Abstract
Physogastry is a phenomenon occurring in Euarthropoda and describes an extreme inflation of (parts of) the trunk. It is best known from ticks, termite queens, or honey-pot ants, but can also be found in several other representatives of Euarthropoda. Physogastry has so far rarely been seen in the fossil record. We describe here an example of physogastry in two lacewing larvae (Neuroptera) enclosed in a single piece of Kachin amber (ca. 100 Ma old). We measured head and trunk ratios of different physogastric and non-physogastric representatives of Euarthropoda. Plotting these ratios shows that the new larvae, which display quite extremely inflated trunks, are very similar to ticks or honey-pot ants, but also to certain lacewing larvae of the group Berothidae (beaded lacewings). Outline analysis of head capsule and mouthparts (stylets) further suggests a position within Berothidae. Physogastry is presumed to be linked with living in confined spaces such as wood galleries or soil. Indeed, at least some larvae of Berothidae are known to live inside termite nests for part of their larval life phase, a habit the new larvae may also have had. The new record represents the oldest case of extreme physogastry in insects known to date.
Similar content being viewed by others
Introduction
Representatives of the group Euarthropoda—spiders, beetles, lobsters, centipedes and all their closer relatives—have to moult in order to grow. Indeed, their epidermis produces a chitinous cuticle, which is, basically, not expandable1. When moulting, the animal has in fact two layers of cuticle, an outer one that will be shed, and an inner one that is also already not truly expandable. In order to gain size during a moult, the inner cuticle is formed with distinct folds and is unfolded when the outer cuticle is moulted, allowing a size gain2,3,4.
In this respect, a rare phenomenon in Euarthropoda seems remarkable, namely the extreme inflation of the trunk, or parts of it, often referred to as physogastry5,6,7,8. The best-known example of extreme inflation is that of ticks: when feeding blood on the host their posterior trunk (hysterosoma) inflates to several times its original size (Fig. 1F)9,10,11. Other well-known extreme cases are the inflated posterior trunks (abdomen, gaster) of termite queens (Fig. 1I)12,13,14,15,16 and honey-pot ants (Fig. 1H)6.
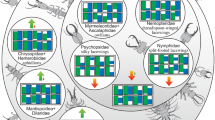
Examples of physogastric/inflated representatives of Euarthropoda and their non-physogastric/non-inflated counterparts and a scatter plot of body ratios (see Supplementary Table 1). The corresponding drawings of the same panel are always to the same scale; note the logarithmic scale; the small letters l and r behind the capital letters refer to the left and right specimen in the respective panel; the arrow marks the fossil physogastric tick reported by Peñalver et al.17. (A–E) Lacewing larvae. (A) Thread-winged lacewings (Crocinae), Josandreva sazi18, larva stage 1 (left) and 3 (right). (B) Fossils of possible beaded lacewings (Berothidae19), possible larva stage 1 (left, specimen 5835, CJW F 3198) and possible larva stage 3 (right, specimen 5833, CJW F 3197). (C) New fossil larva. (D) Beaded lacewings, Lomamyia20, larva stage 1 (left) and 3 (right). (E) Mantis lacewings (Mantispidae), Mantispa uhleri21, larva stage 1 (left) and 3 (right). (F) Ticks (Acari9), unfed female (left) and fed female (right). (G) Gnathiidae, Gnathia africana22, unfed zuphea (left) and fed praniza (right). (H) Honey-pot ant (Formicidae), Myrmecocystus mexicanus “normal” worker (left, bugguide #1588835) and worker in honey-pot state (right, bugguide #567398). (I) Termites (Isopoda), Macrotermes gilvus13, worker (left) and physogastric queen (right). d(head) diameter of head, d(trunk) diameter of trunk, l(head) length of head, l(trunk) length of trunk.
Besides these, there are also less well-known cases of physogastry. Other mites can expand in a fashion similar to that of ticks5,7, but are in fact more comparable to termite queens, as inflation is related to reproduction. Other queens, for example, among ants23,24,25,26 and bees27,28, also show this feature, as do beetles and flies imitating termites and ants (adults29,30,31,32,33; larvae33,34,35). Some beetles also show physogastry related to enlarged ovaries36,37.
More rarely, the trunk can expand in the anterior region only, as in some beetles that have a relation to termites38, and in some isopodan crustaceans (Gnathiidae; Fig. 1G). The latter expand enormously in the larval stages, when feeding on the blood of fish, but also as adult females when carrying their brood22,39,40,41,42; in rare cases the phenomenon has been reported in immatures males43. Another lesser-known case of physogastry is that of certain lacewing larvae displaying straight jaws being representatives of the groups Dilaridae (pleasing lacewings20,44) and Berothidae (beaded lacewings; Fig. 1D;20 [45, their p. 203]). In all cases of physogastry, we need to assume that the cuticle of these animals has special folds, or other structural peculiarities, to allow the extreme change in volume when feeding (e.g., in ticks, honey-pot ants, caterpillars, presumably lacewings46,47,48) or growing oversized gonads (e.g., in termites, ants, beetles49).
