Abstract
Forests in Europe are exposed to increasingly frequent and severe disturbances. The resulting changes in the structure and composition of forests can have profound consequences for the wildlife inhabiting them. Moreover, wildlife populations in Europe are often subjected to differential management regimes as they regularly extend across multiple national and administrative borders. The red deer Cervus elaphus population in the Bohemian Forest Ecosystem, straddling the Czech-German border, has experienced forest disturbances, primarily caused by windfalls and bark beetle Ips typographus outbreaks during the past decades. To adapt local management strategies to the changing environmental conditions and to coordinate them across the international border, reliable estimates of red deer density and abundance are highly sought-after by policymakers, wildlife managers, and stakeholders. Covering a 1081-km2 study area, we conducted a transnational non-invasive DNA sampling study in 2018 that yielded 1578 genotyped DNA samples from 1120 individual red deer. Using spatial capture-recapture models, we estimated total and jurisdiction-specific abundance of red deer throughout the ecosystem and quantified the role of forest disturbance and differential management strategies in shaping spatial heterogeneity in red deer density. We hypothesised that (a) forest disturbances provide favourable habitat conditions (e.g., forage and cover), and (b) contrasting red deer management regimes in different jurisdictions create a differential risk landscape, ultimately shaping density distributions. Overall, we estimated that 2851 red deer (95% Credible Interval = 2609–3119) resided in the study area during the sampling period, with a relatively even overall sex ratio (1406 females, 95% CI = 1229–1612 and 1445 males, 95% CI = 1288–1626). The average red deer density was higher in Czechia (3.5 km−2, 95% CI = 1.2–12.3) compared to Germany (2 km−2, 95% CI = 0.2–11). The effect of forest disturbances on red deer density was context-dependent. Forest disturbances had a positive effect on red deer density at higher elevations and a negative effect at lower elevations, which could be explained by partial migration and its drivers in this population. Density of red deer was generally higher in management units where hunting is prohibited. In addition, we found that sex ratios differed between administrative units and were more balanced in the non-intervention zones. Our results show that the effect of forest disturbances on wild ungulates is modulated by additional factors, such as elevation and ungulate management practices. Overall density patterns and sex ratios suggested strong gradients in density between administrative units. With climate change increasing the severity and frequency of forest disturbances, population-level monitoring and management are becoming increasingly important, especially for wide-ranging species as both wildlife and global change transcend administrative boundaries.
Similar content being viewed by others
Introduction
Transboundary wildlife populations are the norm in a politically divided world, rather than the exception1,2. Such populations are usually subjected to spatially variable management regimes associated with separate jurisdictions. At the same time, they are often under the influence of near-ubiquitous disturbances brought on by rapid environmental changes that transcend administrative boundaries. As pressures on ecosystems mount, natural resource managers and policymakers are encouraged to seek and consider information about population-level processes3. This can be exceedingly difficult to accomplish, for technical and political reasons4,5.
In recent decades, forest disturbances have been increasing in Europe due to alterations to forest structure and composition in combination with climatic change6. Two of the most important disturbance types are windfalls and bark beetle Ips typographus outbreaks7. Bark beetles are of particular concern due to their capacity for causing extensive tree die-offs and economic damage by interrupting the transition of water and nutrients within affected trees8,9,10. Insect outbreaks are closely related to climate change and Norway spruce Picea abies monocultures in Central Europe11,12. These disturbances could impact herbivores through a change in resource availability13 as forest openings provide enhanced foraging opportunities for many species including browsers and grazers14,15,16,17. Forest disturbances due to, for example, logging and fire can increase the nutritional value of ungulate forage, but there are trade-offs for ungulate management specific to the type of forest and disturbances18. While higher forage quality in disturbed areas attracts more ungulates, this may not be favoured as high ungulate concentrations may alter vegetation communities19.
Sexual segregation outside the mating season is common in ungulates20 and the differential use of habitats is a common form by which males and females of the same population spatially segregate21. For example, female red deer Cervus elaphus with calves have been shown to occupy different habitats than males to trade-off between forage and predation risk21,22. Such differences in habitat selection may ultimately result in varying sex ratios across space. This phenomenon will be particularly pronounced in ungulate populations where seasonal variation in environmental conditions leads to migratory behaviour in at least part of the population (i.e., partial migration;23). For managers of wide-ranging ungulates, such as red deer, knowledge of differential distribution patterns of males and females is important to evaluate the damage to agricultural crops and forest regeneration, set harvest quotas, or inform the establishment of spatial management zones with different intensities of human influence, such as recreation or hunting24,25.
The red deer population in the Bohemian Forest Ecosystem spans several administrative boundaries, where it experiences different levels of habitat disturbances and management interventions. While forming a contiguous population along the Czech-German border, red deer are exposed to different forms of management in the constituent national and subnational jurisdictions of the system. In the non-intervention zones of the national parks, forests are allowed to recover after disturbances without human intervention in contrast to the state forest areas and the periphery of the national parks26. With longstanding tradition, several winter enclosures and open feeding sites are located across the Bohemian Forest Ecosystem with regular provisioning of supplementary forage from the first snowfalls until green-up in spring27. In spring, forage provisioning is stopped at open feeding sites, enclosures are opened, and deer can move freely during the growing season. This management tool is used to encourage deer to stay in designated wintering sites to prevent bark stripping and browsing, to help regenerate forest28. Deer are counted every winter in the different enclosures and these annual counts have been traditionally used as an index for changes in relative abundance. However, with ongoing climatic change and thus milder winters, more deer may spend winters outside the fenced enclosures and away from open feeding sites. Policymakers, local managers, and other stakeholders involved in red deer management in the Bohemian Forest Ecosystem have been seeking information about the status of the population, especially estimates of abundance, and learning about the role that forest disturbance may play in red deer density and distribution. Also, the distribution of male and female red deer is of great interest to managers, because the sex ratio in winter at feeding stations and in enclosures appears to differ from the one during the green season, but empirical evidence is lacking.
Here, we used non-invasive faecal DNA sampling and spatial capture-recapture (SCR) analysis to (a) estimate the density distribution of red deer throughout the Bohemian Forest Ecosystem, and (b) test for the effects of forest disturbances and management regimes on red deer density throughout this transboundary ecosystem. The forage maturation hypothesis proposes that ungulate migration is driven by selection for high forage quality29. Therefore, we expected red deer densities in summer to be higher in areas with greater forest disturbances, primarily because of more cover and higher food availability14 due to an abundance of early seral stands. Due to the need for cover and forage when raising offspring, we expected this response to be stronger for females than for males. We also expected the positive effect of forest disturbances on red density to be stronger at higher elevation as human interventions on forest disturbances are generally lower in these areas. We expected local management practices to affect red deer density distribution and that the highest abundance occurs in protected areas where hunting pressure is the lowest.
