Abstract
We analyzed the seasonal variations in the number of renal biopsies and clinical characteristics of primary glomerular disease in Japan using the Japan Renal Biopsy Registry (J-RBR). We retrospectively collected clinical and pathological data of patients with primary glomerular disease who were registered in the J-RBR between 2007 and 2018. Immunoglobulin A nephropathy (IgAN), minimal change nephrotic syndrome (MCNS), membranous nephropathy (MN), and postinfectious acute glomerulonephritis (PIAGN) constituted the four major glomerular disorders included in this study (total, 13,989; IgAN, 9121; MCNS, 2298; MN, 2447; and PIAGN, 123). The number of patients with IgAN or MCNS was higher during summer. However, no overt seasonal variations were observed in patients with MN or PIAGN. Subgroup analyses suggested that in the patients with IgAN, more renal biopsies of severe cases were performed during winter, probably owing to age and blood pressure. Furthermore, more renal biopsies of severe cases were performed during spring and winter in patients with MCNS even after adjusting for the abovementioned host factors. This study suggests that seasonal factors influence the decision to perform renal biopsy as well as the pathogenesis of primary glomerular disease. Thus, our findings may provide important insights regarding the pathophysiology of primary glomerular disease.
Similar content being viewed by others
Introduction
The association between seasonal factors and the onset and prevalence of various diseases have been previously investigated1. Reportedly, respiratory diseases2, hypertension3, and cardiocerebrovascular diseases4,5 tend to develop and worsen during winter, which is also when the rate of respiratory infection peaks6. Furthermore, cold weather alters physiological hemodynamics and hematological factors, thereby contributing to arterial thrombosis7,8. Consequently, acute kidney injury onset and dialysis introduction are also common during winter9,10.
The onset of glomerular disease is believed to be due to the interaction of genetic and environmental factors. Briefly, certain genetic factors predispose individuals toward an immune response that leads to glomerulonephritis, and inflammatory and noninflammatory immune mechanisms are considered to be involved in the pathogenesis of this glomerular injury11. Particularly, infectious and allergic diseases constitute the priamary external factors that influence the pathogenesis of lifetime diseases12. Group A beta-hemolytic streptococcus, a culprit pathogen of epidemic infections during winter, has been causally associated with acute glomerulonephritis13. The persistence of respiratory viruses may contribute to the development and progression of minimal change nephrotic syndrome (MCNS)14. Recent studies suggest that gut microbiota are involved in the pathogenesis of primary glomerular diseases, such as immunoglobulin A nephropathy (IgAN) and membranous nephropathy (MN). Gut microbiota are susceptible to environmental factors, such as personal habits and nutrition15. Additionally, children with idiopathic nephrotic syndrome exhibit a high incidence of allergic diseases, including atopic dermatitis and allergic rhinitis, and the number of cases is reportedly high during spring and autumn16,17. In adults, idiopathic nephrotic syndrome reportedly occurs more frequently during winter. Furthermore, IgAN, which is probably associated with viral infections and tonsillitis, tends to worsen during winter18. Patients with early diabetic nephropathy also exhibit considerably higher proteinuria and albuminuria during autumn and winter than during spring and summer, probably because of increased systolic blood pressure (BP) during winter19.
However, there have only been few studies regarding the relationship between primary glomerulonephritis and seasonal factors closely associated with the development of the infections and allergies, and to the best of our knowledge, there have been no such studies involving a large cohort of patients.
The Japan Renal Biopsy Registry (J-RBR) is a nationwide, multicenter, web-based, prospective renal biopsy registry established in 2007 to record clinical and pathological data of patients undergoing renal biopsy20. J-RBR data is particularly beneficial for analyzing the association of seasonal variations with disease pathogenesis, as Japan is a country with a homogeneous society that experiences four distinct seasons.
This study aimed to analyze the influence of seasonal variations on the number of renal biopsies and the clinical features of primary glomerular disease in Japan using the J-RBR and clarify the relationship between the various types of glomerulonephritis, which are closely related to external factors, and the seasons. We focused on the following four diseases: IgAN, MCNS, MN, and postinfectious acute glomerulonephritis (PIAGN), which are reportedly associated with allergies and infectious diseases and have a high incidence in Japan.