The patchy distribution of physogastry on the phylogenetic tree of Euarthropoda indicates that this ability evolved several times independently (e.g.,30). Fossils can be very informative for reconstructing the evolutionary history of these occurrences, not least by providing minimum ages for them. So far, clear-cut cases of physogastry have not been explicitly reported from the fossil record. We herein report lacewing larvae preserved in ca. 100 million-year-old Kachin amber (Myanmar) displaying extremely enlarged trunks.
Results
Description of the amber piece
The amber piece (PED 1794) includes several inclusions (Fig. 2A,B). Of central interest are two larvae interpreted as lacewings (see further below for identification). Syn-inclusions include a small immature cockroach and two adult beetles.
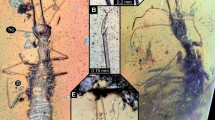
Larva 1 well exposed in both dorsal and ventral views (Figs. 2C, 3A–D); body organised into small head and very large trunk.
PED 1794, Kachin amber, continued. (A–C) Lacewing larva 1, ventral view. (A) Overview. (B) Detail of head. (C) Detail of thorax appendage with empodium (arrow). (D) Lacewing larva 1, dorsal view, red-cyan stereo anaglyph (use red-cyan glasses for the stereo effect). (E) Lacewing larva 2, presumed ventro-lateral view, largely concealed by bubbles. cl claw, fe femur, ta tarsus, ti tibia.
Head capsule rectangular to oval in both dorsal and ventral views (Figs. 2C, 3A,B), widest in the middle, narrower anteriorly and posteriorly, longer than wide (1.5x); details of eyes not apparent; appendage of post-ocular segment 1 (antenna) long and slender, about as long as head capsule, with at least four elements, but joints not well apparent; no external structures of post-ocular segment 2 apparent (intercalary segment); appendages of post-ocular segments 3 and 4 (mandible and maxilla) forming straight stylets (Fig. 3B), shorter than head capsule, strongly tapering towards their anterior tip; distal parts of appendages of post-ocular segment 5 (labial palp) well apparent, elongate, slightly longer than stylets, shorter than antenna (Fig. 3B), with subdivision into elements not well apparent.
Trunk much larger than head, significantly longer (more than 11x), wider than head (more than 6 × at widest point; Figs. 2C, 3A); exact subdivision into segments unclear, but three anterior segments (thorax) well apparent, and nine posterior segments (abdomen) probably present; no distinct set-off neck region between head and trunk.
Thorax segment 1 (prothorax) trapezoid in dorsal view, much wider posteriorly than anteriorly, segments 2 and 3 (meso- and metathorax) slightly widening posteriorly; each segment with a pair of ventral locomotory appendages (legs; Fig. 3A), sub-similar, about as long as segments, inserting far laterally; proximal region of legs not well apparent, subdivision into three major parts, femur, tibia and tarsus distinguishable (Fig. 3C); tarsus with a pair of distal claws and a median trumpet-shaped empodium (white arrow in Fig. 3C).
Abdomen segments appear overall soft and seem to lack sclerites; anterior six(?) segments more or less rectangular in dorsal view, of similar length, slightly wider than thorax segments; posterior segments tapering posteriorly.
Larva 2 not as well exposed as larva 1 (Figs. 2D, 3E); overall morphology very similar to that of larva 1, including size, yet fewer details available. Body preserved less straight, partly twisted, observable mostly in lateral aspect; anterior body region covered from one side by granulated bubble, hindering observation from this orientation.
Quantitative comparison to other lacewing larvae
When plotting ratios of head-to-trunk dimensions of a selection of Euarthropoda (with a main focus on lacewings) (Supplementary Fig. 1; see also Supplementary Table 1, Supplementary Text 1), extant larvae of three major lacewing groups, namely Berothidae, Dilaridae, and Mantispidae, occupy positions in the upper right area of the plot, indicating oversized trunk regions. Some specimens of Ithonidae and Hemerobiidae also plot in this area. Larvae of Mantispidae occupy the most extreme positions. Among the fossil lacewing larvae, some exhibit a rather large trunk, resulting in a far-right position. Some specimens plot even further to the right than the new larva 1 (four cases of putative larvae of Osmylidae and Berothidae), but the new larva plots further up due to the very broad trunk. So far known fossil larvae tend to have more slender trunks.
Quantitative comparison to other cases of physogastry
Comparing the cases of Dilaridae, Berothidae and Mantispidae with other representatives of Euarthropoda reveals that similarly extreme cases occur in termites (Isoptera; Fig. 1I), ants (Formicidae; Fig. 1H) and ticks (Acari; Fig. 1F). Blood-feeding isopodan crustaceans (Gnathiidae; Fig. 1G) can also inflate drastically, but do not reach extreme proportions. The same applies to termitophilous rove beetles (Staphylinidae; Fig. 1).