Methods
Study area
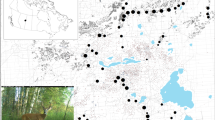
Our study area straddled the Czech-German border, covering three different administrative units in the Bohemian Forest Ecosystem: (1) the Bavarian Forest National Park (BFNP, 245 km2) and (2) the State Forest Neureichenau (SFNR, 152 km2) in Germany, and the majority of (3) the Šumava National Park (SNP, 684 km2) in Czechia (Fig. 1). The two parks are characterised by intermediate elevations with several mountains along the border between Germany and Czechia. The parks are surrounded by low-elevation managed forests, such as SFNR in Germany and the military training area Boletice and the state forest district Boubín on the Czech side (Fig. 1), which are part of regions with lower protection status on both sides of the border (BFNP and the Bohemian Forest Protected Landscape Area30). Both protected landscapes, neighbouring with national parks, form natural buffer zones. The red deer habitat is restricted by law on the German side to an approximately 604-km2 area that is only marginally larger than BFNP and SFNR. Outside of this designated red deer area, all red deer should be culled by law during the regular hunting season. In Czechia, however, the red deer occurrence does not have such a solid border and continues further into the neighbouring Bohemian Forest Protected Landscape Area. The non-intervention zone of the national parks prohibits hunting (herein, no-hunting zone).
Spatial covariates included to model variation in red deer detection probability (A recorded GPS search tracks) and density (B management units, C elevation, D proportion of forest disturbances). The management units in the panel B are: (1) BFNPp: the no-hunting zone of the Bavarian Forest National Park, (2) BFNP: ungulate management zone of the Bavarian Forest National Park, (3) SFNR: the State Forest Neureichenau district, (4) SNPp: the no-hunting zone of Šumava National Park, and (5) SNP: the ungulate management zone of the Šumava National Park and a small part of the State Forest district Boubín in the buffer of the central part of the study area on the Czech side (darker blue area outside the white lines). For visibility, soft edges of the management units are not shown. White and black lines show the three administrative units and the border of the study area used in the spatial capture-recapture analysis, respectively. The inset maps in the top row show the location of the study area (red rectangle) within the mainland Europe. All maps were created using R59.
With an elevation range from 570 to 1453 m, the study area is dominated by coniferous forests (60%), mixed forests (20%), and grasslands including pastures (14%). Other land cover types present in the area include broadleaved forests (6%), shrublands (< 1%), and surfaces covered with buildings and extensive pavement (< 1%;31). Several open areas within the forest were created by bark beetle outbreaks31, which first occurred in 1983, reached a peak in 1996–199732, and have continued since. Forests in the study area are mainly comprised of Norway spruce in moist valleys and transition to mixed forests with an abundance of European beech Fagus sylvatica and silver fir Abies alba at intermediate elevations. Forests at high elevation are less dense and rich in Norway spruce, interspersed with mountain ash Sorbus aucuparia and sycamore Acer pseudoplatanus. In SNP, Norway spruce has replaced natural forests in more extensive areas than in the BFNP33,34.
Red deer dominates the ungulate guild in the study area, but roe deer Capreolus capreolus also occurs, albeit at lower densities. Wild boar Sus scrofa is more common on the Czech than on the German side, but distributions fluctuate. Fallow deer Dama dama and moose Alces alces seldom traverse the study area35. The western part of Czechia is also inhabited by the non-native sika deer C. nippon that is regularly culled in the study area36. However, the threat of hybridization between sika and red deer remains unmanaged37,38. Red deer in the study area are partially migratory39. Natural predators include wolves Canis lupus, with a first pair having recolonised the area in 2016, and Eurasian lynx Lynx lynx, which occasionally prey on red deer females and calves40. Hunting is likely the main cause of mortality for red deer in the Bohemian Forest Ecosystem. Hunting quotas are set based on annual counts in winter enclosures and at feeding stations, the number of red deer hunted in the previous year, and an inventory of browsing damage28. Red deer are hunted mainly from high stands by single hunters or small groups of hunters in autumn, while in late autumn and winter drive hunts outside BFNP may also be practised. In SFNR, red deer are hunted under regular German and Bavarian hunting guidelines. In SNP, hunting is prohibited in approximately 10% of the area. Hunting is prohibited in 75% of BFNP and a large proportion of red deer culling occurs when the animals enter the winter enclosures. The hunting season lasts from June to January in Germany and from August until mid-January (until the end of March for calves) in Czechia. Overall, as of 2018, hunting was prohibited in 23% of the study area (Fig. 1), and hiking in 19% of the study area was restricted to marked trails to reduce human disturbances.
Faecal DNA sampling and genotyping
To guide non-invasive DNA sampling of the red deer population, a 1-km2 grid was generated using ArcGIS Desktop 10.5.1. Due to the large extent of the study area, not all grid cells could be searched and following a simulation study of sampling design trade-offs, we randomly discarded 20% of the grid cells but avoided discarding neighbouring grid cells to limit the size of resulting spatial gaps in sampling. We also omitted 42 grid cells because more than half of their area was covered by human settlements, water bodies, or very steep terrain that was difficult to access by searchers. The final search area included 543 grid cells (Fig. 1).
We collected fresh deer faeces between 1 June and 26 July 2018 (49 sampling days). Although the environmental conditions in early spring are potentially more suitable for faecal DNA sampling of the red deer population, this period overlaps with the partial migration of the red deer39. Therefore, we limited our sampling to summer, when red deer in our study area have established their seasonal home ranges. Surveyors conducted structured search-encounter sampling and GPS-recorded their search tracks and the location of samples. To ensure homogeneous coverage, each grid cell was subdivided into 16 smaller units of 250 × 250 m, which were searched with similar intensity (Fig. 1). Surveyors were advised to walk about 10 km within each 1 km2 grid cell. However, steep terrain that was dangerous to access was skipped. We collected only fresh pellets, but in areas with particularly low numbers of detections, older pellets with a relatively intact surface were also sampled. We further enforced a minimum distance of 2 m between subsequent pellet groups to avoid sampling the same group twice. From each pellet group, two individual pellets were sampled. A new toothpick or latex glove was used for every sample to avoid cross-contamination when transferring the sample into a falcon tube. At the end of each day, samples were placed in a freezer at − 20 °C.
