Abstract
This study analyzed the impact of factors affecting overall survival in patients with pT2N0M0 esophageal squamous carcinoma (ESCC) and developed a nomogram to predict overall survival (OS). We reviewed the clinical data of 413 patients with pathological T2N0M0 ESCC after radical esophagectomy in two hospitals. Data from one institution was used as the training cohort. A nomogram was established using Cox proportional hazard regression for identifying the prognostic factors affecting for OS in ESCC patients. The area under the curve (AUC), calibration curves and decision curve analysis (DCA) were used to evaluate prognostic efficacy, which was validated in an independent validation cohort. In the training cohort (N = 304), the median OS was 69.33 months, and the 3-, 5- and 10-year OS rates were 76.80%, 67.00% and 56.90%, respectively. The median OS of the validation cohort (N = 109) was 73.50 months, and the 3-, 5- and 10-year OS rates were 77.00%, 67.80% and 55.60%, respectively. According to Cox univariate and multivariate analyses, sex, age, tumor length and the number of resected lymph nodes were identified as predictors of OS. We developed nomograms and performed internal and external validation. The time-dependent receiver operating characteristic (ROC) curve and area under the curve (AUC) value, calibration curve and decision curve analysis (DCA) showed good prediction ability of the nomogram. The developed nomogram can effectively predict OS after esophagectomy in patients with pT2N0M0 ESCC.
Similar content being viewed by others
Introduction
Esophageal carcinoma (EC) is an aggressive malignancy with poor prognosis and is the sixth leading cause of cancer death and the eighth most diagnosed cancer worldwide, and its incidence has been increasing over the past several decades1,2. Esophageal squamous cell carcinoma (ESCC) is the most common pathological subtype of esophageal cancer in China, accounting for more than 90% of all esophageal cancer cases3. With the improvement of diagnostic accuracy and medical technology, the prognosis of EC has been significantly improved, but is still unsatisfactory. Surgery is the most important treatment of choice in localized early EC (T1b‐T2 N0‐1 and M0), the failure of postoperative treatment is mainly due to regional recurrence and distant metastasis4. At present, there are few progresses in the research on the stage, treatment and prognosis of stage T2 ESCC. Numerous studies indicated that tumor grade, tumor infiltration depth and lymph node metastasis are important prognostic indicators for T2 stage EC5. The optimum treatment strategy for T2 stage EC has not been determined. The systematic comprehensive treatment mode of preoperative neoadjuvant therapy combined with surgery have attracted more and more attention of clinicians6,7. A retrospective study with T2 stage EC patients, the 5-year survival rate of patients was 64.1%, which was improved compared with surgery8. However, there is still no evidence to prove that there is a significant difference between preoperative treatment and surgery in resection rate, recurrence rate and long-term survival rate.
This study aimed to analyze the relationship between prognostic factors and overall survival (OS) in resected cases of pT2N0M0 ESCC from the First Affiliated Hospital of Anhui Medical University and Hefei Third People’s Hospital through a database to establish a prognostic nomogram for ESCC. Based on the nomogram, related factors affecting the prognosis of EC patients were screened to predict the survival rate. This may be valuable to clinicians for improved treatment decisions to improve clinical outcomes.
Patients selection and methods
Patient selection
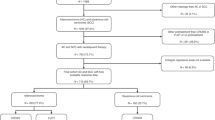
The complete clinicopathological data of 304 previously untreated EC patients who underwent radical esophagectomy were obtained from the First Affiliated Hospital of Anhui Medical University and 109 patients from the Hefei Third People's Hospital between January 2010 and March 2019 were retrospectively analyzed.
The inclusion and exclusion criteria for enrolled patients were as follows: (I) pathological diagnosis was squamous cell carcinoma; (II) underwent radical surgery; (III) pT2N0M0 (staging according to TNM 8th edition); (IV) whose complete postoperative information was available. The exclusion criteria were as follows: (I) age < 18 years; (II) lack of complete clinicopathological or follow-up data; and (III) previous history of other tumors or secondary primary tumors.
