Abstract
Changing climates in the past affected both human and faunal population distributions, thereby structuring human diets, demography, and cultural evolution. Yet, separating the effects of climate-driven and human-induced changes in prey species abundances remains challenging, particularly during the Late Upper Paleolithic, a period marked by rapid climate change and marked ecosystem transformation. To disentangle the effects of climate and hunter-gatherer populations on animal prey species during the period, we synthesize disparate paleoclimate records, zooarchaeological data, and archaeological data using ecological methods and theory to test to what extent climate and anthropogenic impacts drove broad changes in human subsistence observed in the Late Upper Paleolithic zooarchaeological records. We find that the observed changes in faunal assemblages during the European Late Upper Paleolithic are consistent with climate-driven animal habitat shifts impacting the natural abundances of high-ranked prey species on the landscape rather than human-induced resource depression. The study has important implications for understanding how past climate change impacted and structured the diet and demography of human populations and can serve as a baseline for considerations of resilience and adaptation in the present.
Similar content being viewed by others
Introduction
The difficulty of disentangling the interrelated effects of climate impacts and population pressures on the archaeological record has long been recognized1,2,3,4,5,6,7,8,9. The issue is particularly crucial for understanding subsistence shifts reflected in the zooarchaeological record5,8,9,10,11,12: Are changes in species compositions of the zooarchaeological record primarily a product of: (i) changing environments altering species’ natural abundances in those environments, (ii) human populations depleting high-ranked resources resulting in a broadening of the diet leading to the incorporation of lower-ranked resources, or (iii) a combination of the two? Archaeological research has produced a rich body of literature structured around two contrasting positions, one foregrounding climate-driven explanations for changes in faunal representations13,14,15,16,17, the other advocating in favor of overhunting and anthropogenic impacts1,18,19,20,21, and in rare cases, researchers have attempted to separate which factor is decisive for specific species22.
To address the problem, we synthesize spatiotemporal paleoclimate, archaeological, and zooarchaeological data using a combination of ecological theory and methods, specifically those derived from eco-informatics and human behavioral ecology23,24, to test the extent to which climate and human populations affect changes in subsistence observed in the Late Upper Paleolithic (LUP) zooarchaeological record. The framework implemented here allows us to: (i) identify high-ranked prey species for LUP foragers, (ii) estimate the distribution and relative abundances of prey species across Europe, and (iii) overcome issues of nonindependence between climate and human population estimates.
At present, we understand that humans have significant effects on contemporary and historical ecologies and animal populations25,26,27,28, but not how far back such impacts extend in time nor if there is an identifiable point in human prehistory at which human populations began having large-scale effects on mammalian biodiversity. Notably, the Pleistocene-Holocene transition has become an important candidate timeframe for such effects in recent years9,29,30,31,32. Moreover, the question of how humans responded to changes in the environment, whether they be human-induced and/or climate-driven, is of particular relevance in light of ongoing debates on global anthropogenic climate change in the present and future33,34,35.
There is a positive correlation between hunter-gatherer population size and warming temperatures15,36,37, with climatic variables having differential effects on hunter-gatherer populations38, and recent work showing a relatively narrow climate niche of Holocene Homo sapiens35. During the Terminal Pleistocene, the correlation between population and climate variables clouds our ability to deduce causation due to a number of interconnected events of interest, such as faunal extinctions, population collapse and exchange, subsistence shifts, and the species composition of zooarchaeological assemblages1,5,6,13,14,15,18,19,22,39,40. The difficulty of discerning whether human populations or climate are accountable for changing faunal compositions and frequencies observed archaeologically—and which critically inform understandings of past human ecology, adaptation and strategic decision-making—rests in the nonindependence of these variables, particularly for the Terminal Pleistocene where climatic but also faunistic changes were pronounced41,42.
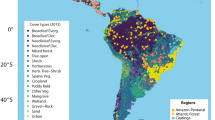
The LUP in Europe (22–9 kya) overlaps with the end of the Pleistocene and the beginning of the Holocene, thus including the Final Paleolithic and the early Mesolithic. The period is characterized by rapid changes in climates43,44,45,46 and non-analog ecologies16,47,48,49,50,51,52,53. Changing climates affected both human and faunal distributions, thereby structuring human diets, population size, and cultural processes36. The period between the Last Glacial Maximum (LGM; ca. 25–22 kya) and the beginning of the Holocene (ca. 11.8 kya) is generally characterized by glacial recession and the opening of northern latitude landscapes for human foragers54,55. Traditionally, this expansion of human populations into Europe’s northern latitudes at the margins of former glaciers has been linked to a fragmentation of societies with regionally distinct economic and cultural expressions56,57,58 (Fig. 1). With its relatively well-resolved archaeological record, high temporal control, and robustly inferred human population growth trajectories, climate change patterns, as well as faunal turnovers and habitat shifts, the period represents a unique window into early human prehistory for disentangling the interrelationships between contemporaneous climate, human populations, and their prey species.
Geographic and chronological scope of the employed dataset. Commensurate with the large-scale, long-term pan-European approach adopted here, the archaeological data on site location, chronological position and zooarchaeological assemblage composition are aggregated under their associated higher-order Cultural Techno-Units (CTUs), yielding six archaeological macro-complexes: the Magdalenian, Epigravettian, Arch-Backed Complex (ABC), Tanged Point Complex (TPC), and early Mesolithic. (A) shows the spatial distribution of all analyzed sites and their CTU affiliation. (B) shows the change in mean terrestrial temperature in Europe from 22 to 9kya, with an SPD used to estimate changes in regional population density represented by the shaded grey area using calibrated radiocarbon dates drawn from the European subset of the P3K14C database. The color-coded bars present the median measured age and the distribution of all dates recorded for each CTU. The map was created in ArcMap 10.3.1 (www.arcgis.com). European GIS-elevation data is based on the SRTM open-source data for Eurasia provided by NASA/JPL59. Coastline, glacial extent and Late Glacial drainage system data are taken from the European prehistoric and historic atlas (EPHA) hosted by the Centre for Baltic and Scandinavian archaeology (ZSBA) in Schleswig, Germany (CC-BY 4.0 license)55.