Genetic analyses were performed according to41. Briefly, after DNA extraction, eight dinucleotide microsatellites and one sex marker42 were amplified in two multiplex PCRs (Table S1). Two negative controls were included in all PCRs to detect potential contamination. Determination of matching genotypes was carried out using GENECAP43. We scrutinised genotypes differing by one (1-MM) or two (2-MM) alleles to detect genotyping errors. For all 1-MM and 2-MM pairs, raw data were re-checked to resolve the mismatches. Genotype pairs with only one mismatch were regarded as originating from the same individual44. Those pairs with 2-MM were considered as originating from different individuals if re-checking of raw data and an additional two PCR repeats did not alter the results and if both samples matched with other samples in the data set45. To confirm the power of the used loci, we calculated the probability of identity and the probability of identity for siblings as a more conservative metric46 and heterozygosity using GIMLET47. We calculated genotyping error rates (allelic dropout and false alleles) as recommended in48 (Eqs. 1 and 3). Samples identified by their genotype as originating from roe or fallow deer were excluded from further analyses. As a result, the data consisted of individual identity, sex, and location associated with non-invasive red deer detections.
Analysis
We built an SCR model in a Bayesian framework49 with two hierarchical levels distinguishing the observation process from the ecological process (see Supporting Information for model definition).
Ecological process
In SCR models, individual locations are defined by their centre of activity or home range. Abundance is then defined as the number of individual activity centres within the region of interest or habitat. Here, we defined the habitat as the area searched for red deer DNA samples surrounded by a 3-km buffer to account for edge effects50 leading to a habitat polygon of 1209 km2 subdivided into 1 × 1 km grid cells (Fig. 1). In SCR analysis, the buffer allows to explicitly account for the possibility to detect individuals that had their activity centre outside of the searched area51. To explore the drivers of red deer density, we modelled the distribution of activity centres as an inhomogeneous Bernoulli point process52,53 whose intensity is proportional to the red deer density and related to a set of covariates:
In this formulation, Ih is the point process intensity in habitat grid cell h, and Rh, Eh, and Uh are covariates describing the proportion of disturbed habitat, the average elevation, and management unit within a 2-km radius of habitat grid cell h, respectively. β coefficients are the effects of covariates (see below) in a given habitat cell on the probability that an individual has its activity centre located in this same cell. The main effects include the proportion of forest disturbances βR, elevation βE, and management region βU (Eq. 1). As disturbances tend to occur at different elevations (e.g., windthrows predominantly at higher elevations54), and elevation as a proxy for plant phenology has been shown to be a main predictor for red deer migration27 and habitat selection30, we included an interaction between elevation and forest disturbances. βER is the interaction terms of disturbance and elevation. In addition, because management regimes differ amongst and within the different administrative units, we considered that red deer density could differ between the five management regions, corresponding to three administrative units and their hunting/no-hunting zones (Fig. 1). βU is the slope for the management region of the habitat cell and is compared to the ungulate management zone of SNP (intercept), where hunting is authorised (Fig. 1).
The proportion of disturbance was generated using the Landsat-based forest disturbance maps created by54. The forest disturbance map is derived from a time-series analysis of Landsat satellite imagery and maps stand-replacing forest disturbances occurring between 1986 and 2014, including natural disturbances by windthrow and bark beetle outbreaks, as well as anthropogenic tree removal, such as salvage logging of windthrows and bark beetle-infested stands, as well as other harvests. We combined the categories provided by54, i.e., bark beetle infestations, windthrows, salvage-logged wind throws, and bark beetle sites between 1995 and 2014, into a single covariate describing the total area of disturbed forest per grid cell. We derived elevation from Shuttle Radar Topographic Mission (SRTM) maps downloaded at 1 × 1 km resolution. Land protection status was provided by the national parks. Management units include (1) ungulate management zone of BFNP, (2) non-intervention zone of BFNP, (3) ungulate management zone of SNP, (4) non-hunting zone of SNP, and (5) SFNR (Fig. 1). We considered a moving window around each habitat cell size to calculate the proportion of each management unit within a 2 km radius, where the values gradually decreased towards the edges from 1 to 0. All spatial covariates were resampled to the habitat resolution (Fig. 1), then standardised before model fitting.
To account for the fact that some individuals in the population may never be detected, we used a data-augmentation approach55. Following this approach, we derived estimates of population size N by summing the number of individuals included in the population, where M is the maximum possible number of individuals in the population.
We modelled individual inclusion in the population through a latent state variable zi, governed by the inclusion parameter ψ for all individuals i in 1:M as:
Observation process
The SCR observation component models how the individual detection probability varies over a set of detectors. Here, we generated detector locations by discretizing the search area into 4122 grid cells of size 400 × 400 m (Fig. 1). We used the partially aggregated binomial model to retain as much information from the collected genetic data as possible56 and further divided each detector grid cell into 16 sub-cells (or less, if some sub-cells did not overlap the suitable red deer habitat based on our knowledge of the study system). We then generated individual spatial detection histories by retrieving the frequency of sub-cells with at least one sample from the focal individual for each detector main grid cell.
We used the half-normal detection function57 and modelled the probability of detecting an individual at a given detector as a decreasing function of the distance between this individual’s activity centre and the detector:
Here, pij is the detection probability of individual i at detector j, p0 is the baseline detection probability, dij is the distance between individual i’s activity centre and detector j, and σ is the scale parameter which dictates how fast the detection probability decreases with distance. To account for spatial variation in detectability, we modelled a detector-specific baseline detection probability:
In this equation, \(\dot{p}_{0_{u}}\) is a separate baseline detection probability for each of the three administrative units (BFNP, SFNR, and SNP) because of the potential variation in sampling effort. Lj is the length of GPS search tracks recorded within detector grid cell j, and βL is the slope parameter describing the linear relationship between effort and detection probability.
Model fitting and post-processing
We fitted sex-specific models using NIMBLE version 0.6-958 and R version 3.5.259 with functions now available in the R package nimbleSCR60. We ran 4 chains of 100,000 iterations each and discarded the first 10,000 samples as burn-in, leading to a total of 360,000 MCMC samples per model to draw inferences from. We assessed convergence by looking at the potential scale reduction value for all parameters and mixing of the chains using trace-plots61. For mapping density, we thinned the posterior samples by 10 and based the maps on 36,000 samples. To obtain estimates of abundance for each administrative unit, we summed the number of model-predicted activity centres that fell within the administrative unit of interest for each iteration of the MCMC chains, thus generating a posterior distribution of the abundance for this area, from which mean abundance estimates can be derived. We mapped sex-specific and total realised density for red deer based on the average model-estimated activity centre locations of individuals. For the prediction of density as a function of covariate effects, we calculated the relative density per cell and multiplied the habitat intensity value by the N estimate in each cell for every MCMC iteration.