Methods
J-RBR system and patient selection
The J-RBR is a nationwide, multicenter registry system that was organized by the Committee for the Standardization of Renal Pathological Diagnosis and the Working Group for the Renal Biopsy Database of the Japanese Society of Nephrology in 200720. Individual patient data, including basic patient information, clinical diagnosis, renal pathological findings, biochemical features, and urinalysis, were uploaded to the J-RBR website using the Internet Data and Information Center for Medical Research system of the University Hospital Medical Information Network (UMIN). The J-RBR is registered under the Clinical Trial Registry of UMIN (registration number, UMIN000000618). This study was approved by the Ethics Committee of the Japanese Society of Nephrology and conducted per the principles of the Declaration of Helsinki (Research of J-RBR in Japanese Society of Nephrology, No.79, J-RBR201904, September 2, 2019). Written informed consent was obtained from all the study participants or the parents if the participant was a child.
This retrospective study study included Japanese patients with primary IgAN, MCNS, MN, or PIAGN who were registered in the J-RBR from July 1, 2007 to January 1, 2018. The baseline characteristics of the patients, including clinical and pathological features at the time of the renal biopsies, were obtained from the J-RBR database.
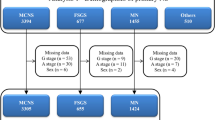
During the registration period, 17,281 patients were registered in the J-RBR. Of these, 3292 patients were excluded because of missing data that were critical for the analysis. Overall, 13,989 patients (IgAN: 9121; MCNS: 2298; MN: 2447; and PIAGN: 123) were finally included in the analysis (Fig. 1).
Patients who were diagnosed with other renal or systemic diseases were excluded.
Clinical measurements and definitions
The clinical data of the patienta, including age, sex, body mass index (BMI), systolic and diastolic BP, serum creatinine (sCr) levels, estimated glomerular filtration rate (eGFR), serum albumin levels, serum total cholesterol levels, and urinary protein excretion (UPE) rate, were evaluated. The eGFR was calculated using a three-variable equation modified for Japanese populations as follows: eGFR = 194 × age−0.287 × sCr−1.094 (× 0.739 if female)22.
The four seasons relevant to the climate in Japan were defined month-wise as follows: spring (March–May), summer (June–August), autumn (September–November), and winter (December–February)23.
To examine the possibility of applying the findings of this study in clinical practice, the ages of the patietns were classified into three categories: children (aged < 18 years), adults (aged 18–64 years), and older individuals (aged ≥ 65 years).
Hematuria was defined as more than five red cells per high power field (HPF) in urinary sediments and graded based on the number of red cells per HPF as follows: 0–4, 5–10, 11–30, and ≥ 30.
Based on the KDIGO 2012 guidelines modified for the Japanese population, the UPE rates at biopsy were classified as normal (< 0.15 g/day or g/gCr; A1), mild (0.15–0.49 g/day or g/gCr; A2), and severe (≥ 0.5 g/day or g/gCr; A3). Additionally, the eGFR at the time of biopsy was classified into five groups: G1, G2, G3a, G3b, G4, and G5 for ≥ 90, 60–89, 45–59, 30–44, 15–29, and < 15 mL/min/1.73 m2, respectively. According to these UPE rates and eGFR values, the chronic kidney disease (CKD) heat map classified renal prognosis as low, medium, high, and very high24,25,26.
Statistical analysis
We first described the baseline characteristics of the entire study population and of the patients in the four groups (IgAN, MCNS, MN, and PIAGN). Continuous variables were presented as the mean and standard deviation or frequencies with percentages (in parentheses). Seasonal differences in the number of kidney biopsies per glomerular disease were compared using the four (seasons) × four (diseases) table chi-squared test. The differences in continuous and categorical variables were assessed using independent-samples t-test or Mann–Whitney U-test and chi-square test or Fisher’s exact test where appropriate, respectively. In addition, multiple comparisons were performed using analysis of variance and applying the Bonferroni correction.
A multivariable linear regression analysis that defined summer as the reference was constructed to identify the possible influence of seasonal variations on the severity of glomerular disease biopsy. In each analysis, the age, sex, and mean BP of the patients were treated as fixed covariates. Statistical significance was defined as a two-sided P-value of < 0.05. All statistical analyses were performed using SPSS v.25.0 (IBM Corp., Armonk, NY, USA).