To address the methodological concerns and answer the question of causality in zooarchaeological assemblage composition change, we implement the following analytical framework:
-
1.
To identify the high-ranked prey species during the study period in Europe, we revisit and update post-encounter return rate estimates derived from the generalized Prey Choice Model (PCM60,61,62) with estimates of pursuit failure.
-
2.
We then estimate past distributions and relative abundances of the top-ranked species through time by creating species-specific spatiotemporal species distribution models (SDMs) over downscaled centennial-scale and spatially explicit paleoclimate data63.
-
3.
Using the archaeological data, we then use the date and location to extract the relevant spatiotemporal variables of temperature and suitability for the different species, thus incorporating the spatial and temporal variation from across Europe during the time.
-
4.
We create a summed probability distribution (SPD) as a proxy for a regional population estimate from the comprehensive radiocarbon database P3K14C64.
-
5.
We combine and extend independent archaeological65,66,67 and zooarchaeological databases68 containing information on site locations, cultural attribution and proportional zooarchaeological abundances (see “Materials and methods”).
-
6.
Mobilizing these datasets, we use a piecewise structural equation model (pSEM) to discern the relative contribution of climate and population to changes observed in zooarchaeological assemblages during the LUP, while controlling for climatic change.
The pSEM69 we build allows us to determine the individual effects of the two variables on recorded zooarchaeological abundances of prey species throughout the LUP while accounting for potential nonindependence of climatic and human variables. Archaeological data on geographic site location, temporal position, and faunal assemblage composition are aggregated into higher-order Cultural Techno-Units (CTUs) serving as primary units of analysis to discern trends and specific inter-variable relationships. These CTUs allow us to comparatively assess the interplay between changing human contexts including foraging patterns, climates, and animal species distributions70,71,72, to chart shifting causal relationships at the human–environment interface. Our findings suggest that changes in zooarchaeological assemblage configurations in LUP Europe are primarily a result of climate-driven habitat shifts of key high-ranked prey species rather than anthropogenic resource depletion.
Post-encounter return rate and pursuit failure
To begin to understand human foraging decisions we must determine the prey species most important to LUP hunter-gatherers’ subsistence, and thus high-ranked. To understand which prey species were high ranked, we (i) identify what mammals were generally present in the ecosystems of the study period, (ii) estimate their (post-encounter) profitability, and (iii) rank them according to their profitability.
Profitability is defined here within the PCM, as post-encounter return rate (PERR;60). PERR is commonly defined as energy divided by handling costs (measured in time) to produce a rate of energy acquisition (LHS of Eq. (1) in “Materials and methods”). Ranking prey species can be difficult, and in the past researchers have simply assumed that the mean body weight of a species corresponds with its ranking since body weight should roughly equal hunting returns9,73,74,75,76. While this may be true for some animal species, especially smaller animals with high pursuit success and low handling costs, the approach is demonstrably not appropriate for larger animals as the relationship between body weight and profitability is non-linear77,78,79,80,81. To derive a more robust measure for PERR and prey rank for mobile game we need to consider the increased handling costs of larger game and the probability of a failed pursuit.
To incorporate the probability of pursuit failure into PERR, it is vital to i) distinguish post-encounter pre-acquisition costs (e.g., pursuit costs) from post-acquisition costs (e.g., butchering, smoking, cooking, etc.), and ii) discount the post-acquisition costs and energy gained by the probability of pursuit failure (Eq. (2) in “Materials and methods”). The result is an elaboration on standard PCM PERR that overcomes over- and underestimating profitability issues present in previous implementations incorporating pursuit failure78,81. By incorporating pursuit failure into our determination of PERR, a forager’s decision to pursue a prey item does not necessarily result in acquisition of that prey item. This probability of failure then feedbacks into the forager’s assessment of the profitability of that prey item, and structures whether that resource is within the diet breadth and should be pursued upon encounter.
Predictions
Predictions from PCM informs our piecewise structural equation models (pSEM). In this way, we obtain three empirical predictions that can be tested against the archaeological record and the model outputs of the pSEM:
-
1.
High-ranked prey species should always be pursued upon encounter, regardless of their abundance62; their abundance in the archaeological record is therefore a measure of their abundance on the wider landscape. Higher-ranked species will consequently have better model fits in our analysis.
-
2.
When the natural abundance of high-ranked prey species is greater on the landscape relative to other low-ranked species, we expect the high-ranked species to make up the majority of the zooarchaeological record, at least if their abundances are not substantially impacted by human hunting.
-
3.
As high-ranked prey species decline in abundance (either from human hunting pressure or habitat shifts resulting from climate change) foragers should incorporate more lower-ranked species according to prey rank order, thus increasing their abundance in the zooarchaeological record. Yet the incorporation of lower-ranked prey items (diet breadth expansion) does not necessarily imply human resource depression but could also reflect changes in natural abundances of high-ranked pretty species due to habitat shifts and climate change.