Ethics declarations
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. No ethical approval was required for collecting scats and no invasive samples have been taken from the animals or humans and all procedures carried out followed the research permit by the administrations of the Bavarian Forest National Park, Šumava National Park, and the Bavarian State Forest.
Results
Faecal DNA sampling and genotyping
During sampling, 3450 km of GPS search tracks were recorded, and 3234 putative red deer faeces were collected. The 1578 (48.8%) successfully genotyped samples were assigned to 1120 red deer individuals (494 females, 560 males, and 66 of unknown sex due to amplification failure of the sex marker). The sample size and success rate are in line with similar studies in which ungulate populations have been sampled using non-invasive genetic methods41,62,63,64. Of the genetically identified individuals that were included in the analysis (n = 1054), 28.5% were detected more than once (33.7% of the detected males and 25.9% of the detected females) with a maximum of six samples from the same individual. Genotyping error rates are reported in Table S2. The mean allelic dropout rate over all loci was 4.2%, whereas the mean false alleles rate was 0.9%. The overall Probability of Identity of the data set was 1.89 × 10–11, and the overall probability of identity for siblings was 0.00016.
Abundance and density estimates
We estimated the red deer population size in our study area at 2851 individuals (95% Credible intervals CI = 2609 to 3119). Sex-specific estimates were 1406 females (95% CI = 1229 to 1612) and 1445 males (95% CI = 1288 to 1626) for the summer of 2018 (Fig. 2, Table S3). The overall sex ratio was even (F:M = 1:1.03), but differed between management zones, with a slight skew towards males in SNP (1:1.07), a strong skew towards males in the SFNR (1:2.06) and a female bias in the BFNP (1:0.78). The overall abundance was higher on the Czech side (2052 red deer, 95% CI = 1836 to 2292) compared to the German side (800 red deer, 95% CI = 680 to 940). Likewise, the average red deer density was higher in Czechia (3.5 km−2, 1.2 to 12.3) compared to Germany (2 km−2, 0.2 to 11; Fig. 2).
Density maps (A–C) and abundance estimates (plot D) for red deer Cervus elaphus across the Bohemian Forest Ecosystem in summer, June and July, 2018. Population estimates are broken down into sex-specific estimates (B male, C female deer) for the three administrative units (BFNP Bavarian Forest National Park, SFNR State Forest Neureichenau, and SNP Šumava National Park). Grey areas in density maps represent regions beyond the sampled extent that belong to the management jurisdictions. Violins in plot (D) show posterior distributions of abundance with 95% credible interval and white dot indicates the medians. All figures were created using R59.
We estimated the average red deer density for the entire study area in the Bohemian Forest Ecosystem at 1.42 females and 1.46 males km−2. The effect of forest disturbances on red deer density was modulated by elevation, changing from negative at low elevations to strongly positive at high elevations (Fig. 3; Table S4). The ungulate management zone of BFNP and SFNR on the German side had lower baseline red deer densities compared to the ungulate management zone of SNP on the Czech side (Female βBFNP = − 2.8, 95% CI = − 4.8 to − 1.4 and Male βBFNP = − 2.2, 95% CI = − 3.8 to − 1; Female βSFNR = − 1.9, 95% CI = − 2.7 to − 1.1 and Male βSFNR = − 1, 95% CI = − 1.5 to − 0.5; Table S4). The non-intervention zone of BFNP had a higher baseline density, compared to the ungulate management zone of SNP as the reference area, but beta coefficients overlapped zero (Table S4). The non-intervention zone of SNP had lower red deer densities compared to the management zone of the SNP, but coefficients overlapped zero (Table S4).
Sex-specific predictions of red deer relative density (individual per km−2) across the Bohemian Forest Ecosystem as a function of the interaction between proportion of forest disturbances and elevation (A male, B female deer). Contour lines in the right-column plots represent relative density of red deer for each sex. All figures were created using R59.
Baseline detection probability was slightly higher for males (Male p0 = 0.00034, 95% CI = 0.00025–0.00045) compared to females (Female p0 = 0.00024, 95% CI = 0.00017–0.00033). Detection probability was positively associated with the length of transects searched for both sexes (Female βL = 0.4, 95% CI = 0.3 to 0.5 and Male βL = 0.5, 95% CI = 0.4 to 0.6). The scale parameter of the half-normal detection function σ was similar between female (σ = 1 km, 95% CI = 0.9 to 1.1) and male red deer (σ = 0.9 km, 95% CI = 0.8 to 0.9). These σ values translate to average summer home-range sizes of 18 km2 (95% CI = 15 to 21) and 15 km2 (95% CI = 13 to 17) for females and males, respectively, during the sampling period.
Discussion
The transboundary population of red deer in the Bohemian Forest Ecosystem along the Czech-German border is characterised by higher densities at higher elevations and in areas with forest disturbances, which are associated with higher forage quality in summer. Red deer density was up to six times higher in areas subjected to forest disturbance than in undisturbed areas, especially at higher elevations (Fig. 3). Red deer density was also higher in the non-intervention zones of the protected areas, compared with areas where hunting was authorised. We also found pronounced sex-specific spatial variation in density (Fig. 2). Both forest disturbances and different ungulate management regimes across the administrative units explained this variation.
Context-dependent effect of forest disturbance management
While forage availability is likely driving habitat quality in forest disturbance gaps, ungulates have to trade-off between forage and predation risk65,66. The positive effect of forest disturbances on red deer density in the Bohemian Forest Ecosystem was context-dependent and most pronounced for males. Specifically, while red deer seemed to avoid disturbed areas at low elevations, disturbances were attractive for red deer at high elevations (Fig. 3). Forest openings created by disturbances have the potential to provide ungulates access to diverse and abundant forage that follows the removal of overstory canopy14,67. In our study area, bark beetle outbreaks have created similar forest gaps. There are several studies documenting the link between canopy removal and improved food availability for deer and other large herbivores in disturbed forest stands67,68,69. In addition, disturbed areas—if they stay unmanaged—provide excellent shelter. While disturbances caused by bark beetle infestations and windthrows remain unmanaged in the non-intervention zones of the national parks, which cover large parts of the high-elevation areas in the study system, disturbances in the management units of the national parks and in SFNR are managed through salvage logging. For forest disturbances, we could not distinguish windthrows and bark beetle infestations from salvage-logged areas as two different treatment groups. However, salvage-logged areas were a small fraction of the managed forest disturbances in the study area.
