community Ecology (Oxford University Press, 2019).
Gause, G. F. The Struggle for Existence (The Williams & Wilkins Company, 1934).
Kennedy, P. Ectomycorrhizal fungi and interspecific competition: Species interactions, community structure, coexistence mechanisms, and future research directions. New Phytol. 187, 895–910 (2010).
Hiscox, J., O’leary, J. & Boddy, L. Fungus wars: Basidiomycete battles in wood decay. Stud. Mycol. 89, 117–124 (2018).
Chesson, P. Mechanisms of maintenance of species diversity. Annu Rev. Ecol. Evol. Syst. 31, 343–366 (2000).
Hiscox, J. et al. Priority effects during fungal community establishment in beech wood. ISME J. 9, 2246–2260 (2015).
Acknowledgements
J.M. is a Ramon y Cajal fellowship (RYC2019-028404-I), whose research is supported by the EPIDEMIOLIVE Project (PID2020-117550RA-I00) launched by the Spanish Government (MICIN). In addition, this research was supported by the Gen4Olive project (H2020 program; Grant no. 101000427) from the European Commission. Finally, the authors thank Paqui Luque and Halil Sucu for their invaluable help in the experiments.
Author information
Authors and Affiliations
Contributions
M.T.G.L., M.S.S., and C.E. performed the experiments, analysed data and contributed to manuscript writing. B.X.C. conducted experiments and contributed to manuscript writing. A.G. and C.M.D. participated to the study design and manuscript editing. A.T. provided funding for the study. J.M. conceived the experiments, provided funding, contributed to data analysis and co-wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Garcia-Lopez, M.T., Serrano, M.S., Camiletti, B.X. et al. Study of the competition between Colletotrichum godetiae and C. nymphaeae, two pathogenic species in olive. Sci Rep 13, 5344 (2023). https://doi.org/10.1038/s41598-023-32585-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-32585-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.