Abstract
Loss of biodiversity and especially insect decline are widely recognised in modern ecosystems. This decline has an enormous impact due to the crucial ecological roles of insects as well as their economic relevance. For comparison, the fossil record can provide important insights on past biodiversity losses. One group of insects, for which a significant decline over the last 100 million years has often been postulated, but not demonstrated quantitatively, is Neuroptera (lacewings). Many adult lacewings are pollinators, while the larvae are mostly predators, which becomes very obvious from their prominent stylet-like mouthparts. We investigated the fossil record of larvae of all neuropteran lineages as well as a large share of extant neuropteran larvae. Based on these, we performed an outline analysis of the head with stylets. This analysis provides a quantitative frame for recognising the decline of lacewings since the Cretaceous, indicating also a severe loss of ecological roles.
Similar content being viewed by others
Introduction
The loss of diversity in the group Insecta is now widely recognised1,2, also by the broader public (e.g.3). Losses of biodiversity in the past, as documented by the fossil record, are thought to be informative to improve our understanding of the general processes leading to such losses (e.g.,4,5,6,7).
To recognise losses, changes in biodiversity need to be assessed accurately. This assessment is especially challenging when comparing diversity in the past to modern diversity. For example: Neuroptera, the group of lacewings, is generally accepted to have been part of the early diversification of Holometabola (wasps, flies, and all their closer relatives) and having been much more diverse in the past than it is today8. However, this supposed loss of lacewing diversity is not easy to demonstrate quantitatively. The modern fauna includes more than 6000 species of lacewings, but only about 1000 fossil species have been recognised so far9. Therefore, species numbers alone do not reflect a case of diversity loss.
Diversity loss could also be investigated at higher taxonomic levels, e.g. ranks such as “family”. However, these higher taxonomic ranks are somewhat arbitrary, as objective criteria when a certain taxonomic rank should be given to a specific monophyletic group are lacking (e.g.10,11,12). Even the number of families of lacewings in the modern fauna is a matter of debate and differs between authors: 1913, 17–188, 169,14,15,16, 1517,18 or 1419; overall 21 names for supposed families can be found (references above;20). Some newer publications have reduced the numbers by 1 (recent example in21), but others have revived long synonymised names22 and even introduced new ones23,24. The resulting range in the numbers of families in the modern fauna (≈13 to 21) is quite large and depends on the taxonomic interpretation of the author(s). Therefore, counting higher taxonomic units is unlikely to represent a reliable tool for recognising a loss of diversity in the present case as well.
In consequence, it seems that the loss of biodiversity in lacewings is perceived in a different way. What has been recognised appears to be a loss of morphologically distinct forms, which indicate that lacewings in the past fulfilled specific ecological functions that are nowadays no longer performed by their successors. Potentially, these roles are nowadays performed by representatives of other, now more diverse groups, such as butterflies25. Hence, the loss of diversity in lacewings is apparently not recognised via taxonomic diversity (related to species richness), but instead via morphological diversity (≈ disparity).
Part of the success of the large group Holometabola (including Neuroptera) has been attributed to the differentiation of ecological function between the adults and their larvae. Therefore, morphological diversity assessments should not be restricted to adults, but need to include larval stages as well. The larvae of lacewings are mostly fierce predators; each lower and upper jaw together form a forward-projecting stylet, which is used to inject venom and digestive fluids into the prey and suck the dissolved body tissues out. Lacewing larvae indeed show a large morphological variation in the modern fauna8,17, but even more so in the past, especially in the Cretaceous, as documented by different inclusions in amber of 130–100 million years of age (e.g.26,27,28,29,30).
Recent quantitative analyses of the morphologies of larvae and their changes over time in individual lineages of Neuroptera have revealed losses of morphological diversity in some lineages28,31, but not in all of these29,32,33. Here, we present the first comprehensive quantitative analysis of morphological diversity of the heads and stylets (mouthparts) of larvae of the entire group of Neuroptera over the last 130 million years, based on a dataset of more than 1,000 specimens. Of these, 230 specimens came from Cretaceous ambers (ca. 130–90 mya), 34 from Eocene ambers (ca. 40–35 mya), 12 from Miocene ambers (25–15 mya), and 787 from the modern fauna, in total 1063 larvae.