Forests recover faster and more homogeneously at salvage-logged than at non-intervention sites in the Bohemian Forest Ecosystem70. In addition, forest recovery is faster in low elevations, leading to a long-lasting increase of habitat quality in the higher compared to the lower elevations71,72. Additionally, the type of vegetation covering disturbed sites differs between high and low elevations, with more grasses and ferns at high elevations, which might also be more attractive to mixed feeders like red deer73. Finally, at low elevations, more open habitats associated with higher hunting pressure at disturbed sites may offset the positive effect of increased forage availability, leading to red deer avoiding disturbed areas at lower elevations. This is in accordance with the pattern of the increased positive association between disturbances and red deer density with elevation (Table S4). We detected similar patterns of positive elevation-disturbance effects on red deer density, but they were more pronounced for male than for female red deer.
Seasonal migration and forage availability
Red deer inhabiting the Bohemian Forest Ecosystem are partially migratory, i.e., only part of the population migrates, while the remainder stays resident on the shared winter range39. Migration behaviour, and hence habitat selection, is strongly affected by forage phenology as suggested by the forage maturation hypothesis29. Specifically, individuals migrating to higher elevations in spring have access to more high-quality forage during the growing season compared to red deer that remain at lower elevations23. In this context, forest disturbances play a crucial role in red deer habitat selection and distribution patterns72, which was supported by our findings. Gaps provided by forest disturbances often increase foraging opportunities due to a higher abundance of plant biomass on the ground14 (but see73). Recent studies suggest that habitat suitability for red deer improved after disturbance for at least 25 years, and these disturbance-related habitat effects generally increase with elevation72. Specifically, different disturbance types occurred along elevational gradients and wind throws were characteristic for higher elevations72. Post-disturbance recovery is also affected by elevational gradients in the Bohemian Forest Ecosystem, further affecting post-disturbance recovery70, with salvage logging mainly occurring at lower elevations. Overall, we observed a pattern that is typical for a partially migratory ungulate population under the predictions of the forage maturation hypothesis29,74,75. These findings are mediated by ungulate management in our study system.
Management interventions and the effect of hunting
Hunting is the main selective force for the red deer in our study area, as for many transboundary ungulate populations in temperate climates76. Hunting has been shown to affect the distribution, and hence density of Cervus spp.25,29,77. In the Bohemian Forest Ecosystem, red deer density in summer was the lowest in SFNR, where population size is regulated by hunting and elevation is lower compared to the two national parks (Fig. 2; Table S3). In contrast, density was more than twice as high in BFNP, which includes a large non-intervention zone. The highest densities were predicted for SNP, where deer are also protected year-round in the non-intervention zone (i.e., hunting is not authorised), but more deer were hunted outside the non-intervention zone compared to the BFNP or SFNR.
We detected a difference in red deer sex ratio amongst the three administrative units with a 1:2 female-to-male ratio in SFNR, compared to 1:1.1 and 1:0.8 in SNP and BFNP, respectively (Table S3). In contrast, the winter enclosure counts in SFNR rather suggest a female-biased sex ratio. During the winter preceding this study, 419 red deer with a sex ratio of 1.16:1 (F:M) in BFNP, 222 red deer (1.98:1) in SFNR, and 562 red deer (1.08:1) in SNP were counted in the enclosures or at open feeding sites (n = 1203). The current hunting regulations protect males older than three years in BFNP, but this has not resulted in a male-biased sex ratio in this area based on our findings (Table S3). The male-biased sex ratio observed in SFNR is most likely the result of a combination of hunting focused on females to regulate population size, differential space use between males and females during summer, and a high proportion of female migrants that use SFNR in winter only. For example, there are extensive forest disturbance areas in SFNR that provide high-quality forage, yet the deer do not use this area and move to the Czech side instead, where hunting pressure is lower. Likewise, telemetry data show pronounced seasonal dynamics in the space use of red deer in the Bohemian Forest Ecosystem, with a considerable proportion of female red deer that spent the winter in the enclosures in SFNR, migrating in spring eastward into the border region between SFNR and SNP, or north to the high-elevation disturbed areas between the two national parks (Peters et al., unpublished data). Migration to the open German-Czech border zone provides access to high-quality forage similar to the forage availability in disturbed areas and seems to support ideal conditions for females raising offspring. Females with calf might also prioritise risk avoidance more than males71 and risk avoidance has been suggested as the main driver for sexual segregation in red deer during the calving season22.
While our study produced actionable information for the transboundary red deer population, managers and decision-makers should be aware that we only report a snapshot representation of the population for a limited time frame. For example, spatial patterns in density during the hunting season, which mainly occurs in autumn, can be expected to differ from those presented here29. Most importantly, our summer abundance estimates differ from the winter counts, which can be due to seasonal movements, raising the question of whether winter counts are appropriate to derive hunting quota in our study system. Our sampling period was constrained by deer migration to the summer habitats in spring and the rutting season and the migration in autumn. Alternatively, sampling that is completed after the rut, but before migration, would better represent the autumn population and distribution, which is most relevant to harvest management. Sampling in this period would also probably yield better DNA quality due to lower ambient temperatures resulting in higher genotyping success rates and would be more feasible from a practical point of view. Furthermore, the Bohemian red deer population extends over a larger area beyond the one we sampled here, especially on the Czech side, and seasonal movements into or out of our study area are likely. Integration of population-level monitoring into an adaptive management framework will require periodic monitoring and an informed choice of the seasons to be prioritised for sample collection. The limitation would be the difficulty of conducting fieldwork and the cost of sample collection and DNA analysis.
Abundance estimates and jurisdictions
Each jurisdiction in the Bohemian Forest Ecosystem manages its part of the red deer population largely autonomously. In our study, the effect of differential management systems became visible at the border between SFNR with low red deer densities and SNP with high densities. However, when different management systems pursue a common goal, such as the two national parks in their neighbouring non-intervention zone of BFNP and the no-hunting zone of SNP, red deer densities are comparable. Our results confirmed that there is a continuous exchange of red deer between Germany and Czechia, especially in the high-elevation core zones of the two national parks. Thus, red deer on both sides of the national and subnational borders are part of one contiguous population. Coordinated population-level monitoring and analysis yielded a model-estimated distribution of activity centres (Fig. 2)—an individual-based representation of the red deer population from which abundance estimates can be extracted for any desired spatial extent and therefore at multiple spatial scales or administrative levels3. The spatially explicit nature of the analysis also accounts for the fact that borders are permeable and that individuals living near them may cross them.