Quantitative comparison of head and stylet shape
The SHAPE analysis of head and stylet outline (see Supplementary Table 2 and Supplementary Text 1 for details on the specimens) resulted in only two principal components (for the results of the principal component analysis, see Supplementary Text 2, Supplementary Files 1, 2). PC1 explains 95.4% of the entire variation and is dominated by the relative length of the stylets. Low values indicate shorter stylets, high values indicate longer stylets. PC2 explains 2.0% of the entire variation and is dominated by the shape of the anterior rim of the head capsule. Low values indicate convex anterior rims, high values indicate concave anterior rims.
Extant larvae of Berothidae and Dilaridae do not strongly separate (Supplementary Fig. 2). However there is a certain area in PC1 (and PC2) occupied only by larvae of Berothidae (i.e. where larvae of Dilaridae do not plot). There are some fossil larvae with straight stylets that plot in this particular area. This is also the case in the new fossil larva (larva 1). The fossil larvae with straight stylets largely overlap with the range of the extant larvae.
Discussion
Identity of the new fossils
Although the new fossils may appear alienating at first, the forward-projecting mouthparts forming stylets and the presence of trumpet-shaped empodia on the legs immediately identify them as larvae of Neuroptera. The overall head shape, with straight stylets, further indicates that the specimens are representatives of Dilaridae or Berothidae19. Both groups seem to be not closer related according to recent phylogenetic reconstructions50,51. Straight stylets are therefore apomorphic for the group Dilaridae as well as another unnamed group, which includes also Berothidae51. Considering the comparatively short stylets, an interpretation as larvae of Berothidae seems more likely, which is supported by the quantitative morphological analysis (Supplementary Fig. 2). The new fossils differ from extant known cases with large trunks by having relatively broader trunks. In extant larvae, the trunks in beaded lacewings (Berothidae) are broader than those of pleasing lacewings (Dilaridae), further suggesting that the new specimens are representatives of Berothidae.
Known larvae of Berothidae from Kachin amber either have non-inflated trunks (Fig. 1B left) or partly inflated ones (Fig. 1B right). Together with the two new fossils (Fig. 1C), it seems likely that the three conditions (without inflated trunk, with partly inflated trunk, with extremely inflated trunk) represent three larval stages, either of a single fossil species or of several closely related ones. We therefore see it as the most likely interpretation that the two new fossils are stage 3 larvae of beaded lacewings.
The phenomenon of physogastry: setting a comparative frame
The term physogastry has been used in different instances in the literature. Mergelsberg8 used it specifically for cases in which the inflation is due to enlarged reproductive organs. Here we use the term in the wider sense, referring to any extreme inflation of the trunk region (as in [52, his p. 16]). This use is better suited for discussing fossil cases, for which the possible causes can only be inferred indirectly. The case in Gnathiidae demonstrates that the results of enlarged trunks due to feeding or reproductive organs are very similar. Also, a differentiation of inflation of anterior and posterior trunk (e.g.,38) is likely to complicate the situation rather than enlightening it. Hence, we use physogastry also for both cases.
It is also relevant to consider which “normal” conditions possible cases of physogastry should be compared to. In most cases, it seems most practical to contrast individuals of the same developmental stage, such as workers in honey-pot ants (Fig. 1H), or unfed ticks (Fig. 1F). In other cases, it may be necessary to consider other morphs of the same species, as in termites (Fig. 1I). In the case of lacewing larvae (Fig. 1A–E), non-inflated and inflated individuals will belong to different larval stages, with non-inflated ones representing stage 1 larvae, while inflated ones most often represent stage 3 larvae. The comparison might therefore appear at first more extreme, yet this is caused by the fact that two moults occurred between the two stages, while in the case of honey-pot ants, for example, no moulting is involved. Also, comparisons over wider phylogenetic areas is important for further discussions (see discussion in53). Another aspect is how noticeable physogastric individuals can be throughout different groups of Euarthropoda. In general, physogastry becomes apparent as such due to a very large-sized trunk in comparison to the head. Indeed, simple head vs. trunk ratios (width and diameter) provided a plot (Fig. 1), the upper right area of which mostly includes individuals that have traditionally been considered to be physogastric such as late stage larvae of Berothidae (Fig. 1B, D), but especially termite queens (Fig. 1I). The new fossil (Fig. 1C) plots a bit off from the few known examples of physogastric lacewing larvae from the extant fauna, but clearly is among other individuals that appear physogastric. We therefore consider the new fossils as clear cases of physogastry.
Physogastry in holometabolan larvae and lacewings
We assume that cases of physogastry are probably underestimated among holometabolan larvae. Many grubs and caterpillars in their later stages factually have very long and broad trunks and are rather soft, which would then qualify for physogastry as delineated above.