Cases from the First Affiliated Hospital of Anhui Medical University served as the training cohort, and those from the Hefei Third People's Hospital served as the validation cohort.
Written informed consent was waived due to the retrospective design and the absence of any intervention.
Data collection
We retrospectively collected the demographic characteristics and clinicopathological characteristics of 413 patients from the two hospitals, including age, sex, tumor location, tumor length, tumor grade, the number of lymph nodes dissected, chemotherapy and radiotherapy. The patients were followed up every 3 months during the first 2 years after the operation, every 6 months after 2 years, and every year after 5 years. Follow-up examinations included routine laboratory examinations, computed tomography (CT), ultrasound of superficial lymph nodes, barium meal of the upper digestive tract and/or positron emission tomography (PET)/CT.
The primary study outcome was OS, which was calculated from the date of esophagectomy to the date of death or the last follow-up. Patients with a survival time of 0 months were excluded. Survival status was determined by querying patient hospitalization data and telephone follow-up. The end time of follow-up is the October 2021.
According to the Ministry of Health (Ethics review on biomedical research involving human subjects), WMA (Declarations of Helsinki) and CIOMS (International ethical guidelines for biomedical research involving), all methods were performed in accordance with the relevant guidelines and regulations.
Statistical analysis
Statistical analysis was conducted using IBM SPSS Statistics (version 20.0, USA) and R language version 4.1.1. All clinicopathological factors were transformed into categorical variables based on the cutoff values determined by the X-tile software 3.6.1. All time events were estimated using the Kaplan–Meier method and compared using log-rank tests. We used univariate and multivariate Cox proportional hazard regression-adjusted potential confounding variables to calculate adjusted risk ratios (HRs) and associated 95% confidence intervals (CIs). The proportional hazard assumption was assessed using Cox models that allowed time-dependent HRs combined with a curve of S (t) * log [- log (t)]. P-values < 0.05 were considered statistically significant.
Multivariate analyses were applied to identify prognostic factors, and the nomogram was developed with corresponding values given the selected prognostic factors. Each independent prognostic factor in the nomogram was assigned a score, and the total score was calculated from the patient data to predict the 3-, 5-, and 10-year OS rates.
Next, we used the bootstrap method to use internal validation to estimate the prediction accuracy of the nomogram, which was presented as the time-dependent receiver operating characteristic (ROC) curve and the area under the curve (AUC) value. The calibration curve and decision curve analysis (DCA) were used to verify the prediction effect of the model. Then, we performed validation training for external validation to evaluate the performance of the prediction model.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the First Affiliated Hospital of Anhui Medical University and the Third People's Hospital of Hefei City and agreed to be published.
Results
Descriptive statistics
The baseline characteristics are summarized in Table 1. Between January 2010 and March 2019, a total of 413 pT2N0M0 ESCC patients were recruited from the First Affiliated Hospital of Anhui Medical University (training cohort, n = 304) and the Hefei Third People's Hospital (validation cohort, n = 109). The patients included more males than females (75.79% vs. 24.21%), and the median age was 65 years (range, 37–78 years). More than half of the patients had primary tumors located in the middle of the esophagus (66.10%), with a median tumor length of 3 cm, and tumor grade mostly well differentiated (moderate to well differentiation, 73.37%). Less than 10 lymph nodes were removed in 63.20% of the patients. A total of 14.29% of patients received adjuvant radiotherapy, and 20.58% received adjuvant chemotherapy. The demographic and clinical factors were largely consistent between the training and validation cohorts.
The median OS of the training cohort was 69.33 months, and the 3-, 5-, and 10-year OS rates were 76.80%, 67.00%, and 56.90%, respectively. The median OS in the validation cohort was 73.50 months, and the 3-, 5-, and 10-year OS rates were 77.00%, 67.80%, and 55.60%, respectively.