Different sampling methods and analytical approaches have been used to estimate red deer population size78. In our study area, traditional count-based methods are the standard method to obtain abundance indices, which are often unable to capture the sampling process78,79. A recent study by80 used camera trap-based random encounter models and distance sampling to estimate red deer density during the same study period in part of our study area, the Bavarian Forest National Park and part of Šumava National Park (Fig. 1). Confidence intervals for the density estimates with the distance sampling method (mean = 2.55 km−2, 95% CI = 1.64–3.82), random encounter model (mean = 2.27 km−2; 95% CI = 1.60–3.13), and our SCR analysis (Table S3) overlap largely. However, our modelling approach provided more precise estimates. In addition, because SCR models estimate individual locations, deriving density is possible at any spatial resolution and extent (Fig. 2).
Implications beyond the study system
In multiple ways, the case of the Bohemian Forest red deer population is representative of managed transboundary wildlife populations in Europe and elsewhere. Populations of large mammals are frequently shared by multiple jurisdictions, including nations. Regardless of how much they differ in their goals and actions, management on different sides of a border becomes intertwined through its impacts on the shared population. This is not limited to ungulates; similar situations are faced by large carnivore managers in Northern and Central Europe, where carnivore populations are shared by several countries4,81. Despite differences in management objectives and strategies in Germany and Czechia, we recommend coordinated monitoring and joint analysis to produce population-level estimates of abundance and density. Achievement of specific management goals relies on collaboration between management units sharing a functionally linked deer population.
Population size and density are perhaps the most fundamental measures used in wildlife monitoring and for setting management goals. Yet, they can be challenging to obtain, and decision-makers often end up relying on proxies or indices of questionable reliability82,83. Technical advancements and the rising popularity of non-invasive monitoring methods have made population-level monitoring more accessible84. The resulting data, especially if collected across several years, in combination with analytical methods that account for imperfect and variable detectability, can yield absolute estimates of abundance, and thus can be used to show the effects of management practices or the change of ecological processes, such as seasonal migrations or imbalanced sex ratios over time. Such methods will become increasingly important since wildlife managers are not only challenged by the administrative separation of the population, but also by the ubiquitous pressures associated with ongoing human-caused global change. Management tools introduced decades ago and successfully used in the past may lose efficiency in the future. For example, in our study landscape, the enclosure system used to manage the red deer population in winter as a damage mitigation tool might lose its efficiency in milder winters because of climate change. Likewise, the expansion of disturbed areas caused by windthrows and bark beetle outbreaks may offer emerging forage areas for red deer in winter. Thus, traditional wildlife management interventions need to be updated with evidence-based sustainable practices.
Data availability
Data analysed in this study are available upon reasonable request from the last author (Wibke Peters).
References
Liu, J., Yong, D. L., Choi, C.-Y. & Gibson, L. Transboundary frontiers: An emerging priority for biodiversity conservation. Trends Ecol. Evol. 35, 679–690 (2020).
Mason, N., Ward, M., Watson, J. E., Venter, O. & Runting, R. K. Global opportunities and challenges for transboundary conservation. Nat. Ecol. Evol. 4, 694–701 (2020).
Bischof, R. et al. Estimating and forecasting spatial population dynamics of apex predators using transnational genetic monitoring. Proc. Natl. Acad. Sci. USA 117, 30531–30538 (2020).
Bischof, R., Brøseth, H. & Gimenez, O. Wildlife in a politically divided world: Insularism inflates estimates of brown bear abundance. Conserv. Lett. 9, 122–130 (2016).
Linnell, J. D. et al. Border security fencing and wildlife: The end of the transboundary paradigm in Eurasia?. PLoS Biol. 14, e1002483 (2016).
Senf, C. & Seidl, R. Mapping the forest disturbance regimes of Europe. Nat. Sustain. 4, 63–70 (2021).
Seidl, R. et al. Small beetle, large-scale drivers: How regional and landscape factors affect outbreaks of the European spruce bark beetle. J. Appl. Ecol. 53, 530–540 (2016).
Seidl, R., Rammer, W., Jäger, D. & Lexer, M. J. Impact of bark beetle (Ips typographus L.) disturbance on timber production and carbon sequestration in different management strategies under climate change. For. Ecol. Manag. 256, 209–220 (2008).
Seidl, R. et al. Modelling natural disturbances in forest ecosystems: A review. Ecol. Model. 222, 903–924 (2011).
Kausrud, K. et al. Population dynamics in changing environments: The case of an eruptive forest pest species. Biol. Rev. 87, 34–51 (2012).
Seidl, R. et al. Forest disturbances under climate change. Nat. Clim. Change 7, 395–402 (2017).
Marini, L. et al. Climate drivers of bark beetle outbreak dynamics in Norway spruce forests. Ecography 40, 1426–1435 (2017).
Pickett, S. & White, P. (eds) The Ecology of Natural Disturbance and Patch Dynamics 385–455 (Academic Press, 1985).
Kuijper, D. P. et al. Do ungulates preferentially feed in forest gaps in European temperate forest?. For. Ecol. Manag. 258, 1528–1535 (2009).
Ivan, J. S., Seglund, A. E., Truex, R. L. & Newkirk, E. S. Mammalian responses to changed forest conditions resulting from bark beetle outbreaks in the southern Rocky Mountains. Ecosphere 9, e02369 (2018).
Lehnert, L. W., Bässler, C., Brandl, R., Burton, P. J. & Müller, J. Conservation value of forests attacked by bark beetles: Highest number of indicator species is found in early successional stages. J. for Nat. Conserv. 21, 97–104 (2013).
Przepióra, F., Loch, J. & Ciach, M. Bark beetle infestation spots as biodiversity hotspots: Canopy gaps resulting from insect outbreaks enhance the species richness, diversity and abundance of birds breeding in coniferous forests. For. Ecol. Manag. 473, 118280 (2020).
Hayes, T. A., DeCesare, N. J., Peterson, C. J., Bishop, C. J. & Mitchell, M. S. Trade-offs in forest disturbance management for plant communities and ungulates. For. Ecol. Manag. 506, 119972 (2022).