The larvae of the group Holometabola (including beetles, bees, butterflies, flies, but also lacewings) are in principle 'eating machines'54,55. It is therefore not surprising that some evolved the ability to expand the trunk volume quite dramatically. Factually, many holometabolan larvae, including different lacewings, show a tendency towards physogastry (e.g., Psychopsidae56; Osmylidae57) Yet, extreme cases in lacewings, in which the term physogastry has been applied, are those of later stage larvae of pleasing lacewings (Dilaridae58) and beaded lacewings (Berothidae20). Surprisingly, the term has not been applied for later larvae of mantis lacewings (Mantispidae), although our comparison demonstrates the trunk is comparatively even more dramatically inflated (Fig. 1E). In fact, in the comparative frame used herein mantis lacewing larvae are more extreme than, for example, termite queens. Most likely, the mantis lacewing case is more extreme due to the fact that even the thorax segments are inflated. Indeed, in contrast, in most other cases of physogastry, the anterior trunk segments (for example, in Gnathiidae, termites, or ants) remain largely unaffected (Fig. 1G–I). Extant larvae of Berothidae and Dilaridae, as well as the new fossil cases, display enlarged anterior trunk segments as well, which seems to represent a special case among these lacewing larvae.
Physogastry in the fossil record
So far, clear-cut cases of physogastry seem to not have been explicitly reported from the fossil record. Still, it is worth to consider some of the candidate groups. Termites are present already in the Cretaceous59, yet there is so far no known case of a physogastric queen. It is not totally surprising, given that not all termite queens exhibit extreme physogastry60. Indeed, the phenomenon appears to be especially expressed in termites specialised in foraging61, but not in wood-dwelling ones. During the Cretaceous, termites were likely wood-dwelling forms, and physogastric termite queens might have not evolved yet. Similarly, highly specialised ants are well known in the Cretaceous62, but no case of physogastric queens has ever been reported.
Few lineages of Diptera also exhibit physogastry as adults in relation to blood-feeding, e.g., bird flies63. While there are fossil representatives of some of these lineages, none of them has shown clear-cut signs of physogastry63.
Fossil ticks are still rare, and most specimens known so far are non-inflated individuals64,65; none of them has been referred to by the term 'physogastric'. Yet, inflated ticks have been reported by Shi et al.66 and Poinar67. Most recently, Peñalver et al.17 reported ticks on dinosaurs, including a female inflated specimen. This specimen plots in the 'physogastric area' (arrow in Fig. 1), with an even slightly wider and longer trunk than in the new fossils.
Possible relatives of Gnathiidae have been reported from the Jurassic68. Several such fossils (of the group Urda) are, for example, known from the Solnhofen Lithographic Limestones [68, their Table 1], yet none of these fossils has been reported to show inflation of the trunk region.
Some already reported fossil larvae of the group Berothidae have a moderately enlarged trunk, most likely representing stage 2 larvae. These specimens include fossils from the Cretaceous [19, their Figs. 6, 12] as well as from the Eocene [19, their Fig. 2] [69, their Figs. 8–11].
It appears that most lineages nowadays known to exhibit physogastry lack a clear case in the fossil record, at least in the Cretaceous. The ticks reported by Shi et al.66 and Peñalver et al.17 seem to be the only exceptions, yet without using the term physogastry. The new fossil larvae therefore represent the only second case of extreme physogastry in the Cretaceous and the oldest one for the group Insecta.
Function of physogastry and life style of the new fossils
The function of physogastry in lacewing larvae is still difficult to appreciate, even in the extant representatives. Most lacewing larvae are predators, a lifestyle difficult to reconcile with a strongly enlarged trunk. In other cases, physogastry is clearly coupled to a reduced or almost absent mobility6,70.
For pleasing lacewing larvae, a life style in wood galleries of other holometabolan larvae20,58,71 and soil58,72 was inferred. Beaded lacewing larvae live in termite nests, mantis lacewings larvae in egg sacs of spiders or in nests of eusocial hymenopterans (wasps, bees73, their p. 103).
Badano et al.58 suggested that the 'enlarged trunk' morphology may be functionally coupled to life in a confined space. In larvae of checker beetles, living either in bee nests or in wood galleries of other holometabolan larvae, also an inflation of the trunk, in later larval stages, is well apparent74. A similar phenomenon is found in certain larvae of ground beetles living in soil as parasites of leaf beetle immatures75. Living with termites also seems to be coupled to a certain degree to physogastry, as is the case for some beetles and flies8,76.
It is not fully clear whether fossil larvae of beaded lacewings also lived in termite nests (see discussion in69), but it seems likely to have been the case. Fossils reported herein are not preserved together with termites (but with a cockroach and beetles), indicating that these specimens were outside a termite nest. A point to consider is that mantis lacewings living in bee nests will be killed by bee workers if they moult to the adult stage inside the nest [73, their p. 103]. It can then be expected that beaded lacewings living in termite nests would face the same situation, and would have to leave the nest at a certain time in their ontogeny. Gurney20 discussed whether extremely physogastric beaded lacewing larvae could be close to pupation, but remained inconclusive. Cocoons of beaded lacewings have been found under loose bark or even outside wood, under a log or branch77. This indicates that the late stage larvae leave the termite nest at a certain point. It can then be legitimately speculated that the new fossil larvae indeed originally lived in a termite nest, but left it in order to pupate outside.