The fundamental rationale of this PCM approach is that LUP foraging decisions are not directly reflected in the zooarchaeological record but are mediated by cost–benefit structures and the relative economic significance of different prey species. The same cost–benefit patterns of decision making are present throughout the LUP with differences in zooarchaeological assemblages stemming from differences between spatially and temporally distinct habitats. Both empirical convergence with and divergence from these expectations yield important insights on human–environment interactions in the period between the LGM and the Holocene.
Results
Prey ranking and post-encounter rates
Using African fauna post-encounter return data79, we estimate PERR using Eq. (2) (see Supplementary Information 1)82 for European species6. Excluding carnivores, the seven prey species with the highest PERR are, in order of decreasing importance, reindeer (Rangifer tarandus), boar (Sus scrofa), fallow deer (Dama dama), red deer (Cervus elaphus), ass (Equus hydruntinus), elk (Alces alces), and horse (Equus ferus) (Table 1).
In the analyses that follow we focus primarily on five of the top seven prey items listed above excluding fallow deer and ass. In the study period, ass is only represented in paleontological databases, making modeling its paleo-distribution difficult and beyond the scope of this paper. Fallow deer are not present in Europe during the LUP. For the purpose of the pSEM and the detailed analysis of LUP prey choices, we moreover concentrate on the top three species returned from the remaining list of ranked prey items: reindeer, boar and red deer.
Species’ habitat suitability and faunal composition by CTU
We combine the archaeological, zooarchaeological, population estimate, and habitat suitability datasets based on their shared CTU, distilling the disparate data into estimates of average species availability (based on the SDM results) and average zooarchaeological composition (See Table 1 in Supplementary Information 2)82.
Using the combined dataset, we can compare the habitat envelope of individual CTUs with regard to prey species suitability and the average composition of associated zooarchaeological assemblages to confront our theory-derived predictions (Fig. 2). There are notable differences between the analyzed CTUs both in terms of their animal habitat suitability profiles and the averaged relative zooarchaeological frequency of high-ranked prey species within them. The Magdalenian and especially the Tanged Point Complex (TPC) focus disproportionally on reindeer despite their habitats being suitable for other lower-ranked species, notably elk and red deer. In fact, reindeer is zooarchaeologically represented in all CTUs even when suitability scores are extremely low such as in the Mesolithic and this is consistent with PCM predictions. The same is true for boar, which is consistently taken by LUP hunter-gatherers, even when boar habitat suitability is low as in the TPC, the Arch-backed Complex (ABC) and the Magdalenian. This similarly suggests that prey ranking mediates foraging patterns. In the Mesolithic the pattern shifts as boar becomes abundant in the zooarchaeological record, paralleled by a marked increase in boar habitat suitability. Elk and horse are never the dominant species in the archaeological record even in CTU contexts in which their habitat suitability scores are elevated, reflecting their low prey ranking. The pattern for red deer is interesting and may reflect its intermediate ranking in the diet breadth of LUP hunter-gatherers. Red deer is the most frequent prey species in the averaged faunal samples from the Epigravettian, ABC and Mesolithic and this might reflect their abundances in the landscape as well as a possible inclination toward broader-spectrum diets in these CTUs, which are also all associated with a notable woodland component in their environments56,83,84,85. Taken together, this larger pattern illustrates the trade-offs between habitat suitability, prey abundances, and species profitability.
Comparison of zooarchaeological NISP proportions and climate-inferred habitat suitability for the top-ranked prey species. (A) presents the averaged zooarchaeological assemblage compositions attributable to each species for each CTU. (B) shows the reconstructed average habitat suitability for each species of each CTU. Each bar represents the zooarchaeological abundance (A) and the habitat suitability (B) for the corresponding top-ranked prey species colored by CTU affiliation. Note that these bars are not directly comparable between species. In comparing (A) and (B), it is evident that LUP foragers were not targeting species based on which species are most abundant, but on the species’ prey rank, with a preference for higher-ranked prey species.
pSEM: climate-population dynamics
Figure 3A outlines the structure of the piecewise structural equation model (pSEM) we use to address issues of non-independence between the main predictor variables. The model fits for each separate inter-variable relationship for the three top-ranked prey species (reindeer, boar, and red deer) are given in Fig. 3A. In all analyzed cases, human population density (P) correlates strongly with regional average summer temperature highs (C) (β = 0.91, p = 0.03). Other statistically significant relationships are highlighted in bold and pertain to the link between species habitat suitability and zooarchaeological abundance in the case of—as expected following the PCM—reindeer (β = 1.47, p = 0.028) and boar (β = 0.75, p = 0.068). The comparison between LUP populations and climate-driven species habitat suitability as predictor variables for the observed faunal abundances in the zooarchaeological record reveals that population (P) has a negligible effect on the observed patterns relative to climate (C).
Relationship between climate and human population during the LUP based on the piecewise structural equation model (pSEM). (A) describes the structure of the pSEM used, wherein the zooarchaeological record is considered the product of the natural abundance of animal prey species in an occupied habitat and the effects of human population pressures on the available prey resources. The natural abundances of a species in an occupied habitat are then further influenced by human population densities and both are affected by regional climatic trends. (B) shows the model outputs for climate (C) and population (P) for each of the three considered top-ranked prey species (reindeer, boar, and red deer). (C) outlines the structure of the red deer-specific model, using an pSEM in which the abundance of red deer in the zooarchaeological record is structured by the abundance of reindeer and boar in the zooarchaeological record, which is regarded to be a product of human population density, in turn considered to be a product of regional climate. (D) shows the results of the red deer model.