Boulanger, V. et al. Ungulates increase forest plant species richness to the benefit of non-forest specialists. Glob. Chang. Biol. 24, e485–e495 (2018).
Ruckstuhl, K. & Neuhaus, P. Sexual Segregation in Vertebrates (Cambridge University Press, 2006).
Main, M. B., Weckerly, F. W. & Bleich, V. C. Sexual segregation in ungulates: New directions for research. J. Mammal. 77, 449–461 (1996).
Bonenfant, C. et al. Multiple causes of sexual segregation in European red deer: Enlightenments from varying breeding phenology at high and low latitude. Proc. R. Soc. Lond. Ser. B 271, 883–892 (2004).
Bischof, R. et al. A migratory northern ungulate in the pursuit of spring: jumping or surfing the green wave?. Am. Nat. 180, 407–424 (2012).
Coppes, J., Burghardt, F., Hagen, R., Suchant, R. & Braunisch, V. Human recreation affects spatio-temporal habitat use patterns in red deer (Cervus elaphus). PLoS ONE 12, e0175134 (2017).
Meisingset, E. L. et al. Spatial mismatch between management units and movement ecology of a partially migratory ungulate. J. Appl. Ecol. 55, 745–753 (2018).
Zeppenfeld, T. et al. Response of mountain Picea abies forests to stand-replacing bark beetle outbreaks: Neighbourhood effects lead to self-replacement. J. Appl. Ecol. 52, 1402–1411 (2015).
Rivrud, I. M., Heurich, M., Krupczynski, P., Müller, J. & Mysterud, A. Green wave tracking by large herbivores: An experimental approach. Ecology 97, 3547–3553 (2016).
Möst, L., Hothorn, T., Müller, J. & Heurich, M. Creating a landscape of management: Unintended effects on the variation of browsing pressure in a national park. For. Ecol. Manag. 338, 46–56 (2015).
Rivrud, I. M. et al. Leave before it’s too late: Anthropogenic and environmental triggers of autumn migration in a hunted ungulate population. Ecology 97, 1058–1068 (2016).
Heurich, M. et al. Country, cover or protection: What shapes the distribution of red deer and roe deer in the Bohemian Forest Ecosystem?. PLoS ONE 10, e0120960 (2015).
Pflugmacher, D., Rabe, A., Peters, M. & Hostert, P. Mapping pan-European land cover using Landsat spectral-temporal metrics and the European LUCAS survey. Remote. Sens. Environ. 221, 583–595 (2019).
Lausch, A., Fahse, L. & Heurich, M. Factors affecting the spatio-temporal dispersion of Ips typographus (L.) in Bavarian Forest National Park: A long-term quantitative landscape-level analysis. For. Ecol. Manag. 261, 233–245 (2011).
Krojerová-Prokešová, J., Barančeková, M., Šustr, P. & Heurich, M. Feeding patterns of red deer Cervus elaphus along an altitudinal gradient in the Bohemian Forest: effect of habitat and season. Wildl. Biol. 16, 173–184 (2010).
Cailleret, M., Heurich, M. & Bugmann, H. Reduction in browsing intensity may not compensate climate change effects on tree species composition in the Bavarian Forest National Park. For. Ecol. Manag. 328, 179–192 (2014).
Janík, T. et al. The declining occurrence of moose (Alces alces) at the southernmost edge of its range raise conservation concerns. Ecol. Evol. 11, 5468–5483 (2021).
Saggiomo, L. et al. Evaluating the management success of an alien species through its hunting bags: The case of the sika deer (Cervus nippon) in the Czech Republic. Acta Univ. Agric. Silvic. Mendelianae Brunensis 69, 30 (2021).
Bartos, L., Hyanek, J. & Zirovnicky, J. Hybridization between red and sika deer. Zool. Anzeiger Jena 207, 271–287 (1981).
Krojerová-Prokešová, J. et al. Genetic differentiation between introduced central European sika and source populations in Japan: Effects of isolation and demographic events. Biol. Invasions 19, 2125–2141 (2017).
Peters, W. et al. Large herbivore migration plasticity along environmental gradients in Europe: Life-history traits modulate forage effects. Oikos 128, 416–429 (2019).
Belotti, E. et al. Patterns of lynx predation at the interface between protected areas and multi-use landscapes in Central Europe. PLoS ONE 10, e0138139 (2015).
Ebert, C., Sandrini, J., Welter, B., Thiele, B. & Hohmann, U. Estimating red deer (Cervus elaphus) population size based on non-invasive genetic sampling. Eur. J. Wildl. Res. 67, 27 (2021).
Gurgul, A., Radko, A. & Słota, E. Characteristics of X-and Y-chromosome specific regions of the amelogenin gene and a PCR-based method for sex identification in red deer (Cervus elaphus). Mol. Biol. Rep. 37, 2915–2918 (2010).
Wilberg, M. J. & Dreher, B. P. GENECAP: A program for analysis of multilocus genotype data for non-invasive sampling and capture-recapture population estimation. Mol. Ecol. Notes 4, 783–785 (2004).
Ruell, E. W., Riley, S. P., Douglas, M. R., Pollinger, J. P. & Crooks, K. R. Estimating bobcat population sizes and densities in a fragmented urban landscape using noninvasive capture–recapture sampling. J. Mammal. 90, 129–135 (2009).
Paetkau, D. An empirical exploration of data quality in DNA-based population inventories. Mol. Ecol. 12, 1375–1387 (2003).
Waits, L. P., Luikart, G. & Taberlet, P. Estimating the probability of identity among genotypes in natural populations: cautions and guidelines. Mol. Ecol. 10, 249–256 (2001).
Valière, N. GIMLET: A computer program for analysing genetic individual identification data. Mol. Ecol. Notes 2, 377–379 (2002).
Broquet, T. & Petit, E. Quantifying genotyping errors in noninvasive population genetics. Mol. Ecol. 13, 3601–3608 (2004).
Royle, J. A., Nichols, J. D., Karanth, K. U. & Gopalaswamy, A. M. A hierarchical model for estimating density in camera-trap studies. J. Appl. Ecol. 46, 118–127 (2009).
Efford, M. G. Estimation of population density by spatially explicit capture–recapture analysis of data from area searches. Ecology 92, 2202–2207 (2011).
Efford, M. Density estimation in live-trapping studies. Oikos 106, 598–610 (2004).
Illian, J., Penttinen, A., Stoyan, H. & Stoyan, D. Statistical Analysis and Modelling of Spatial Point Patterns (Wiley, 2008).