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee where the studies were conducted (IRB approval number: the Japanese Society of Nephrology, No. 79, September 2, 2019, and the Jikei University School of Medicine, 31–284(9783)) and per the principles of the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all study participants or the parents if the participant is a child.
Results
Clinical characteristics
Table 1 summarizes the clinical characteristics of all the 13,989 patients at the time of their renal biopsy. Overall, the mean age of the patients was 43.8 ± 21.0 years (53.6% men). The mean eGFR was 74.6 ± 30.9 mL/min/1.73 m2. In total, 9,121 (65.2%) patients had IgAN, 2,298 (16.4%) had MCNS, 2,447 (17.5%) had MN, and 123 (0.88%) had PIAGN (Fig. 2).
Frequency of the primary glomerular diseases. The number of renal biopsies was the highest in patients with IgAN (65.20%) and the lowest in patients with PIAGN (0.88%). IgA nephropathy (IgAN), minimal change nephrotic syndrome (MCNS), membranous nephropathy (MN), and postinfectious acute glomerulonephritis (PIAGN).
Seasonal variations in the number of renal biopsies
The number of kidney biopsies per season was as follows: 3,374 (24.1%) during spring, 4,015 (28.7%) during summer, 3,246 (23.2%) during autumn, and 3,354 (24.0%) during winter. The seasonal variation was significant (P < 0.001), and the number of renal biopsies was the highest during summer (Fig. 3a). Figure 3b displays the distribution of the number of renal biopsies across the seasons for the four glomerular diseases.
Seasonal variations in IgAN
The seasonal variations in the biochemical and clinical parameters of the patients with IgAN are summarized in Table 2. The number of kidney biopsies was significantly (P < 0.001) higher during summer in patients with IgAN than those with other diseases. Significant seasonal differences were observed in age (P < 0.001), BMI (P < 0.001), systolic BP (P < 0.001), diastolic BP (P < 0.001), mean BP (P < 0.001), serum creatinine levels (P < 0.001), and UPE (P < 0.001), with the lowest peak values for these parameters occurring during summer. The distribution of age, UPE rates, and the CKD heat map categories significantly varied across the seasons. Cases involving younger patients were more common during summer compared with older patients, whereas adult cases were less common during summer and more common during autumn and winter. Regarding UPE, there were fewer cases involving severe proteinuria and more cases involving mild proteinuria during summer, contrary to the trend observed during winter. In the CKD heat map categories, the low-risk group was more common during summer and less common during autumn and winter. The high-risk and very high-risk groups were less common during summer.
Seasonal variations in MCNS
Seasonal variations in the biochemical and clinical parameters of patients with MCNS are summarized in Table 3. Significant seasonal differences were detecetd in age (P < 0.001), BMI (P < 0.001), systolic BP (P < 0.001), diastolic BP (P < 0.001), mean BP (P < 0.001), serum creatinine levels (P < 0.01), and UPE (P < 0.001), with the lowest peak values for these parameters occurring during summer. The distribution of age, UPE rates, and CKD heat map categories significantly varied across the seasons. Cases involving younger patients were more common and during summer than those involving adult patients. Regarding UPE, there were fewer cases involving severe proteinuria and more cases involving mild proteinuria during summer, contrary to the trends observed during spring. In the CKD heat map categories, the low-risk group was more common during summer and less common during spring and winter, whereas the opposite trend was observed in the high-risk group.
Seasonal variations in MN
Seasonal variations in the biochemical and clinical parameters of the patients with MN are summarized in Table 4. The number of kidney biopsies was significantly lower during summer in the patients with MN than those with other diseases. Significant seasonal differences were detected in systolic BP (P < 0.001), diastolic BP (P < 0.001), mean BP (P < 0.001), and UPE (P < 0.001), with the lowest peak values for these parameters occurring during summer. The distribution of age categories significantly varied across the seasons. Cases involving younger patients were more common during summer than during winter. In UPE and CKD heat map categories, no significant differences were found in the analyses of seasonal patterns.