Results
Comparing diversity of morphological groups through time
Ideally, we would compare the diversity for each node along the phylogenetic tree of Neuroptera. However, there are certain challenges to this approach. First, uncertainties concerning the relationships of different ingroups still remain, for example, Ascalaphidae, the group of owlflies, may represent an ingroup of Myrmeleontidae, the group of antlions, or vice versa21,27. Yet, the monophyly of a group including Ascalaphidae and Myrmeleontidae is beyond doubt (hence collectively referred to as “owllions”34).
More challenging is especially the fact that we cannot reliably identify the closer relationships of many of the fossil larvae. We therefore need to compare the diversity for larger groups that in some cases do not represent natural (monophyletic) groups, but rather share an ecological role or represent functional groups, e.g. aphidlions (see discussion in29), sometimes with a shared distinct morphology such as larvae with straight stylets in Mantispoidea and Dilaridae33. Fossils can be identified as aphidlions or as possessing straight jaws30 and can then be compared to their modern counterparts.
After an initial analysis we excluded larvae of dustywings (Coniopterygidae). Their head shapes strongly differ from those of the other larvae (more or less triangular) and therefore strongly polarise the overall morphospace, disguising differences among the other larvae. Also so far there is no clear larva of Coniopterygidae in the Cretaceous, making the comparison more challenging. Coniopterygidae has been resolved as sister group to all other lacewings9,14,15, the comparison in the following therefore concentrates on the sister group to Coniopterygidae, Euneuroptera, the true lacewings14.
Clear losses of diversity through time
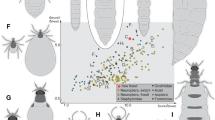
Of the four recognisable ingroups of Myrmeleontiformia, the group of antlion-like lacewings, three show a significant loss of larval diversity since the Cretaceous (Welch's two sample t-test, p-value < 0.001 for all comparisons), only the larvae of owllions seem to have diversified after the Cretaceous (Fig. 1; Suppl. Fig. 1; Suppl. Text 1), as already indicated34. Yet, in the Cretaceous there were numerous now extinct early offshoots of the larger group of antlion-like lacewings (Myrmeleontiformia26,35,36,37,38) with heads and mouthparts to a certain extent comparable to those of modern owllion larvae; these similarities are detectable, for example, in bearing prominent teeth or in similar values in some of the principal components (PCs; especially PC1, but also PC2; Fig. 1). During the Cretaceous, the lineage of split-footed lacewings (Nymphidae) was more diverse and seems to have occupied some areas of the morphospace that are in the modern fauna occupied by owllions (Fig. 1; indicated in34). The diversification of owllions therefore fills some of the space previously occupied by split-footed lacewings, but not all of it. In consequence, the group Myrmeleontiformia has lost morphological diversity of larvae since the Cretaceous, despite the later diversification of owllions.
Simplified diversity changes of lacewing larvae in the last 130 million years; relative relationships simplified as Venn diagram. The overall changes of diversity for the different lacewing groups are presented as arrows, with green arrows pointing to diversity increase, orange arrows pointing to diversity decrease; small arrows indicating a diverging diversity change in an ingroup in contrast to the larger group. The boxes indicate the range for the total sum of variance of the bootstrapped and rare-fractioned data sets, and for each principal component separately of the untreated data sets. In total, the diversity of lacewing larvae has decreased (orange arrow at the bottom). Sketches show simplified morphologies of different representatives based on various sources; drawings produced by the authors. ex extant, K Cretaceous, PC principal component, sum VAR sum of variance. More differentiated plot with four time slices in Suppl. Fig. 1.
The larvae of the three other functional groups in Neuroptera (Osmyloidea, aphidlions, and the straight-jawed larvae of Dilaridae and Mantispoidea) clearly have further diversified after the Cretaceous (Fig. 1). Also, in some lacewing groups the larvae most likely possessed still more plesiomorphic traits in the Cretaceous, lacking the characteristic features of their modern counterparts. Therefore, larvae of these lineages could not yet be identified. Example groups are Sisyridae and Ithonidae.