Zhang, W. et al. A flexible and efficient Bayesian implementation of point process models for spatial capture–recapture data. Ecology 104, e3887 (2023).
Oeser, J., Pflugmacher, D., Senf, C., Heurich, M. & Hostert, P. Using intra-annual Landsat time series for attributing forest disturbance agents in Central Europe. Forests 8, 251 (2017).
Royle, J. A., Dorazio, R. M. & Link, W. A. Analysis of multinomial models with unknown index using data augmentation. J. Comput. Graph. Stat. 16, 67–85 (2007).
Milleret, C. et al. Using partial aggregation in spatial capture recapture. Methods Ecol. Evol. 9, 1896–1907 (2018).
Royle, J. A., Chandler, R. B., Sollmann, R. & Gardner, B. Spatial Capture-Recapture (Academic Press, 2014).
de Valpine, P. et al. Programming with models: Writing statistical algorithms for general model structures with NIMBLE. J. Comput. Graph. Stat. 26, 403–413 (2017).
R Development Core Team. R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, 2020). https://www.R-project.org/.
Bischof, R. et al. nimbleSCR: Spatial Capture-Recapture (SCR) Methods Using ‘Nimble’. R Package Version 0.1.0 (2020).
Brooks, S. P. & Gelman, A. General methods for monitoring convergence of iterative simulations. J. Comput. Graph. Stat. 7, 434–455 (1998).
Brinkman, T. J., Person, D. K., Chapin, F. S. III., Smith, W. & Hundertmark, K. J. Estimating abundance of sitka black-tailed deer using DNA from fecal pellets. J. Wildl. Manag. 75, 232–242 (2011).
Goode, M. J. et al. Capture–recapture of white-tailed deer using DNA from fecal pellet groups. Wildl. Biol. 20, 270–278 (2014).
Brazeal, J. L., Weist, T. & Sacks, B. N. Noninvasive genetic spatial capture-recapture for estimating deer population abundance. J. Wildl. Manag. 81, 629–640 (2017).
Dupke, C. et al. Habitat selection by a large herbivore at multiple spatial and temporal scales is primarily governed by food resources. Ecography 40, 1014–1027 (2017).
Rettie, W. J. & Messier, F. Hierarchical habitat selection by woodland caribou: Its relationship to limiting factors. Ecography 23, 466–478 (2000).
Smolko, P., Veselovská, A. & Kropil, R. Seasonal dynamics of forage for red deer in temperate forests: Importance of the habitat properties, stand development stage and overstorey dynamics. Wildl. Biol. 2018, 1–10 (2018).
Stone, W. E. & Wolfe, M. L. Response of understory vegetation to variable tree mortality following a mountain pine beetle epidemic in lodgepole pine stands in northern Utah. Vegetatio 122, 1–12 (1996).
Pec, G. J. et al. Rapid increases in forest understory diversity and productivity following a mountain pine beetle (Dendroctonus ponderosae) outbreak in pine forests. PLoS ONE 10, e0124691 (2015).
Senf, C., Müller, J. & Seidl, R. Post-disturbance recovery of forest cover and tree height differ with management in Central Europe. Landsc. Ecol. 34, 2837–2850 (2019).
Lone, K., Loe, L. E., Meisingset, E. L., Stamnes, I. & Mysterud, A. An adaptive behavioural response to hunting: Surviving male red deer shift habitat at the onset of the hunting season. Anim. Behav. 102, 127–138 (2015).
Oeser, J., Heurich, M., Senf, C., Pflugmacher, D. & Kuemmerle, T. Satellite-based habitat monitoring reveals long-term dynamics of deer habitat in response to forest disturbances. Ecol. Appl. 31, e2269 (2021).
Ewald, J. et al. Estimating the distribution of forage mass for ungulates from vegetation plots in Bavarian Forest National Park. Tuexenia 34, 53–70 (2014).
McNaughton, S. Ecology of a grazing ecosystem: The Serengeti. Ecol. Monogr. 55, 259–294 (1985).
Fryxell, J. M. Forage quality and aggregation by large herbivores. Am. Nat. 138, 478–498 (1991).
Frid, A. & Dill, L. Human-caused disturbance stimuli as a form of predation risk. Conserv. Ecol. 6, 060111 (2002).
Ciuti, S. et al. Human selection of elk behavioural traits in a landscape of fear. Proc. R. Soc. B 279, 4407–4416 (2012).
Forsyth, D. M. et al. Methodology matters when estimating deer abundance: A global systematic review and recommendations for improvements. The J. Wildl. Manag. 86, e22207 (2022).
Stephens, P. A., Pettorelli, N., Barlow, J., Whittingham, M. J. & Cadotte, M. W. Management by proxy? The use of indices in applied ecology. J. Appl. Ecol. 52, 1–6 (2015).
Henrich, M. et al. Deer behavior affects density estimates with camera traps, but is outwighted by spatial variability. Front. Ecol. Evol. 10, 881502 (2022).
Gervasi, V., Linnell, J. D., Brøseth, H. & Gimenez, O. Failure to coordinate management in transboundary populations hinders the achievement of national management goals: The case of wolverines in Scandinavia. J. Appl. Ecol. 56, 1905–1915 (2019).
Moqanaki, E. M., Jiménez, J., Bensch, S. & López-Bao, J. V. Counting bears in the Iranian Caucasus: Remarkable mismatch between scientifically-sound population estimates and perceptions. Biol. Conserv. 220, 182–191 (2018).
Gopalaswamy, A. M. et al. How “science” can facilitate the politicization of charismatic megafauna counts. Proc. Natl. Acad. Sci. USA 119, e2203244119 (2022).
Tourani, M. A review of spatial capture–recapture: Ecological insights, limitations, and prospects. Ecol. Evol. 12, e8468 (2022).
Acknowledgements
This project was funded by the program Ziel ETZ Free State of Bavaria – Czechia 2014-2020 (INTERREG V). R.B., C.M., and P.D. were partially funded by the Research Council of Norway (NFR 286886, project WildMap). We thank the field staff and volunteers that helped with collecting the faecal samples.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
F.F., W.P., T.P., and M.H. coordinated the data collection. C.E. led the genetic analysis. M.T. led the analysis and writing of the manuscript with contributions from all co-authors. All co-authors contributed to the drafts and gave final approval for submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tourani, M., Franke, F., Heurich, M. et al. Spatial variation in red deer density in a transboundary forest ecosystem. Sci Rep 13, 4561 (2023). https://doi.org/10.1038/s41598-023-31283-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-31283-7
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.