Seasonal variations in PIAGN
Seasonal variations in the biochemical and clinical parameters of the patients with PIAGN are summarized in Table 5. Significant seasonal differences were detected in systolic BP (P < 0.028), diastolic BP (P < 0.036), mean BP (P < 0.02), serum creatinine levels (P < 0.03), and eGFR (P < 0.02). Serum creatinine levels during autumn were the highest, and the eGFR values during autumn were lower than those during spring. In the distributions of age, UPE rates, and CKD heat map categories, no significant differences were found during the analysis of seasonal patterns.
Association between the seasons and the eGFR and UPE rates of the glomerular diseases
Table 6 shows the results of the multivariate linear regression analysis of eGFR and UPE rates without adjusting for the host factors. Both eGFR values and UPE rates were calculated with reference to summer as they tend to be lower during summer than during other seasons27. Multiple linear regression analyses revealed that summer was associated with higher eGFR in patients with IgAN, MCNS, and MN. Alternatively, proteinuria analyses indicated that summer was associated with low levels of proteinuria in patients with MCNS and MN. However, after adjusting for age, sex, BMI, and mean BP, multiple regression analyses revealed that spring (P = 0.038) and winter (P = 0.014) were associated with higher proteinuria in patients with MCNS and that spring (P = 0.036) was associated with lower eGFR compared with summer in patients with MN (Table7).
Discussion
Furthermore, we examined whether seasonal variatoins affected the clinical characteristics of the patients at the time of biopsy.
The overall analysis revealed that the number of renal biopsies was significantly higher during summer, particularly for patients with IgAN. This may be because school urinalysis and physical examinations are often conducted during spring in Japan28, and admissions for kidney biopsies are more common during the summer holidays than during the other periods of the year. Thus, it is reasonable to assume that IgAN and MCNS are more common in younger individuals presenting with mild cases during summer, similar to the results of the present study.
The analyses of seasonal variations in patients with IgAN in this study revealed a significant increase in the number of renal biopsies in adult patients during autumn and winter. The number of patients with severe proteinuria was particularly high during winter. Previous retrospective studies involving patients with IgAN have reported high proteinuria exacerbation during autumn and winter29. Similarly, patients with diabetic nephropathy and pediatric MCNS are reportedly more prone to proteinuria and albuminuria exacerbations during autumn and winter compared with summer. The mechanism underlying this worsening of proteinuria during autumn and winter is not well understood; however, the lack of seasonal variations in the degree of occult urine suggests that age, BP, and renal function may be responsible for the worsening of proteinuria30. BP rises during winter owing to vasoconstriction and falls during summer because of vasodilation due to temperature changes31. Our results also indicate that BP increases in autumun and winter, which supports the results reported in previous studies3. However, differences in BP values are trivial and may not be clinically important. Furthermore, other unknown factors may be involved. In addition, IgAN may be associated with the exacerbation of proteinuria due to preceding upper respiratory tract infections, which tends to occur more frequently during winter18.
The seasonal variation in the patients with MCNS in this study was characterized by a higher number of renal biopsies during summer in young people than in adults. Regarding proteinuria, there were more cases of mild proteinuria during summer and more cases of severe proteinuria during spring than during the other seasons. Several previous studies have reported seasonal variations in patients with pediatric MCNS characterized by a peak during autumn for primary MCNS cases and a peak during spring for recurrent MCNS cases, suggesting an association with the amount of mite allergen in hay fever and house dust17,18. A previous study reported that prior respiratory viral infections are associated with recurrent nephrotic syndrome, irrespective of the type of virus32. Herein, no exacerbation of proteinuria was observed during autumn; however, proteinuria exacerbated during spring, as reported previously, suggesting some type of spring allergic factor.
Our analyses did not detect any significant seasonal variations in the patients with MN and PIAGN. To the best of our knowledge, there have been no reports regarding seasonal variations in patients with MN, and although our study suggests that renal dysfunction is more common during spring than during the other seasons, it was difficult to identify a mechanism or hypothesis to support this finding. For PIAGN, a causal relationship with streptococcal infections has been suggested, which increases the number of cases and exacerbates the disease during winter32,33. However, the results of the present study did not appear to be statistically significant owing to the small number of study participants3. This may be due to the low absolute number of PIAGN cases in our study owing to the widespread use of antibiotics in recent years.