Despite all these diversifications, the overall diversity of the entire group Neuroptera has decreased after the Cretaceous (Fig. 1). Again, this loss relates to larvae that are not representatives of modern lineages. Extinct supersting larvae36,37 have similarities with some modern larvae of Osmyloidea33 and may have been replaced by them; others seem to have no clear equivalent in the modern fauna.
Beyond the diversity loss
Besides the fact that several morphologies of lacewing larvae have been lost since the Cretaceous, many morphologies have persisted since then (Fig. 2), indicated by a strong overlap in morphospace occupation. In addition, the modern fauna includes only few morphologies that have not yet been present in the Cretaceous. Most obvious examples are the modern larvae of Sisyridae (spongilla flies) with their extremely long stylets, which are used to parasitise on sponges, and the straight-jawed larvae of modern mantis lacewings (Mantispidae). These morphologies, only present today, are likely the result of diversification events after the Cretaceous, representing gains in diversity.
Overview on morphological diversity of lacewing larvae from the Cretaceous to today, illustrated with selected larval morphologies. Several morphologies are only known from the Cretaceous (left part), a certain number is known in the Cretaceous and the modern fauna (middle), while relatively few are only known in the modern fauna. Drawings based on various sources and produced by the authors.
Discussion
Limitations of the approach
The comparison performed here remains asymmetric due to the nature of the fossil record, with a less clear signal from the less productive outcrops (Eocene and Miocene ambers). However, although our view back in time is limited to a smaller sample size and to very specific regions of the world, we can still recognise the larger morphological diversity of lacewing larvae in the Cretaceous. Taking biases into account, it is likely that the actual morphological diversity in the past was even larger than indicated by the current data.
The approach used here will likely only pick up strongly expressed cases of loss, as it might be not very sensitive due to the asymmetry of the sampling bias. For the sub-samples of the Miocene and Eocene, the (mostly) smaller diversity compared to the modern faunas is probably an artefact caused by the small sub-sample sizes. Cases in which a smaller sub-sample reveals a larger diversity (as for the Cretaceous) than the modern sub-sample likely represent a true signal. One might argue that the Cretaceous summarises a diversity of a longer time span. Yet in fact the vast majority of the Cretaceous samples (and therefore also the diversity) originates from a single locality, Kachin amber (Suppl. Table 1).
It proves to be important not only to compare fossils within their closely related lineages, but also in the wider frame14, p. 545. Some of the persisting morphologies (present in the past and today) may represent surviving old morphologies as in larvae of dragon lacewings (Nevrorthidae), yet others may represent cases of convergent evolution in which new evolving morphologies in one lineage replace extinct ones from another lineage34. Such more complex patterns will only be recognisable in larger-scaled analyses, as the one performed here.
Overall a complex pattern, but a net loss of diversity
The approach applied here reveals an overall loss of morphological diversity in lacewings in a quantitative frame, but also reveals more complex aspects of it. While some lineages within Neuroptera undergo a decline, other lineages have diversified, partly “taking over”, or better convergently evolved some of the extinct morphologies and likely also ecological functions. We can also recognise that some modern-appearing fossil larvae, that can be identified as representing modern lineages, differ from their modern counterparts and represent extinct or replaced morphologies as well.
The results presented here can for the first time support the generally accepted loss of diversity in lacewings by quantitative and statistically supported measures. Furthermore it demonstrates that larvae can be well used for making such comparisons.
Given the variety of ecological functions which holometabolan larvae perform, the habit of excluding them from diversity studies is unfortunate. Quantitative morphology offers not only a tool for including larvae into a comparative frame as demonstrated here, it can also identify changes in diversity that can not be picked up quantitatively in a taxonomic frame.