Figure 3B shows the individual results for each animal-CTU pairing regarding climate (C) and human population (P) as explanatory variables. Consistent with the observations on the relation between CTU, habitat suitability, and zooarchaeological frequencies, climate is a good predictor for reindeer (β = 1.47, p = 0.028) and boar (β = 0.75, p = 0.068). The pSEM performance is considerably weaker regarding the link between climate and red deer hunting (β = 0.65, p = 0.185) and this may suggest that red deer is a strategic compromise or a second-choice target but occurs in appreciable abundance in most environments. The individual species-specific results of the effects of LUP population density yield a different pattern. There is no relationship for boar (β = 0.31, p = 0.44), reindeer (β = − 0.82, p = 0.108), or red deer (β = 1.03, p = 0.117).
pSEM: red deer
The abundance of red deer within the zooarchaeological record is not strongly driven by the species’ habitat suitability (β = 0.65, p = 0.185) nor human population density (β = 1.03, p = 0.117; cf. Figure 3A,B). Since we expect lower-ranked prey items to be incorporated into the diet as the habitat suitability and relative natural abundance of higher-ranked resources decreases, we can test for this effect by using a slightly adjusted pSEM (Fig. 3C). Interestingly, we find that as the proportion of the zooarchaeological record attributable to reindeer and boar (the two highest-ranked prey species) declines, there is a significant increase in the proportion of red deer observed in the zooarchaeological record (Fig. 3D; β = − 1.0255, p = 0.033), and that while climate generally influences human populations, human population density does not seem to have an effect on the abundance of the combination of boar and reindeer (β = 0.495, p = 0.41). This strongly suggests red deer were hunted in habitats where higher-ranked resources (reindeer and boar) were less abundant, but not because of human hunting pressure on these two high-ranked prey species.
Figure 4 presents the standardized beta values for all considered prey species. We can use these values to qualitatively examine whether species habitat suitability and human population density produce the kind of patterns predicted by PCM. Since the incorporation of lower-ranked resources such as red deer, horse and elk into LUP diets is not only structured by the natural abundances of these resources and the strength of human impacts, but also by the abundance of higher-ranked resources, we expect that the standardized beta values for habitat suitability will reflect this, with weaker values corresponding to lower prey ranks. The data follows this expected pattern. Higher-ranked prey items have stronger positive standardized betas regarding the effect of climate-driven habitat suitability. The shape of the trajectory qualitatively matches expectations under PCM constraints. The standardized beta values for the effect of human population take a different shape. They are strongly negative for reindeer and reach their positive peak for red deer before strongly declining again, but neither of these trends are significant (threshold of p < 0.1). This suggests that anthropogenic resource depression did not drive changes in subsistence and thus the zooarchaeological record during this time.
Relationship between top-ranked prey species habitat suitability and LUP human population impacts measured by standardized beta coefficients and standard error estimates for each individual species-specific pSEM. Squares represent the calculated coefficients for species habitat suitability. Theory predicts that as we descend in prey rank beta coefficients decrease while the error increases suggesting that models are performing worse the lower the prey rank, in turn suggesting a positive relationship between predictive strength and prey rank. Circles represent the coefficients for human population. There is a clear parabolic effect but note that the coefficients alone suggest zooarchaeological abundances of reindeer decline as populations expand and zooarchaeological abundances of red deer increase as population increase, although these relationships are non-significant in our models. The remaining values yield standard errors overlapping with 0.
Discussion
The analyses presented in this paper suggest that changes in LUP foraging patterns, as seen in the zooarchaeological record of the period, were primarily climate-driven. Our results show that general changes in the composition of zooarchaeological assemblages throughout the LUP appear to be a product of climate-driven habitat shifts of prey species in almost all cases, not anthropogenic resource depression. Separating the effects of the two has been challenging for archaeologists1,3,5,6, yet by deploying a pSEM, we are able to account for the linkages between climate, human populations, and prey species abundances in the landscape specific for the LUP, and measure the impact that each of them have on zooarchaeological species composition. The results lead to the following observations which can be discussed in relation to our PCM-derived theoretical predictions.
High-ranked prey species should always be pursued upon encounter, regardless of their abundance, and their abundance in the archaeological record is therefore a measure of prey abundance on the landscape. If true, higher-ranked species will have better model fits, and this is reflected in the reindeer and boar data which shows better model fits than lower-ranked prey items such as red deer. The prediction is clearly reflected in the pSEM outputs which produce stronger beta values, smaller standard errors, and smaller p-values for the relationships between the habitat suitability and zooarchaeological abundances of reindeer and boar when compared to other species (cf. Fig, 3B). The pattern also extends beyond boar and reindeer to the other analyzed prey species as shown by the comparison of standardized beta values, where beta values decrease as standard error estimates increase as one descends in prey rank (cf. Fig. 4). This observation suggests that the presence and frequency of lower-ranked prey species in the zooarchaeological record is a result of a variable other than that species’ strict abundance on the landscape. Based on PCM, we expect the other variable to be the abundance of higher-ranked prey items, which is supported by our analysis of red deer zooarchaeological abundances relative to reindeer and boar zooarchaeological abundances (cf. Fig. 3C,D). This corroborates the hypothesis of constrained rationality, i.e., that the structure of the large mammal zooarchaeological record during the LUP is strongly shaped by cost–benefit regimes of human decision making and reflects the differential availability of high-ranked prey species in local environments.