Notably, the clinical features of the disease are influenced by various factors and not only seasonal variations. Therefore, we assessed seasonal variations in clinical features adjusted for age, sex, body size, and BP. Notably, when adjusted for the abovementioned factors, the seasonal factor was weaker in all the glomerular diseases, and only MCNS exhibited proteinuria exacerbation during spring and winter. These results support previous evidence stating that certain infectious or allergic factors contribute to MCNS exacerbation during spring and winter16,17.
Previous studies have reported that ambient temperature, humidity, and air pollution (NO2, SO2, and PMs) with seasonal variations can cause dehydration and abnormal immune responses that have been associated with the risk of developing urolithiasis, acute kidney injury, CKD, and urinary tract infections34,35. Although, to the best of our knowledge, no studies have yet demonstrated an association between primary glomerular diseases and these external factors, it is possible that these factors have influenced the seasonal variation observed in this study.
This study has several limitations. First, it was a national study, and thus, its findings cannot be internationally generalized. Second, the institutions providing data to the J-RBR are not evenly distributed throughout Japan. Third, as the analysis was based on renal biopsy data, there are no uniform indications for renal biopsies, which may have caused sampling bias and data collection not reflecting disease incidence and severity. Moreover, location-specific data, such as temperature and regional differences, were not assessed. Further studies are warranted regarding the time of disease onset and treatment initiation to clarify the strong causal role of seasonal factors in the development of glomerular diseases.
Although there are various limitations in exploring the mechanisms and causes of seasonal variations in glomerular diseases, the greatest strength of this study is the large sample of patients collected from multiple facilities across Japan.
In conclusion, the results of this study demonstrate seasonal variations in the number of renal biopsies in Japan and indicate that seasonal variations in disease severity exist, particularly in patients with IgAN and MCNS. In both cases, the number of kidney biopsies increases during summer. In patients with IgAN, increase in the number of severe cases during winter may be largely owing to age and BP. Conversely, increase in the number of severe cases of patients with MCNS during winter may be due to seasonal variations. Understanding the seasonal variations in the number of renal biopsies will help establish a better presystem for renal biopsy decision and help identify triggers in the patient background, such as BP, lifestyle, infections, and allergies. Furthermore, our findings may provide important insights for the future understanding of the pathophysiology of primary glomerular diseases.
Data availability
These data in the present study are available from the corresponding author upon reasonable request.
References
Stewart, S., Keates, A. K., Redfern, A. & McMurray, J. J. V. Seasonal variations in cardiovascular disease. Nat. Rev. Cardiol. 14, 654–664. https://doi.org/10.1038/nrcardio.2017.76 (2017).
Jenkins, C. R. et al. Seasonality and determinants of moderate and severe COPD exacerbations in the TORCH study. Eur. Respir. J. 39, 38–45. https://doi.org/10.1183/09031936.00194610 (2012).
Iwahori, T. et al. Seasonal variations in home blood pressure: findings from nationwide web-based monitoring in Japan. BMJ Open 8, e017351. https://doi.org/10.1136/bmjopen-2017-017351 (2018).
Arntz, H. R. et al. Diurnal, weekly and seasonal variations of sudden death. Population-based analysis of 24,061 consecutive cases. Eur. Heart J. 21, 315–320. https://doi.org/10.1053/euhj.1999.1739 (2000).
Rothwell, P. M., Wroe, S. J., Slattery, J. & Warlow, C. P. Is stroke incidence related to season or temperature? The Oxfordshire Community Stroke Project. Lancet 347, 934–936. https://doi.org/10.1016/s0140-6736(96)91415-4 (1996).
Woodhouse, P. R., Khaw, K. T., Plummer, M., Foley, A. & Meade, T. W. Seasonal variations of plasma fibrinogen and factor VII activity in the elderly: Winter infections and death from cardiovascular disease. Lancet 343, 435–439. https://doi.org/10.1016/s0140-6736(94)92689-1 (1994).
Kawahara, J., Sano, H., Fukuzaki, H., Saito, K. & Hirouchi, H. Acute effects of exposure to cold on blood pressure, platelet function and sympathetic nervous activity in humans. Am. J. Hypertens. 2, 724–726. https://doi.org/10.1093/ajh/2.9.724 (1989).