Cox regression analysis
In the univariate Cox analysis, sex, tumor length, and the number of lymph nodes harvested were prognostic predictors of OS, and the primary location, differentiation, postoperative adjuvant radiotherapy or chemotherapy did not affect the prognosis. Multivariate analysis showed that the male sex, age over 65 years, tumor length > 2 cm, and the number of harvested lymph nodes < 10 were independent factors affecting OS (Table 2). Finally, a survival curve of those risk factors was displayed using the Kaplan–Meier method suing R software (Fig. 1).
Nomogram development and internal validation
Based on the results of multivariate analysis, all independent predictors of OS were integrated to construct a prognostic nomogram, which was used to calculate the OS rates at 3, 5, and 10 years (Fig. 2).
Then, we performed internal validation of the nomogram and found that the nomogram had a concordance index (C-index) of 0.68. The 3-, 5-, and 10-year AUC values for ROC were 0.66 (95% CI 0.59–0.73), 0.69 (95% CI 0.61–0.74) and 0.79 (95% CI 0.71–0.86), respectively (Fig. 3A). The calibration curves were also applied to verify the predicted effect of the nomogram, and indicated that the calibration plot was highly consistent between the predicted survival and actual survival rates (Fig. 3B–D).
External validation
The nomogram was externally validated using an independent validation cohort. The C-index of the nomogram was 0.69. The 3-, 5-, and 10-year AUC values for ROC were 0.73 (95% CI 0.64–0.83), 0.72 (95% CI 0.62–0.83) and 0.75 (95% CI 0.61–0.90), respectively (Fig. 4A), and the time-dependent AUC curve showing the performance of the nomogram in predicting OS in the validation cohort was plotted (Fig. 4B). Considering the 3-year OS rate, the calibration plot showed good conformity between the predicted and actual probability for OS (Fig. 4C). Finally, we performed DCA to assess the clinical utility of the nomogram, suggesting that was clinically valuable in predicting survival (Fig. 4D).
Discussion
In recent decades, the prognosis of EC has gradually improved in many countries. EC treatment ranges from simple surgery to surgery-based comprehensive treatment and from postoperative adjuvant therapy to preoperative neoadjuvant therapy; however, the treatment of T2 stage ESCC is still inconclusive. Compared with other stages, the treatment and prognosis of T2 ESCC are more controversial. Previous studies have shown that many clinicopathological factors are associated with the prognosis of ESCC9,10. As we retrospectively analyzed 413 patients with pathological T2N0 from two different hospitals. The results showed that some factors, namely the male sex, age over 60 years, tumor length > 2 cm, and the number of intraoperative lymph nodes dissected < 10 affected survival while, the degree of differentiation, tumor location and postoperative adjuvant therapy had no significance on prognosis (Table 2). In the present study, sex was an independent risk factor affecting OS after T2N0 ESCC in that the survival rate of females was significantly higher than that of males. Previous studies have also reported that the survival rate of women after esophageal cancer is higher, and may be related to the poor lifestyle habits, such as smoking and drinking, of male patients11,12. Similarly, We found that age also affects survival after esophageal cancer surgery. The poor nutritional status, decreased body resistance, and difficulty in recovering from surgery associated with older age may affect the prognosis.