Methods
Key resources table
Reagent or resource | Source | Identifier |
|---|---|---|
Deposited data | ||
Shapes and templates for shapes | Details in Suppl. Table 1 | N/A |
Software and algorithms | ||
R software-environment ver. 4.1.2 | R Core Team 202139 | RRID:SCR_001905; https://cran.r-project.org/ |
Custom R scripts for analysis and statistical tests | This study | |
SHAPE | Iwata and Ukai 200240 | |
Quantification and statistical analysis
The data basis for this study were the outlines from earlier studies (see Key resources table; Suppl. Table 1; Suppl. Text 2). The biological shape was quantified by elliptic Fourier analysis (EFA), which applies the principle of the Fourier transformation to translate the two-dimensional outline into a mathematical object. We achieved this with the SHAPE software package40. The outline is translated into harmonics describing the shape41; we used 20 harmonics. The normalized elliptic Fourier descriptors (NEFDs) representing the specimens were aligned automatically based on the first harmonic. The results were analysed with a principal component analysis (PCA). The resulting principal components (PCs) were then used as input data for further statistical analysis and graphical interpretation. For further details, see Suppl. Text 1 and 3 and Suppl. Files 1–6.
All further analyses were performed offline using custom scripts in the R-statistics environment (ver. 4.1.039). For initial visualization of the morphospace, PCs were plotted against each other in scatterplots, using ggplot2 (ver. 3.3.542). We then calculated the range of values each ingroup of Neuroptera occupied for each PC, respectively for each time subset. Lastly, we calculated the sum of variances for each ingroup of Neuroptera, comparing the time subsets, using the package dispRity (ver. 1.6.043; Suppl. Table 2; Suppl. Fig. 2). For this approach, we first bootstrapped each data set 10,000 times and applied rare-faction-based correction for differences in sample sizes. We used the sample size of the smaller group (often the fossil group) for this correction. We then tested the groups for significant differences in the sum of variance metric, using Bonferroni corrected pairwise Welch two sample t-tests.
Data availability
This paper analyzes existing, publicly available data, listed in Suppl. Table 1. All data reported in this paper will be shared by the lead contact upon request. Joachim T. Haug (joachim.haug@palaeo-evo-devo.info).
Code availability
All original code has been deposited at GitHub and is publicly available as of the date of publication. A link to the repository is listed in the key resources table. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Material availability
This study did not generate new unique reagents.
References
Wagner, D. L. Insect declines in the Anthropocene. Ann. Rev. Entomol. 65, 457–480 (2020).
Wagner, D. L., Grames, E. M., Forister, M. L., Berenbaum, M. R. & Stopak, D. Insect decline in the Anthropocene: Death by a thousand cuts. Proc. Nat. Acad. Sci. 118, e2023989118 (2021).
Jarvis, B. The insect apocalypse is here. New York Times Mag. 2, 41 (2018).
Calosi, P., Putnam, H. M., Twitchett, R. J. & Vermandele, F. Marine metazoan modern mass extinction: improving predictions by integrating fossil, modern, and physiological data. Ann. Rev. Mar. Sci. 11, 369–390 (2019).
Cowie, R. H., Bouchet, P. & Fontaine, B. The sixth mass extinction: Fact, fiction or speculation?. Biol. Rev. 97, 640–663 (2022).
Muscente, A. D. et al. Quantifying ecological impacts of mass extinctions with network analysis of fossil communities. Proc. Nat. Acad. Sci. 115, 5217–5222 (2018).
Rull, V. Biodiversity crisis or sixth mass extinction? Does the current anthropogenic biodiversity crisis really qualify as a mass extinction?. EMBO Rep. 23(1), e54193 (2022).
Aspöck, U. & Aspöck, H. Verbliebene Vielfalt vergangener Blüte. Zur Evolution, Phylogenie und Biodiversität der Neuropterida (Insecta: Endopterygota). Denisia 20 (Kat. Oberöst. Landesmus. N. S. 66), 451–516 (2007).
Winterton, S. L. et al. Evolution of lacewings and allied orders using anchored phylogenomics (Neuroptera, Megaloptera, Raphidioptera). Syst. Entomol. 43, 330–354 (2018).
Ereshefsky, M. Linnaean ranks: Vestiges of a bygone era. Philos. Sci. 69, S305–S315 (2002).