Balaforlu, B. et al. Seasonal variations of C-reactive protein and atherosclerotic cardiovascular events in hemodialysis patients. Ren. Fail. 32, 825–831. https://doi.org/10.3109/0886022X.2010.494800 (2010).
Selby, N. M. Acute kidney injury changes with the seasons. Nephrol. Dial. Transplant. 33, 1281–1283. https://doi.org/10.1093/ndt/gfy070 (2018).
Maeoka, Y. et al. Seasonal variations in hemodialysis initiation: A single-center retrospective analysis. PLoS ONE 12, e0178967. https://doi.org/10.1371/journal.pone.0178967 (2017).
Couser, W. G. & Johnson, R. J. The etiology of glomerulonephritis: Roles of infection and autoimmunity. Kidney Int. 86, 905–914. https://doi.org/10.1038/ki.2014.49 (2014).
Boland, M. R., Shahn, Z., Madigan, D., Hripcsak, G. & Tatonetti, N. P. Birth month affects lifetime disease risk: A phenome-wide method. J. Am. Med. Inform. Assoc. 22, 1042–1053. https://doi.org/10.1093/jamia/ocv046 (2015).
White, C. B., Bass, J. W. & Yamada, S. M. Rapid latex agglutination compared with the throat culture for the detection of group A streptococcal infection. Pediatr. Infect. Dis. 5, 208–212. https://doi.org/10.1097/00006454-198603000-00010 (1986).
Zhang, H. et al. New insight into the pathogenesis of minimal change nephrotic syndrome: role of the persistence of respiratory tract virus in immune disorders. Autoimmun. Rev. 15, 632–637. https://doi.org/10.1016/j.autrev.2016.02.007 (2016).
Ivanova, M. et al. 17th IHIW component “Immunogenetics of Ageing”—New NGS data. Hum. Immunol. 80, 703–713. https://doi.org/10.1016/j.humimm.2019.07.287 (2019).
Toyabe, S., Nakamizo, M., Uchiyama, M. & Akazawa, K. Circannual variation in the onset and relapse of steroid-sensitive nephrotic syndrome. Pediatr. Nephrol. 20, 470–473. https://doi.org/10.1007/s00467-004-1780-x (2005).
Odaka, J., Kanai, T., Uehara, R., Kusano, E. & Yamagata, T. Seasonal variations in first episode of childhood idiopathic steroid-sensitive nephrotic syndrome and adult minimal change nephrotic syndrome. Clin. Exp. Nephrol. 19, 146–147. https://doi.org/10.1007/s10157-014-0966-1 (2015).
Inagaki, K. et al. Seasonal proteinuria changes in IgA nephropathy patients after proteinuria remission. PLoS ONE 12, e0187607. https://doi.org/10.1371/journal.pone.0187607 (2017).
Wada, Y. et al. Seasonal variations of urinary albumin creatinine ratio in Japanese subjects with Type 2 diabetes and early nephropathy. Diabet. Med. 29, 506–508. https://doi.org/10.1111/j.1464-5491.2011.03472.x (2012).
Sugiyama, H. et al. Committee for Standardization of Renal Pathological Diagnosis and Working Group for Renal Biopsy Database, Japanese Society of Nephrology, Tokyo, Japan: the first nationwide, web-based, and prospective registry system of renal biopsies in Japan. Clin. Exp. Nephrol. 15, 493–503. https://doi.org/10.1007/s10157-011-0430-4 (2011).
Sugiyama, H. et al. Committee for Standardization of Renal Pathological Diagnosis; Committee for Kidney Disease Registry; Japanese Society of Nephrology. Japan renal biopsy registry and Japan kidney disease registry: committee report for 2009 and 2010. Clin. Exp. Nephrol. 17, 155–173. https://doi.org/10.1007/s10157-012-0746-8 (2013).
Matsuo, S. et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 53, 982–992. https://doi.org/10.1053/j.ajkd.2008.12.034 (2009).
Kitano, N., Suzuki, H. & Takeuchi, T. Patient age and the seasonal pattern of onset of Kawasaki’s disease. N. Engl. J. Med. 378, 2048–2049. https://doi.org/10.1056/NEJMc1804312 (2018).