Material and methods
Material
The single amber piece which is the focus of this study is from Kachin amber, Myanmar, and therefore considered of Cretaceous age, about 100 million years66,78,79. The specimen was legally purchased via the online platform ebay.com from the trader burmite-researcher. The specimen is now deposited in the Palaeo-Evo-Devo Research Group Collection of Arthropods, Ludwig-Maximilians-Universität München, Germany, under repository number PED 1794.
Documentation methods
The amber piece was documented using a Keyence VHX-6000 digital microscope with standard procedures. Composite imaging was performed as well as HDR. Different backgrounds and light settings (polarised vs. non-polarised light) were used to find the combination providing the best contrast (for details, see80 and references therein).
Measurements
For comparison of the new specimens with other physogastric representatives of Euarthropoda as well as their non-physogastric counterparts, we performed several measurements based on illustrations in the literature and in the database bugguide.net (for details, see Supplementary Table 1 and Supplementary Text 1). Our measurements included the length and diameter of the anterior body region and of the remaining body. The anterior body region corresponds to the head capsule for representatives of Insecta, the cephalothoracic shield in representatives of Isopoda, as well as the gnathosoma in representatives of Acari. Hence, this region basically represents the “functional head” in all cases. Forward-projecting mouthparts, antennae, and other similar structures were not included. The remaining part of the body represents the trunk. However, larva 2 discussed in this paper could not be measured reliably.
Quantitative morphology via elliptic fourier transformation
We re-used parts of data of the head and stylet shape from Haug et al.19. Specimens included are extant larvae of the groups Berothidae and Dilaridae as well as fossil larvae potentially representing the same two groups. Additionally, of two specimens depicted in Badano et al.58 the outlines of the head capsule and stylets were redrawn in Adobe Illustrator CS2. Finally, of one of the newly reported larvae the outline of head capsule and stylets were also redrawn and included into the data set. All these outline drawings were transformed into bitmap files and included into an Elliptic Fourier Analysis (EFA) performed with the software SHAPE81, followed by a principal component analysis (PCA) (for details, see19,82).
Data availability
All data is included in this paper.
References
Ross, H. H. A Textbook of Entomology (Krieger Publishing Company, 1991).
Andersen, S. O. Biochemistry of insect cuticle. Ann. Rev. Entomol. 24, 29–59 (1979).
Haug, C. & Rötzer, M. A. I. N. The ontogeny of Limulus polyphemus (Xiphosura s. str., Euchelicerata) revised: looking “under the skin”. Dev. Genes Evol. 228, 49–61 (2018).
Saltin, B. D., Haug, C. & Haug, J. T. How metamorphic is holometabolous development? Using microscopical methods to look inside the scorpionfly (Panorpa) pupa (Mecoptera, Panorpidae). Spixiana 39, 105–118 (2016).
Bussaman, P. et al. Fast population growth in physogastry reproduction of Luciaphorus perniciosus (Acari: Pygmephoridae) at different temperatures. J. Econ. Entomol. 110, 1397–1403 (2017).
Kusnezov, N. Brachymyrmex physogaster n. sp. aus Argentinien und das Problem der Physogastrie bei den Ameisen. Zool. Anz. 165, 382–388 (1960).
Lan, Q., Lu, Z., Ke, B., Liao, J. & Fan, Q. H. Temperature and humidity effects on physogastric development and reproduction of the mushroom mite Dolichocybe perniciosa (Acari: Dolichocybidae). Syst. Appl. Acarol. 22, 1843–1848 (2017).
Mergelsberg, O. Über den Begriff der Physogastrie. Zool. Anz. 106, 97–105 (1934).
Chmelař, J. et al. Sialomes and mialomes: a systems-biology view of tick tissues and tick–host interactions. Trends Parasitol. 32, 242–254 (2016).
Perner, J. et al. RNA-seq analyses of the midgut from blood-and serum-fed Ixodes ricinus ticks. Sci. Rep. 6, 36695 (2016).
Takayama, N. & Takagaki, Y. Tick anaphylaxis triggered by pulling out the tick. Acute Med. Surg. 7(1), e503 (2020).
Havlíčková, J. et al. (3R, 6E)-nerolidol, a fertility-related volatile secreted by the queens of higher termites (Termitidae: Syntermitinae). Z. Naturforsch. C 74, 251–264 (2019).
Lee, C. C., Neoh, K. B. & Lee, C. Y. Colony size affects the efficacy of bait containing chlorfluazuron against the fungus-growing termite Macrotermes gilvus (Blattodea: Termitidae). J. Econ. Entomol. 107, 2154–2162 (2014).
Scheffrahn, R. H. Distribution, diversity, mesonotal morphology, gallery architecture, and queen physogastry of the termite genus Calcaritermes (Isoptera, Kalotermitidae). ZooKeys 148, 41–53 (2011).