Lambertz, M. & Perry, S. F. Chordate phylogeny and the meaning of categorial ranks in modern evolutionary biology. Proc. R. Soc. B Biol. Sci. 282, 20142327 (2015).
Laurin, M. The subjective nature of Linnaean categories and its impact in evolutionary biology and biodiversity studies. Contrib. Zool. 79, 131–146 (2010).
Gepp, J. Erforschungsstand der Neuropteren. Larven der Erde (mit einem Schlüssel zur Larvaldiagnose der Familien, einer Übersicht von 340 beschriebenen Larven und 600 Literaturzitaten). Progress in World's Neuropterology. In Proceedings of the First International Symposium on Neuropterology, Graz. 183–239 (1984).
Engel, M. S., Winterton, S. L. & Breitkreuz, L. C. Phylogeny and evolution of Neuropterida: Where have wings of lace taken us?. Ann. Rev. Entomol. 63, 531–551 (2018).
Vasilikopoulos, A. et al. An integrative phylogenomic approach to elucidate the evolutionary history and divergence times of Neuropterida (Insecta: Holometabola). BMC Evol. Biol. 20, 64 (2020).
Winterton, S. L., Hardy, N. B. & Wiegmann, B. M. On wings of lace: Phylogeny and Bayesian divergence time estimates of Neuropterida (Insecta) based on morphological and molecular data. Syst. Entomol. 35, 349–378 (2010).
MacLeod, E.G. A comparative morphological study of the head capsule and cervix of larval Neuroptera (Insecta). Ph.D. Dissertation (Harvard University, 1964).
Oswald, J. D. & Machado, R. J. P. Biodiversity of the Neuropterida (Insecta: Neuroptera: Megaloptera, and Raphidioptera). in Insect Biodiversity: Science and Society. 2nd Ed. Vol. II (Foottit, R. G. & Adler, P. H. Eds.). 627–671 (Wiley, 2018).
Khramov, A. V., Bashkuev, A. S. & Lukashevich, E. D. The fossil record of long-proboscid nectarivorous insects. Entomol. Rev. 100, 881–968 (2020).
Monserrat, V. J. Nuevos datos sobre algunas especies de Nemopteridae y Crocidae (Insecta: Neuroptera). Heteropterus Rev. Entomol. 8, 1–33 (2008).
Machado, R. J. P. et al. Owlflies are derived antlions: anchored phylogenomics supports a new phylogeny and classification of Myrmeleontidae (Neuroptera). Syst. Entomol. 44, 418–450 (2019).
Ardila-Camacho, A., Martins, C. C., Aspöck, U. & Contreras-Ramos, A. Comparative morphology of extant raptorial Mantispoidea (Neuroptera: Mantispidae, Rhachiberothidae) suggests a non-monophyletic Mantispidae and a single origin of the raptorial condition within the superfamily. Zootaxa 4992(1), 1–89 (2021).
Jones, J. R. Total-evidence phylogeny of the owlflies (Neuroptera, Ascalaphidae) supports a new higher-level classification. Zool. Scr. 48, 761–782 (2019).
Prost, A. & Popov, A. A first comprehensive inventory of Ascalaphidae, Palparidae, and Myrmeleontidae (Insecta: Neuroptera) of Northeastern Nigeria with description of two new species and an overview of genus Bankisus Navás. Hist. Nat. Bulg. 43(5), 51–77 (2021).
Labandeira, C. C. et al. The evolutionary convergence of mid-Mesozoic lacewings and Cenozoic butterflies. Proc. R. S. London B 283, 20152893 (2016).
Badano, D., Engel, M. S., Basso, A., Wang, B. & Cerretti, P. Diverse Cretaceous larvae reveal the evolutionary and behavioural history of antlions and lacewings. Nat. Commun. 9, 3257 (2018).
Badano, D. et al. X-ray microtomography and phylogenomics provide insights into the morphology and evolution of an enigmatic Mesozoic insect larva. Syst. Entomol. 46, 672–684 (2021).
Haug, G. T., Haug, C., van der Wal, S., Müller, P. & Haug, J. T. Split-footed lacewings declined over time: indications from the morphological diversity of their antlion-like larvae. PalZ 96, 29–50 (2022).