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 3, 1–59 (2013)
Tomino, Y. Diagnosis and treatment of patients with IgA nephropathy in Japan. Kidney Res. Clin. Pract. 35, 197–203. https://doi.org/10.1016/j.krcp.2016.09.001 (2016).
Okabayashi, Y. et al. Distribution of nephrologists and regional variation in the clinical severity of IgA nephropathy at biopsy diagnosis in Japan: a cross-sectional study. BMJ Open 8, e024317. https://doi.org/10.1136/bmjopen-2018-024317 (2018).
Narita, K., Hoshide, S., Fujiwara, T., Kanegae, H. & Kario, K. Seasonal variations of home blood pressure and its association with target organ damage. Am. J. Hypertens. 33, 620–628. https://doi.org/10.1093/ajh/hpaa027 (2020).
Murakami, M., Yamamoto, H., Ueda, Y., Murakami, K. & Yamauchi, K. Urinary screening of elementary and junior high-school children over a 13-year period in Tokyo. Pediatr. Nephrol. 5, 50–53. https://doi.org/10.1007/BF00852844 (1991).
Masugata, H. et al. Seasonal variations in estimated glomerular filtration rate based on serum creatinine levels in hypertensive patients. Tohoku J. Exp. Med. 224, 137–142. https://doi.org/10.1620/tjem.224.137 (2011).
Narita, K., Hoshide, S. & Kario, K. Seasonal variations in blood pressure: current evidence and recommendations for hypertension management. Hypertens. Res. 44, 1363–1372. https://doi.org/10.1038/s41440-021-00732-z (2021).
MacDonald, N. E., Wolfish, N., McLaine, P., Phipps, P. & Rossier, E. Role of respiratory viruses in exacerbations of primary nephrotic syndrome. J. Pediatr. 108, 378–382. https://doi.org/10.1016/s0022-3476(86)80876-9 (1986).
Hahn, R. G., Knox, L. M. & Forman, T. A. Evaluation of poststreptococcal illness. Am. Fam. Phys. 71, 1949–1954 (2005).
Link-Gelles, R. et al. Characteristics of intracranial group A streptococcal infections in US children, 1997–2014. J. Pediatr. Infect. Dis. Soc. 9, 30–35. https://doi.org/10.1093/jpids/piy108 (2020).
Kuźma, Ł, Małyszko, J., Bachórzewska-Gajewska, H., Kralisz, P. & Dobrzycki, S. Exposure to air pollution and renal function. Sci. Rep. 11, 11419. https://doi.org/10.1038/s41598-021-91000-0 (2021).
Borg, M., Bi, P., Nitschke, M., Williams, S. & McDonald, S. The impact of daily temperature on renal disease incidence: An ecological study. Environ. Health 16, 114 (2017).
Acknowledgements
The authors are grateful to all the colleagues who collected the data for the J-RBR in the various facilities (Supplementary Appendix). This study was supported in part by the committee of the Japanese Society of Nephrology, and by a Grant-in-aid for Intractable Renal Diseases Research, Research on Rare and Intractable Diseases, Health and Labour Sciences Research Grants from the Ministry of Health, Labour and Welfare of Japan. This manuscript has been edited for English language, grammar, punctuation, and spelling by Enago, the editing brand of Crimson Interactive Pvt. Ltd under Normal Editing.
Author information
Authors and Affiliations
Consortia
Contributions
G.K. performed data collection. G.K., N.T., N.U., K.F., and H.S.6 designed the study and analyzed the data. T.Y., A.S., H.S.5 and H.Y. provided advice on primary glomerular diseases. N.T., K.F., and H.S.6 advised on statistical analysis. N.T., N.U., K.F., H.S.6 and T.Y. supervised the interpretation of the results. The manuscript was written by G.K., N.T., K.F., and H.S.6. The final version was approved by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kanzaki, G., Tsuboi, N., Yokoo, T. et al. Seasonal variations in renal biopsy numbers and primary glomerular disease features based on the Japan renal biopsy registry. Sci Rep 13, 5123 (2023). https://doi.org/10.1038/s41598-023-32182-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-32182-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.