Sieber, R. & Leuthold, R. H. Development of physogastry in the queen of the fungus-growing termite Macrotermes michaelseni (Isoptera: Macrotermitinae). J. Insect Physiol. 28, 979–985 (1982).
Sillam-Dussès, D. et al. The role of the glucose-sensing transcription factor carbohydrate-responsive element-binding protein pathway in termite queen fertility. Open Biol. 6(5), 160080 (2016).
Peñalver, E. et al. Ticks parasitised feathered dinosaurs as revealed by Cretaceous amber assemblages. Nat. Comm. 8, 1924 (2017).
Monserrat, V. J. Estadios larvarios de los neurópteros ibéricos I: Josandreva sazi (Neur. Plan., Nemopteridae). Speleon 26, 39–51 (1983).
Haug, J. T. et al. Changes in the morphological diversity of larvae of lance lacewings, mantis lacewings and their closer relatives over 100 million years. Insects 12, 860 (2021).
Gurney, A. B. Notes on Dilaridae and Berothidae, with special reference to the immature stages of the Nearctic genera (Neuroptera). Psyche 54, 145–169 (1947).
Redborg, K. E. & MacLeod, E. G. The developmental ecology of Mantispa uhleri Banks (Neuroptera: Mantispidae). Ill. Biol. Monogr. 53, 1–130 (1985).
Smit, N. J., Basson, L. & Van As, J. G. Life cycle of the temporary fish parasite, Gnathia africana (Crustacea: Isopoda: Gnathiidae). Folia Parasitol. 50, 135–142 (2003).
Guerrero, R. J., Delabie, J. H. & Dejean, A. Taxonomic contribution to the aurita group of the ant genus Azteca (Formicidae: Dolichoderinae). J. Hymenopt. Res. 19, 51–65 (2010).
Hagan, H. B. The reproductive system of the army-ant queen. Eciton. Am. Mus. Nov. 1663, 1–12 (1954).
Meyer, G. F. Untersuchungen an einer parasitischen Ameise. Insect. Soc. 2, 163–171 (1955).
Tschinkel, W. R. & Howard, D. F. Queen replacement in orphaned colonies of the fire ant, Solenopsis invicta. Behav. Ecol. Sociobiol. 3, 297–310 (1978).
Nogueira-Ferreira, F. H., Silva-Matos, E. V. & Zucchi, R. Interaction and behavior of virgin and physogastric queens in three Meliponini species (Hymenoptera, Apidae). Genet. Mol. Res. 8, 703–708 (2009).
Oldroyd, B. P. & Aanen, D. K. Entomology: a bee farming a fungus. Curr. Biol. 25, R1072–R1074 (2015).
Kleisner, K. & Markoš, A. Semetic rings: towards the new concept of mimetic resemblances. Theory Biosci. 123, 209–222 (2005).
Parmentier, T. Guests of social insects. In Encyclopedia of Social Insects (ed. Starr, C.) 1–15 (Springer, 2020).
Roisin, Y. & Pasteels, J. M. Coptophysa and Coptophysella, two new genera of physogastric termitophilous staphylinids associated with Coptotermes in Papua New Guinea (Coleoptera: Staphylinidae). Bull. l’Inst. R. Sci. Nat. Belg. (Entomol.) 60, 179–184 (1990).
Warren, E. Termites and termitophiles. S. Afr. J. Sci. 16(1), 93–112 (1919).
Wasmann, E. Kleine Mitteilungen. Entomol. Nachr. XXIII(2), 25–32 (1897).
Arndt, E. Phylogenetische Untersuchungen larvalmorphologischer Merkmale der Carabidae (Insecta: Coleoptera). Stuttg. Beitr. Naturk. Ser. A Biol. 488, 1–56 (1993).
Prell, H. Biologische Beobachtungen an Termiten und Ameisen. Zool. Anz. 38, 243–253 (1911).
Klausnitzer, B. Beobachtungen zur Lebensweise von Meloe proscarabaeus Linnaeus, 1758 (Coleoptera: Meloidae). Gredleriana 5, 209–216 (2005).
Lückmann, J. & Klausnitzer, B. Die Verwendung der Ölkäfer (Coleoptera, Meloidae) in der Medizin vom Altertum bis in die Gegenwart. Denisia 30, 815–831 (2010).
Kanao, T., Eldredge, K. T. & Maruyama, M. A defensive body plan was pre-adaptive for termitophily in the rove beetle tribe Termitohospitini (Staphylinidae: Aleocharinae). BioRxiv https://doi.org/10.1101/083881 (2016).
Chong, Y. T., Hatai, K. & Ransangan, J. Life cycle of Caecognathia coralliophila (Crustacea, Isopoda, Gnathiidae) in hatchery reared tiger grouper, Epinephelus fuscogutattus. Bull. Eur. Assoc. Fish Pathol. 35, 177–184 (2015).