Clinicians believe that the differentiation degree of tumor cells can reflect their ability of malignant invasion; the lower the degree of differentiation is, the higher the degree of malignancy, and the likelihood of early metastasis and postoperative recurrence. Several studies have shown that well-differentiated tumors have a better prognosis, while some other studies suggest no significant correlation between tumor differentiation and overall survival13,14. Therefore, the association between tumor differentiation and prognosis remains controversial, we found no such correlation in our study. There also exists no consensus on whether tumor location affects postoperative survival in EC15; in our study, we found no significance between tumor location and postoperative survival. We also found that the number of lymph nodes harvested during surgery was an independent risk factor for OS, and that prognosis was poor when the number of lymph nodes dissected was < 10. Studies have reported that the more lymph nodes removed during surgery, the better the prognosis, which is in line with our findings16. A considerable number of patients with pathological T2N0 stage after radical esophageal cancer have insufficient lymph node dissection, which may affect the prognosis with underestimation of the stage. Therefore, the National Comprehensive Cancer Network (NCCN) guidelines recommend the dissection of at least 15 lymph nodes to allow adequate lymph node staging in patients undergoing esophagectomy.
The retrospective results showed that adjuvant radiotherapy could improve the median survival of patients by 4–6 months, and the 3-year OS rate ranged from 2.9 to 3.3%17,18. In the current consensus, adjuvant therapy is not recommended for pathological T2N0 esophageal cancer. In the present study, some patients still received postoperative adjuvant therapy for the following reasons: (1) patient-related factors: the choice of postoperative adjuvant therapy is affected by factors such as patient intention, economy, and physical fitness; (2) surgery-related factors: there is a high possibility of insufficient mediastinal lymph node dissection in surgical operations and routine cervical lymph node dissection is not performed; 63.20% of patients had < 10 lymph nodes dissected in this study, which may affect the accuracy of postoperative staging; (3) oncologist-related factors: due to the poor overall prognosis of esophageal cancer, doctors have high prognostic expectations for patients with early staging and choose adjuvant therapy under the condition of controllable side effects, hoping to improve the survival rate. The study showed that postoperative radiotherapy or chemotherapy for pT2N0 ESCC could not improve survival, which was consistent with previous reports. Clinicians expect a more reasonable and effective treatment plan to further improve the prognosis because of the poor survival rate after surgery alone19. Therefore, despite a lack of consensus on postoperative adjuvant therapy, there are still exploratory studies on the choice of adjuvant therapy. Previous randomized controlled trials have shown that postoperative radiotherapy can improve the disease-free survival rate and local–regional recurrence rate of pT2-3N0 patients after radical surgery20. Although the OS rate improved, the difference was not statistically significant. Another study also showed that postoperative chemotherapy significantly improves 5-year OS in patients with stage I–III ESCC21.
Nomograms serve as reliable tools to assist clinical decision-making and can also be used as a reference for treatment strategies. Nomograms turn complex regression equations into visual graphs with readable results for easy evaluation, and have gradually gained increasing attention and application in medical research because of their intuitive and easy-to-understand characteristics. Nomograms have been reported to predict survival outcomes after radical esophagectomy in patients with EC22,23. In the present study, we collected the clinical data of the patients for Cox univariate and multivariate analyses and developed a nomogram based on the four factors of the multivariate analysis. Using the developed nomogram, the OS rate can be predicted based on the clinicopathological characteristics of a specific patient. Nomograms show 3-, 5-, and 10-year OS rates against the total score. Clinical prediction models require internal and external validation to ensure the performance of individual risk predictions24,25. Therefore, we divided the data from the two hospitals into training and validation cohorts. The internal validation of the nomogram model and the external validation of the cohorts showed good reliability and accuracy.
The nomogram had some deficiencies. First, it was established based on a retrospective database and was not verified in a prospective data center. There were certain contamination factors and unavoidable treatment bias. For example, the combination of high-risk factors may result in more lymph node resection or systematic adjuvant treatment. In our study, we used an independent validation cohort from another hospital to verify the nomogram, demonstrating its clinical utility. Second, the prognostic factors we included are limited to the common features related to the postoperative pathology of EC and postoperative adjuvant treatment options. The indicators are relatively simple, and some potential features or biomarkers can be considered to further improve the verification standard.