Haug, J. T. et al. The diversity of aphidlion-like larvae over the last 130 million years. Insects 13, 336 (2022).
Pérez-de la Fuente, R., Engel, M. S., Delclòs, X. & Peñalver, E. Straight-jawed lacewing larvae (Neuroptera) from Lower Cretaceous Spanish amber, with an account on the known amber diversity of neuropterid immatures. Cretac. Res. 106, 104200 (2020).
Haug, G. T. et al. The decline of silky lacewings and morphological diversity of long-nosed antlion larvae through time. Palaeont. Electron. 23(2), a39 (2020).
Haug, G. T. et al. The morphological diversity of long-necked lacewing larvae (Neuroptera: Myrmeleontiformia). Bull. Geosci. 96, 431–457 (2021).
Haug, J. T. et al. Changes in the morphological diversity of larvae of lance lacewings, mantis lacewings and their closer relatives over 100 million years. Insects 12, 860 (2021).
Haug, C. et al. The morphological diversity of antlion larvae and their closest relatives over 100 million years. Insects 13, 587 (2022).
Haug, C., Zippel, A., Hassenbach, C., Haug, G. T. & Haug, J. T. A split-footed lacewing larva from about 100-million-year-old amber indicates a now extinct hunting strategy for neuropterans. Bull. Geosci. 97, 453–464 (2022).
Haug, J. T., Müller, P. & Haug, C. A 100-million-year old predator: a fossil neuropteran larva with unusually elongated mouthparts. Zool. Lett. 5, 29 (2019).
Haug, J. T., Baranov, V., Müller, P. & Haug, C. New extreme morphologies as exemplified by 100 million-year-old lacewing larvae. Sci. Rep. 11, 20432 (2021).
Zippel, A. et al. Long-headed predators in Cretaceous amber—Fossil findings of an unusual type of lacewing larva. Palaeoentomology 4, 475–498 (2021).
R Core Team. R: A Language and Environment for Statistical Computing. https://www.R-project.org/ (R Foundation for Statistical Computing, 2021).
Iwata, H. & Ukai, Y. SHAPE: A computer program package for quantitative evaluation of biological shapes based on elliptic Fourier descriptors. J. Hered. 93, 384–385 (2002).
Dujardin, J. P. Outline-based morphometrics, an overlooked method in arthropod studies?. Infect. Gen. Evol. 28, 704–714 (2014).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Springer, 2016).
Guillerme, T. dispRity: A modular R package for measuring disparity. Methods Ecol. Evol. 9, 1755–1763 (2018).
Acknowledgements
This study was supported by the German Research Foundation (DFG HA 6300/6-1) and by the Volkswagen Foundation with a Lichtenberg professorship to JTH. We are grateful to Gideon T. Haug, Neuried, for assistance with the illustrations. We thank all people dedicating their free time to providing free and open source software. We are grateful to J. Matthias Starck, Munich, for his long-standing support. This is LEON publication #49.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
F.B. performed the statistical analyses. J.T.H. prepared the figures. All authors collected the data, discussed the results and wrote the main manuscript text.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haug, C., Braig, F. & Haug, J.T. Quantitative analysis of lacewing larvae over more than 100 million years reveals a complex pattern of loss of morphological diversity. Sci Rep 13, 6127 (2023). https://doi.org/10.1038/s41598-023-32103-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-32103-8
This article is cited by
-
The history of short-tailed whip scorpions: changes in body size and flagellum shape in Schizomida
Swiss Journal of Palaeontology (2024)
-
Mesozoic larva in amber reveals the venom delivery system and the palaeobiology of an ancient lineage of venomous insects (Neuroptera)
Scientific Reports (2024)
-
Morphological diversity in true and false crabs reveals the plesiomorphy of the megalopa phase
Scientific Reports (2024)
-
Convergent evolution and convergent loss in the grasping structures of immature earwigs and aphidlion-like larvae as demonstrated by about 100-million-year-old fossils
Swiss Journal of Palaeontology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.