Diniz, D. G. et al. A note on the occurrence of praniza larvae of Gnathiidae (Crustacea, Isopoda) on fishes from Northeast of Pará, Brazil. An. Acad. Bras. Ciênc. 80, 657–664 (2008).
Ghory, F. S., Kazmi, Q. B. & Baloch, A. B. First report of larval forms of Paragnathia sp. (Crustacea: Isopoda: Gnathidae) from Pakistani waters (Makri Creek). Pak. J. Mar. Sci. 19, 45–54 (2010).
Smit, N. J., Van As, J. G. & Basson, L. A redescription of the adult male and praniza of Gnathia africana Barnard, 1914 (Crustacea, Isopoda, Gnathiidae) from southern Africa. Folia Parasitol. 46, 229–240 (1999).
Wägele, J. W. Description of the postembryonal stages of the Antarctic fish parasite Gnathia calva Vanhöffen (Crustacea: Isopoda) and synonymy with Heterognathia Amar & Roman. Polar Biol. 7, 77–92 (1987).
Monserrat, V. J. Nuevos datos sobre algunas pequeñas familias de neurópteros (Insecta: Neuroptera: Nevrorthidae, Osmylidae, Sisyridae, Dilaridae). Heteropt. Rev. Entomol. 5, 1–26 (2005).
Aspöck, U. & Mansell, M. W. A revision of the family Rhachiberothidae Tjeder, 1959, stat. n. (Neuroptera). Syst. Entomol. 19(3), 181–206 (1994).
Hackman, R. H. Expanding abdominal cuticle in the bug Rhodnius and the tick Boophilus. J. Insect Physiol. 21, 1613–1623 (1975).
Starck, J. M. et al. Morphological responses to feeding in ticks (Ixodes ricinus). Zool. Lett. 4, 20 (2018).
Wolfgang, W. J. & Riddiford, L. M. Cuticular morphogenesis during continuous growth of the final instar larva of a moth. Tissue Cell 13, 757–772 (1981).
Bordereau, C. Ultrastructure and formation of the physogastric termite queen cuticle. Tissue Cell 14, 371–396 (1982).
Vasilikopoulos, A. et al. An integrative phylogenomic approach to elucidate the evolutionary history and divergence times of Neuropterida (Insecta: Holometabola). BMC Evol. Biol. 20, 64. https://doi.org/10.1186/s12862-020-01631-6 (2020).
Winterton, S. L. et al. Evolution of lacewings and allied orders using anchored phylogenomics (Neuroptera, Megaloptera, Raphidioptera). Syst. Entomol. 43, 330–354. https://doi.org/10.1111/syen.12278 (2018).
Schmitz, H. Beschreibung von Termitophora velocipes (Wasmann m litt.), einer termitophilen Phoride aus Vorderindien. Entomol. meddel. 10, 9–16 + 1 pl (1913).
Nagler, C., Høeg, J. T., Haug, C. & Haug, J. T. A possible 150 million years old cirripede crustacean nauplius and the phenomenon of giant larvae. Contrib. Zool. 86, 213–227 (2017).
Badano, D. et al. X-ray microtomography and phylogenomics provide insights into the morphology and evolution of an enigmatic Mesozoic insect larva. Syst. Entomol. 46, 672–684 (2021).
Gauweiler, J., Haug, C., Müller, P. & Haug, J. T. Lepidopteran caterpillars in the Cretaceous: were they a good food source for early birds?. Palaeodiversity 15, 45–59 (2022).
Tillyard, R. J. Studies in Australian Neuroptera. No. 7. The life-history of Psychopsis elegans (Guérin). Proc. Linn. Soc. N. S. W. 43, 787–818 (1918).
Gepp, J. D. Bachhaft Osmylus fulvicephalus—240 Jahre nach seiner Beschreibung durch Johannes Antonius Scopoli—Österreichs Insekt des Jahres (Osmylidae, Neruoptera). Carinth. II Mitt. Naturhist. Landesmus. Kärnten 193, 325–334 (2003).
Badano, D., Di Giulio, A., Aspöck, H., Aspöck, U. & Cerretti, P. Burrowing specializations in a lacewing larva (Neuroptera: Dilaridae). Zool. Anz. 293, 247–256 (2021).
Engel, M. S., Barden, P., Riccio, M. L. & Grimaldi, D. A. Morphologically specialized termite castes and advanced sociality in the early cretaceous. Curr. Biol. 26, 522–530 (2016).
Weil, T. Caste Differentiation in Lower Termites (Doctoral dissertation; Universität Regensburg, 2008).
Nozaki, T. & Matsuura, K. Evolutionary relationship of fat body endoreduplication and queen fecundity in termites. Ecol. Evol. 9, 11684–11694 (2019).
Barden, P. & Grimaldi, D. A diverse ant fauna from the mid-Cretaceous of Myanmar (Hymenoptera: Formicidae). PLoS ONE 9(4), e93627 (2014).