When the local natural abundance of high-ranked prey species in the environment is greater, we expect high-ranked species to be proportionally represented in the zooarchaeological record, at least if their abundances are not simultaneously downregulated by human hunting pressure. This expectation is also broadly met given the results reported above, although with the caveat that we cannot independently control for absolute species abundances in each ecological context. Nonetheless, we observe that in the most suitable habitats for reindeer, LUP people take the most reindeer, and reindeer zooarchaeological frequencies in the worst reindeer habitats are lowest. The same is true for boar except for minor deviations in the poorest of boar habitats. A notable outlier to this pattern is the ABC red deer signal but this can be resolved by PCM-derived decision-making: As high-ranked prey animal species decline in abundance hunter-gatherers should incorporate more lower-ranked species into their diet in prey rank order. The incorporation of lower-ranked prey thereby not necessarily implies human-induced resource depletion but might similarly reflect changes in the species’ natural abundances. Our analysis of red deer zooarchaeological abundances relative to the higher-ranked species, reindeer and boar, support that in habitats less suitable for higher-ranked prey species, and thus have higher-ranked prey species in less abundance, hunter-gatherers incorporate lower-ranked resources in greater abundances. The finding is a clear example of diet breadth expansion10, however not as a product of human hunting pressure, but instead a product of environmental change.
The convergence between PCM-derived theoretical predictions and pSEM outputs confirms that on a macro-archaeological scale, pan-European trends in large mammal species compositions documented in the LUP zooarchaeological record are overwhelmingly shaped by local ecological conditions, reflecting the sorts of energetic and foraging return trade-offs documented in contemporary75,86,87,88,89,90,91 and prehistoric hunter-gatherer populations5,73,92,93. The changes noted in the structure of LUP faunal assemblages through time and space can thus primarily be linked to major climate shifts marking the end of the last glacial period with their associated effects on the distribution and composition of local environments and broader ecosystems14,15,16,17. This indicates a degree of adaptive plasticity among LUP populations in adjusting their foraging patterns to local conditions.
While human populations clearly impact species and habitats at greater population densities28,94 and have modified environments for thousands of years95,96, evidence for substantive super-regional impacts of human populations on animal abundances during the European Terminal Pleistocene is currently lacking. This suggests that population densities were not sufficiently high to depress faunal resources. It does not necessary mean that smaller scale, regional impacts are not discernible9,97, however, only that to detect these is outside of the scope of this study. Effective human population sizes during the LUP were relatively small when compared to later prehistoric or historic periods98. Such small population levels would in themselves reduce the potential ecological impact of human foragers on animal abundances, community structures, or broader ecosystem processes. Our results corroborate this view and show that in Europe there is no macro-archaeological evidence for population-induced changes in prey species abundances during the LUP. This provides a baseline against which earlier and later anthropogenic hunting impacts may be compared. It also raises the question of scale-dependence and conflicting observations, as earlier evidence for possible hunting pressure and lower-level human ecosystem impacts has been reported in the literature99,100,101,102, yet these findings are based on detailed osteological observations or isotope data, and their scales chiefly local. The seeming incommensurability of these with our findings may reflect different processes and especially analytical scales; ephemeral ecological impacts of Paleolithic foragers are likely to have occurred but are drowned out by larger-scale trends of climate change. Our results therefore do not call into question evidence for human ecological influences in the Pleistocene on local or even regional scales but rather caution that we cannot draw general conclusions from site-specific or regional observations.
A key limitation of this study is the discrepancy between the zooarchaeological and archaeological data. We here mitigate this shortcoming by agglomerating these data together by CTU. The result is a concatenation of the variation present within the archaeological record that may yet better help us understand the continuum of material and behavior across time and space. Better resolved zooarchaeological data with well-dated faunal assemblages would allow us to break away from relying on coarse archaeological attributions of CTUs and develop a better understanding of the spatiotemporal structure of variation observed within the zooarchaeological record. Further limitations include the potential underestimation of behavioral plasticity and attendant ecological preferences in animal species such as reindeer103,104, the extrapolation across vastly different ecosystem within Europe including but not limited to the Mammoth steppe in the north and east, and the likely impact of the changing availability and profitability of other resources, such as plants, fish, birds and other small game56,105. Unfortunately, marine resources, birds, and plants are not a part of our data set, and the small game component is only composed of marmots (Marmota marmota), hares (Lepus sp.), rabbits (Oryctolagus cuniculis), and beaver (Castor fiber). That said, in combination, these species make up a comparatively small part of the diet in the assemblages, ranging from an average of 6% for Mesolithic sites and less than 1% at Epigravettian and ABC sites68. As to whether the number of small game present in the data is real or a product of preservation or researcher bias is unknown, and recent studies have demonstrated the potential importance of the mass capture of small game resources to LUP hunters106, while others argue for the relevance of coastal or even marine resources accessible from the shore107,108.
In summary, we here assessed the impact of climate and human population changes on subsistence shifts as reflected in zooarchaeological records during the Late Upper Paleolithic in Europe. Building on the Prey Choice Model to derive the profitability of different prey species, a species distribution model to determine these prey species’ natural distributions, and a structural equation model to account for variable nonindependence, we generated a new analytical framework to quantify the relative contribution of climate change and human population pressure on the formation of the zooarchaeological record of LUP Europe. We find that regional changes recorded in zooarchaeological assemblages throughout the LUP are most likely a product of climate-driven species habitat shifts mediated through cost–benefit decisions of prey rank, rather than anthropogenic depression of key resources.