Conclusion
We developed and validated an individualized survival prediction nomogram for predicting OS in patients with ESCC. We demonstrated that the constructed nomogram showed accuracy and validity in predicting the prognosis of ESCC after esophagectomy, may be used to help identify high-risk patient populations. The nomogram may be valuable in clinical settings and can be further improved.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- EC:
-
Esophageal carcinoma
- ESCC:
-
Esophageal squamous cell carcinoma
- LNM:
-
Lymph node metastasis
- OS:
-
Overall survival
- CT:
-
Computed tomography
- PET:
-
Positron emission tomography
- HRs:
-
Hazard ratios
- CIs:
-
Confidence intervals
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under curve
- DCA:
-
Decision curve analysis
- NLN:
-
Number of lymph node
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. https://doi.org/10.3322/caac.21660 (2021).
Morgan, E. et al. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: New estimates from GLOBOCAN 2020. Gastroenterology 163, 649-658.e642. https://doi.org/10.1053/j.gastro.2022.05.054 (2022).
Allemani, C. et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 391, 1023–1075. https://doi.org/10.1016/s0140-6736(17)33326-3 (2018).
Mariette, C. et al. Pattern of recurrence following complete resection of esophageal carcinoma and factors predictive of recurrent disease. Cancer 97, 1616–1623. https://doi.org/10.1002/cncr.11228 (2003).
Hou, X. et al. The impact of tumor cell differentiation on survival of patients with resectable esophageal squamous cell carcinomas. Ann. Surg. Oncol. 22, 1008–1014. https://doi.org/10.1245/s10434-014-4067-x (2015).
Semenkovich, T. R. et al. Comparative effectiveness of upfront esophagectomy versus induction chemoradiation in clinical stage T2N0 esophageal cancer: A decision analysis. J. Thorac. Cardiovasc. Surg. 155, 2221-2230.e2221. https://doi.org/10.1016/j.jtcvs.2018.01.006 (2018).
Kidane, B. et al. Neoadjuvant therapy vs upfront surgery for clinical T2N0 esophageal cancer: A systematic review. Ann. Thorac. Surg. 108, 935–944. https://doi.org/10.1016/j.athoracsur.2019.04.008 (2019).
Kountourakis, P. et al. Combined modality therapy of cT2N0M0 esophageal cancer: The University of Texas M. D. Anderson Cancer Center experience. Cancer 117, 925–930. https://doi.org/10.1002/cncr.25651 (2011).
Davies, A. R. et al. Factors associated with early recurrence and death after esophagectomy for cancer. J. Surg. Oncol. 109, 459–464. https://doi.org/10.1002/jso.23511 (2014).
Wang, Y., Zhu, L., Xia, W., Wu, L. & Wang, F. The impact of adjuvant therapies on patient survival and the recurrence patterns for resected stage IIa–IVa lower thoracic oesophageal squamous cell carcinoma. World J. Surg. Oncol. 16, 216. https://doi.org/10.1186/s12957-018-1516-1 (2018).
Kauppila, J. H., Wahlin, K., Lagergren, P. & Lagergren, J. Sex differences in the prognosis after surgery for esophageal squamous cell carcinoma and adenocarcinoma. Int. J. Cancer 144, 1284–1291. https://doi.org/10.1002/ijc.31840 (2019).
Shen, W. B. et al. Analysis of the causes of failure after radical surgery in patients with (P)T (3)N (0)M (0) thoracic esophageal squamous cell carcinoma and consideration of postoperative radiotherapy. World J. Surg. Oncol. 15, 192. https://doi.org/10.1186/s12957-017-1259-4 (2017).
Situ, D. et al. Do tumor location and grade affect survival in pT2N0M0 esophageal squamous cell carcinoma?. J. Thorac. Cardiovasc. Surg. 146, 45–51. https://doi.org/10.1016/j.jtcvs.2013.01.034 (2013).
Guo, W. et al. Should stage T2 esophageal squamous cell carcinoma be subclassified?. Ann. Surg. Oncol. 21, 2540–2545. https://doi.org/10.1245/s10434-014-3636-3 (2014).