Grimaldi, D. A. The bird flies, genus Carnus: species revision, generic relationships, and a fossil Meoneura in amber (Diptera, Carnidae). Am. Mus. Nov. 3190, 1–30 (1997).
de la Fuente, J. The fossil record and the origin of ticks (Acari: Parasitiformes: Ixodida). Exp. Appl. Acarol. 29, 331–344 (2003).
Estrada-Pena, A. & de la Fuente, J. The fossil record and the origin of ticks revisited. Exp. Appl. Acarol. 75, 255–261 (2018).
Shi, G. et al. Age constraint on Burmese amber based on U-Pb dating of zircons. Cretac. Res. 37, 155–163 (2012).
Poinar, G. Jr. Fossilized mammalian erythrocytes associated with a tick reveal ancient piroplasms. J. Med. Entomol. 54, 895–900 (2017).
Nagler, C., Hyžný, M. & Haug, J. T. 168 million years old “marine lice” and the evolution of parasitism within isopods. BMC Evol. Biol. 17, 76 (2017).
Wedmann, S., Makarkin, V. N., Weiterschan, T. & Hörnschemeyer, T. First fossil larvae of Berothidae (Neuroptera) from Baltic amber, with notes on the biology and termitophily of the family. Zootaxa 3716, 236–258 (2013).
Hecker, H. Das Zentralnervensystem des Kopfes und seine postembryonale Entwicklung bei Bellicositermes bellicosus (Smeath.) (Isoptera). Acta Trop. 23, 297–352 (1966).
Ferro, M. L. It’s the end of the wood as we know it: insects in veteris (highly decomposed) wood. In Saproxylic Insects (ed. Ulyshen, M. D.) 729–795 (Springer, 2018).
MacLeod, E. G. & Spiegler, P. E. Notes on the larval habitat and developmental peculiarities of Nallachius americanus (McLachlan) (Neuroptera: Dilaridae). Proc. Entomol. Soc. Wash. 63, 281–286 (1961).
Maia-Silva, C., Hrncir, M., Koedam, D., Machado, R. J. P. & Imperatriz-Fonseca, V. L. Out with the garbage: the parasitic strategy of the mantisfly Plega hagenella mass-infesting colonies of the eusocial bee Melipona subnitida in northeastern Brazil. Naturwissenschaften 100, 101–105 (2013).
White, R. A. Jr. & Franklin, R. T. External morphology of larval Thanasimus dubius (Fabricius) (Coleoptera: Cleridae). Coleopt. Bull. 36, 143–152 (1982).
Capogreco, J. V. Immature Lebia viridis Say (Coleoptera: Carabidae): bionomics, descriptions, and comparisons to other Lebia species. Coleopt. Bull. 43, 183–194 (1989).
Komárek, S. Mimicry, Aposematism and Related Phenomena. Mimetism in Nature and the History of its Study (Lincom Europa, München, 2003).
Brushwein, J. R. Bionomics of Lomamyia hamata (Neuroptera: Berothidae). Ann. Entomol. Soc. Am. 80, 671–679 (1987).
Cruickshank, R. D. & Ko, K. Geology of an amber locality in the Hukawng Valley, northern Myanmar. J. Asian Earth Sci. 21, 441–455 (2003).
Yu, T. et al. An ammonite trapped in Burmese amber. Proc. Nat. Acad. Sci. 116, 11345–11350 (2019).
Haug, J. T., Müller, P. & Haug, C. A 100-million-year old predator: a fossil neuropteran larva with unusually elongated mouthparts. Zool. Lett. 5, 29 (2019).
Iwata, H. & Ukai, Y. SHAPE: a computer program package for quantitative evaluation of biological shapes based on elliptic Fourier descriptors. J. Hered. 93, 384–385 (2002).
Braig, F., Haug, J. T., Schädel, M. & Haug, C. A new thylacocephalan crustacean from the Upper Jurassic lithographic limestones of southern Germany and the diversity of Thylacocephala. Palaeodiversity 12, 69–87 (2019).
Acknowledgements
We thank Olivier Béthoux, Paris, and one anonymous reviewer for helpful comments on the manuscript. This study was supported by the German Research Foundation (DFG HA 6300/6-1) and by the Volkswagen Foundation with a Lichtenberg professorship to JTH. We thank J. Matthias Starck, Munich, for his long-standing support. Our thanks go to all people dedicating their free time to providing free and open source software. This is LEON publication #43.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Conceptualization: J.T.H.; Methodology: J.T.H., C.H.; Formal analysis and investigation: J.T.H., C.H.; Writing-original draft preparation: J.T.H., C.H.; Writing-review and editing: J.T.H., C.H.; Funding acquisition: J.T.H.; Resources: J.T.H., C.H.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haug, J.T., Haug, C. 100 Million-year-old straight-jawed lacewing larvae with enormously inflated trunks represent the oldest cases of extreme physogastry in insects. Sci Rep 12, 12760 (2022). https://doi.org/10.1038/s41598-022-16698-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-16698-y
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.