Materials and methods
Archaeological background
In general, Europe during the LUP was colder, wetter, and had greater seasonal variation than the Europe of the contemporary period46,109,110,111,112. In addition, climate over longer timescales was more volatile, punctuated by a series of glacial and interglacial periods (cf. Fig. 1). Climate regimes differed across the continent and Europe was generally characterized by a diversity of ecosystems. Even though the extension of these ecoclimatic contexts is subjected to dynamic re-adjustments over time, Northern Eurasia was broadly associated with the western extremity of the Mammoth steppe113, while the greater Mediterranean and Iberia formed distinct environmental contexts114. Situated at the westernmost fringes of the Mammoth steppe ecozone, Western France likely also harbored some unique ecoclimatic conditions115,116. For a detailed review of climate during the study period see54,55.
Archaeologically, the study period is characterized by a suite of hunter-gatherer techno-complexes and cultural units of varying chronological and spatial scope46,58,117, of which it is difficult to determine at which level these units are meaningful for human behavior118,119,120. To avoid this issue, we use a coarse-grain level of observation above traditional technocomplexes, resulting in six macro-archaeological units corresponding to broad differences in lithic techno-typology and referred to as Cultural Techno-Units (CTUs) throughout this paper. The Late Upper Paleolithic sensu stricto comprises two CTUs, the Epigravettian in Eastern Europe, south of the Alps and along the Mediterranean rim, and the Magdalenian north of the Alps and in Northwestern Europe55,121,122,123,124. Both complexes have traditionally been subdivided into Early, Middle, and Late phases, sometimes including a Final or Terminal phase overlapping with subsequent Final Paleolithic complexes. The subsequent Final Paleolithic is represented by the Arch-backed Complex (ABC)55,124,125,126,127,128, the Tanged Point Complex (TPC)126,129,130,131,132,133 and the very late Flat Blades and Bladelets Technocomplex (BBT; omitted here due to small sample size) dated to the Pleistocene-Holocene transition56,134,135,136,137. The Mesolithic, finally, synthesizes the plethora of earliest Holocene complexes documented and dated in the study region138,139,140,141.
Each of these macro-complexes is briefly described below.
Magdalenian
Includes all technocomplex designations labeled as Magdalenian including Final and Terminal Magdalenian facies as well as Magdalenian-descendant complexes such as the British Cresswellian142 and the Hamburgian143 of the North European Plain and Southern Scandinavia (including the latter’s later Havelte phase). The Magdalenian is generally characterized by invested laminar technologies with integrated or separate bladelet production systems144. Blade products mainly serve as blanks for domestic tools whereas bladelets are often backed and then used as inserts for modular tools and hunting equipment such as the spear thrower. Final Magdalenian complexes and Magdalenian-descendent complexes are distinguished by the emergence of special point types manufactured on blades such as the diagnostic shouldered points of the Hamburgian or the obliquely truncated Cresswell and Cheddar points of the Cresswellian. Reindeer and horse have been argued to be the main staple prey122,145, including ibex and snowy owl in mountain-near areas146,147. The Hamburgian has been hypothesized to index specialized reindeer economies148.
Epigravettian
Includes all technocomplex designations labeled as Epigravettian including regional variants such as the Romanellian of Italy149,150 and the Bouverian of northwestern Italy and southern France151,152. Similar to the Magdalenian, the Epigravettian is a complex characterized by invested laminar technologies with a generalized separation between blade-based domestic tools and bladelet-based implements123,153, although debate about its precise definition remain154. In contrast to the Magdalenian, however, Epigravettian variants are distinguished by backed blades and bladelets reminiscent of Gravettian character. The Late Epigravettian is often accompanied by geometric microlithic forms155. Prey animals include a variety of animals including boar, deer, bovids, and rugged terrain ungulates156.
ABC
Mainly comprises two technocomplexes: the Azilian of Western Europe and the so-called Federmessergroups (FMG) of Central and Eastern Europe125. Both technocomplexes are characterized by specialized bladelet technologies and a proclivity towards finely retouched as well as backed bladelets, often with a curved or arched edge. The ABC is often regarded to reflect woodland adaptations based on bow-and-arrow technologies157. The Azilian is currently interpreted to develop from the preceding Magdalenian124, whereas the status of the FMG and its relationship to the Azilian is contentious. The ABC seems to be associated with a broadening of human diets and mixed prey preferences, including species such as horse, reindeer and various woodland species158.
TPC
Includes the following technocomplexes: the Ahrensburgian and Epiahrensburgian of Northern, Central and Eastern Europe130,133,159, the Bromme complex of Southern Scandinavia160,161, following conservative assessments, and the Swiderian of East-Central and Eastern Europe131,133,162,163. The TPC is defined by the appearance of large, often robust and small tanged points accompanied by more or less developed blade technologies, some of which support highly invested bidirectional reduction systems. These points are characterized by pragmatic, often localized retouch and are highly variable in shape and size. TPC is mainly a phenomenon of the North European Plain and has traditionally been seen as an adaptation to high-mobility animals in open steppe landscapes such as reindeer, or thick-skinned species such as elk and perhaps beaver at the edges of boreal-tundra environments46,157.
(F)BBT
Mainly comprises two technocomplexes: the Belloisian or Long Blade Industries of Northern France134,137 and Britain and the Laborian of Western Europe135. This macro-unit is defined by its emphasis on blade production without large stemmed phenotypes, a focus on the broad face of laminar cores and resulting flat blade products, the use of large plain blades as knives and smaller retouched geometric implements56,135,164. BBT is associated with prey species such as boar, aurochs and deer and other species indicating the exploitation of closed landscapes135. BTT does not feature in the main analysis (see Archaeological background).