Ding, X. et al. A meta-analysis of lymph node metastasis rate for patients with thoracic oesophageal cancer and its implication in delineation of clinical target volume for radiation therapy. Br. J. Radiol. 85, e1110-1119. https://doi.org/10.1259/bjr/12500248 (2012).
Castro, C. et al. An explanatory and predictive model of the variation in esophageal cancer incidence on the basis of changes in the exposure to risk factors. Eur. J. Cancer Prevent. 27, 213–220. https://doi.org/10.1097/cej.0000000000000422 (2018).
Li, Y. et al. Predicting the value of adjuvant therapy in esophageal squamous cell carcinoma by combining the total number of examined lymph nodes with the positive lymph node ratio. Ann. Surg. Oncol. 26, 2367–2374. https://doi.org/10.1245/s10434-019-07489-3 (2019).
Worni, M. et al. Does surgery improve outcomes for esophageal squamous cell carcinoma? An analysis using the surveillance epidemiology and end results registry from 1998 to 2008. J. Am. Coll. Surg. 215, 643–651. https://doi.org/10.1016/j.jamcollsurg.2012.07.006 (2012).
Wong, A. T. et al. The impact of adjuvant postoperative radiation therapy and chemotherapy on survival after esophagectomy for esophageal carcinoma. Ann. Surg. 265, 1146–1151. https://doi.org/10.1097/sla.0000000000001825 (2017).
Deng, W. et al. Postoperative radiotherapy in pathological T2-3N0M0 thoracic esophageal squamous cell carcinoma: Interim report of a prospective, phase III, randomized controlled study. Oncologist 25, e701–e708. https://doi.org/10.1634/theoncologist.2019-0276 (2020).
Duan, J. et al. Prognostic nomogram for previously untreated patients with esophageal squamous cell carcinoma after esophagectomy followed by adjuvant chemotherapy. Jpn. J. Clin. Oncol. 46, 336–343. https://doi.org/10.1093/jjco/hyv206 (2016).
Su, D. et al. Prognostic nomogram for thoracic esophageal squamous cell carcinoma after radical esophagectomy. PLoS ONE 10, e0124437. https://doi.org/10.1371/journal.pone.0124437 (2015).
Zheng, Y. et al. Predicting prognosis in resected esophageal squamous cell carcinoma using a clinical nomogram and recursive partitioning analysis. Eur. J. Surg. Oncol. 44, 1199–1204. https://doi.org/10.1016/j.ejso.2018.04.011 (2018).
Collins, G. S., Reitsma, J. B., Altman, D. G. & Moons, K. G. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. BMJ 350, g7594. https://doi.org/10.1136/bmj.g7594 (2015).
Moons, K. G. et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): Explanation and elaboration. Ann. Intern. Med. 162, W1-73. https://doi.org/10.7326/m14-0698 (2015).
Acknowledgements
Thank you to all participants.
Funding
This work were supported by Anhui Medical University Basic Medicine and Clinical Medicine Cooperation Research Promotion Program, Hefei, China (grant number: 2019xkjT025), and the Key Project Foundation of Natural Science Research in Universities of Anhui Province (No. KJ2021A0300).
Author information
Authors and Affiliations
Contributions
M.K. designed the study, analyzed the clinical data, and drafted the manuscript. X.M.W. and L.Y.Z. collected the clinical data and conducted patient follow-up. Y.C.W. and M.W.Y. analyzed the data and interpretation, M.Z. reviewed the manuscript. All authors read and approved the final manuscript. All participants gave their consent to have their data recorded and analyzed anonymously.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kang, M., Wang, Y., Yang, M. et al. Prognostic nomogram and risk factors for predicting survival in patients with pT2N0M0 esophageal squamous carcinoma. Sci Rep 13, 4931 (2023). https://doi.org/10.1038/s41598-023-32171-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-32171-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.