Mesolithic
Includes all technocomplex designations labeled as Mesolithic, early Mesolithic or Epipalaeolithic falling into the study period (up to 9k cal. BP) as well as regional variants such as the Beuronian of Southern Germany138, the Maglemose of Northern Germany, Britain and Southern Scandinavia140, the Sauveterrian of France141 and the Microlaminar Epipalaeolithic on the Iberian Peninsula165. The Mesolithic is generally characterized by the proliferation of microlithic technology, either bladelet-based or supported by incipient microburin technology. The main locus of variation pertains to the position and type of retouch and the attendant design choices as well as variation in geometric microlith forms. The Mesolithic is also accompanied by a surge of blade tools dedicated to woodworking. Mesolithic diets are chiefly broad spectrum and include a variety of species including deer, boar, aurochs, elk, beaver and sometimes reindeer166,167.
Prey ranking and prey choice model
To begin to understand human foraging decisions, the prey species most important to LUP hunter-gatherers’ subsistence, and thus high-ranked, must be determined. To understand which prey species were high ranked, we need to (i) identify what large mammals were generally present in the ecosystems of the study period, (ii) estimate their (post-encounter) profitability, and iii) rank them according to their profitability.
Profitability is defined here within the PCM, as post-encounter return rate (PERR)62,168,169,170. Ranking prey species can be difficult, and in the past researchers have simply assumed that the mean body weight of a species corresponds with its ranking since body weight should roughly equal hunting returns9,73,74,75,76. While this may be true for some animal species, especially smaller animals easily captured, the approach is demonstrably not appropriate for larger animals as the relationship between body weight and profitability is non-linear77,78,79,80,81. To derive a more robust measure for PERR additional factors need to be incorporated, however. PERR is commonly defined as energy divided by handling costs (measured in time) to produce a rate of energy acquisition [LHS of Eq. (1)]. The standard prey choice decision-making equation then predicts that upon encounter a prey item is pursued/acquired if the prey item is within a given diet breadth. This is reflected in Eq. (1) wherein, e is energy, λ is prey density, and h is handling cost:
After ranking the n prey types by PERR, prey items are added to the equation in order of increasing rank until the condition of Eq. (1) is met. The highest j that satisfies Eq. (1) is the lowest-ranked prey type in the diet and thus defines the total breadth of the diet62. PCM assumes that if the condition of Eq. (1) is met, the forager takes the prey item. Guaranteed acquisition is an assumption meant to simplify the model and generally results in no issues, but when we talk about mobile game species, there is always the chance that a forager will fail to acquire a pursued prey item.
To incorporate the probability of pursuit failure into PERR, it is vital to (i) discount the energy gained, (ii) distinguish post-encounter pre-acquisition costs (e.g., pursuit costs) from post-acquisition costs (e.g., butchering, smoking, cooking, etc.), and (iii) discount the post-acquisition costs and energy gained by the probability of pursuit failure. In mathematical notation, this results in Eq. (2) wherein e is energy, p is the probability of failed pursuit, h is post-acquisition handling costs, and c is post-encounter pre-acquisition costs:
Prey rank analysis of LUP Europe
To understand what prey species were important to LUP foragers and may have structured their land-use, diet, and material culture, we first need to know the species that lived in LUP Europe. We draw our list of species from Pushkina and Raia6 (Supplementary Information 6)82. This table provides only bodyweight data, thus creating an issue of determining PERR. In order to overcome this obstacle, we draw on the African faunal data used in Lupo and Schmitt79 (Supplementary Information 5)82. While many of the African species are bovids, we assume that they are characteristically similar to European species, which are primarily cervids, in regards to the variables considered in PCM. We created a series of linear models to predict the parameters within our PERR equation [see Eq. (2)] for our LUP prey species based on weight (see Supplementary Information 1)82. While weight cannot accurately predict PERR or prey rank, it can predict accurately energy and handling costs. Using these predicted values for each LUP prey species, we then calculated each PERR.
Using the Lupo and Schmitt79 data, we created a series of linear models to predict the parameters necessary to determine PERR for the species list in Pushkina and Raia6. The linear models for each have varying levels of success. We can confidently estimate parameters e (p < 0.001) and h (p < 0.001) and to a lesser extent c (p = 0.085), but p (p = 0.225) is difficult to determine. That said, it appears that we often overestimate c and p for smaller prey species and underestimate them for larger prey species, meaning our parameters are conservative estimates for c and p. Estimates of prey rank and PERR are listed in Table 1. While many of these species have similar PERR (of which we are only able to estimate), with the top five species being within 1,000 kcals/hr, it is important to remember that this is a rate, and that the comparison of PERR is standardized by time, meaning that these differences increase as handling times increase.
Archaeological site database
Sites dating to between the LGM and the Final Paleolithic were extracted from the Cologne CRC806 E1 database on European Late Upper Paleolithic/Magdalenian sites as originally published in Kretschmer65. The database was merged with the published information on direct radiocarbon dates obtained from the same sites also compiled by Kretschmer65. This integrated dataset was supplemented by sites listed in the CRC806 E1 database on the European Final Paleolithic66, again discarding all sites for which no direct dates could be obtained. All sites were checked for the availability of radiocarbon dates as recorded in the latest version of the Paleolithic Europe Radiocarbon Database (v28) curated by the University of Leuven67. The resulting list of dated sites in the study period with geospatial coordinates was then individually reclassified using the CTUs described above. Finally, sites and radiocarbon dates were added manually to CTUs for which the sample size was low by referring to the published and latest archaeological literature. All data is found in Supplementary Information 382. To avoid double-counting of individual sites, we filtered them by spatial location and assigned century. Dates that originate from the same site and fall within the same century were removed from our dataset and the analysis (n = 2132; Fig. 1A).
Zooarchaeological data
We draw in our zooarchaeological data from Boyle68. This dataset originally features relative proportions of prey species identified in zooarchaeological assemblages across Europe (n = 1258). Unfortunately, most of these data do not have associated spatial or temporal data, therefore we instead use their CTU association as the common denominator with which to pair them with the archaeological data. We re-classify the individual faunal observations contained in Boyle68 according to the higher-order archaeological units (provided as ‘Class3’ in the dataset) and remove all other entries. This results in a total sample of 804 observations (Supplementary Information 4)82.
Our analysis focuses on faunal resources, and while meat may have provided a significant portion of the calories for the LUP (if ethnographic observations of high latitude foragers and mean temperature are any indication of meat-dependence71,171), plants and small game may be more important for understanding the subsistence and diet breadth during this time. Being limited to faunal elements means that the archaeological record may have filtered out large portions of human behavior, particularly those associated with plant and marine foraging. Strictly focusing on animal resources may produce a biased perspective on subsistence and human lifeways, of which we need to be particularly aware.
Species distribution modeling
Next, we selected the seven prey species with the highest PERR derived from the fitted parameters and created five species distribution models (SDM) for each species, excluding fallow deer (Dama dama) and ass (Equus hydruntinus). Fallow deer are not present in Europe during the LUP and the species of ass is only present in paleontological databases. To create each species’ SDM we used procedures outlined in the Wallace package172 and modern species data derived from GBiF173. The Wallace package uses a machine learning method, known as maximum entropy, to model presence points to pseudo-absence points, to determine the potential niche space of a species relative to the variables provided174.
Our SDMs used modern species data from the Northern Hemisphere and therefore used modern climate data from BioCLIM175. Our choice of variables is limited by the variables found in our paleoclimate data, so we selected mean temperature (bio01), low temperature (bio05), high temperature (bio06), and precipitation (bio12) as our variables for defining the climatic niche these species occupy at present. All SDM code are found in Supplementary Information 182. All models are limited in their feature expansion to linear quadratic fits to avoid overfitting. Model regularization multipliers were selected based on mean and variation in AUC values produced from a four-fold cross validation (Table 2 in Supplementary Information 2)82. After creating the SDMs, we then hindcast the models over the CHELSA TraCE21k paleoclimate data. The CHELSA TraCE21k data are downscaled TRaCE21k data. The downscaling uses the CHELSA global climate model algorithm63. For our use, these data come in 100-year time slices from 22 to 9 kya using the same variables as our SDM (mean temperature, low temperature, high temperature, and precipitation). By using modern species data, we are assuming that the climate niches occupied by modern species adequately reflect the climate niches occupied by those same species during the terminal Pleistocene. While surely some shifts have occurred, particularly due to modern habitat destruction and impacts, we believe the climatological range represented in the modern data provides an adequate estimate of the range of habitats occupied by the different species. That said, our climate-based SDM models likely overestimate the habitat space of species, representing closer to a potential range rather than the realized range176.
Using our archaeological site database (n = 2465), which includes both spatial and temporal data, we then assigned each site (i) temporally to the century time slice of data for the CHELSA TraCE21k data using its median calibrated age rounded to the nearest century and (ii) spatially to a raster cell using its latitude/longitude. Next, we extracted the corresponding spatiotemporal cell value for the site. This creates the climate data for each site in our data. We then applied the SDM models over these climate values at our sites to determine how suitable each site is for each of the prey species.
Summed probability distribution
To estimate regional population densities in Europe, we created a summed probability distribution (cf. Fig. 1b) P3K14C database 64. After downloading the database, we truncated the data to sites in Europe with radiocarbon dates ranging from 22kya to 9.1 kya. To account for multiple dates at the same site, we binned dates from the same site within 200 years and then applied a 200-year local mean smoother177. The resulting database provides relative ,population estimates throughout the LUP while avoiding potential edge effects177,178.
The combined (archaeological) dataset
Last, we combined the datasets based on CTU association. We calculated the central tendency of each variable for each CTU. These include the central tendency of habitat suitability of each species for each CTU, the mean proportion of each species in the zooarchaeological record, the mean SPD value, and the mean maximum temperature of the warmest month (Table 1 in Supplementary Information 2)82. We used the central tendency because the zooarchaeological database lacks temporal and spatial data, and the archaeological data is missing zooarchaeological data. To overcome this issue, we distill the data to a compatible resolution, which is by CTU. This means that we are taking the central tendency of the habitat suitability of spatial and temporal positions for each CTU and pairing them with the mean zooarchaeological proportions of each species for each CTU. For a closer look at the variation and standard error of our estimates of these variables within the CTUs, see Supplementary Information 182.
Statistical analysis
We use a partial structural equation model (pSEM) to address issues of non-independence between our predictor variables69. The pSEM allows us to accurately assess the impacts of our variables on the zooarchaeological assemblage. Our model structure assumes that climate change effects both human populations and reindeer habitat suitability and thus reindeer populations, human populations impact reindeer populations, and that human populations and reindeer habitat suitability create the zooarchaeological record (Fig. 4A). Each model within the pSEM uses quasibinomial logistic regression.
Additionally, we recorded standardized betas and errors (Fig. 4B) produced by each pSEM model to test the assumption that the zooarchaeological record is structured by high-ranked prey species, with the prediction of declining importance in that species’ habitat as we decline in rank. Standardize betas represent the strength of a trend and the errors account for the uncertainty around that estimated trend.
Data availability
All data and code are available as supplementary information on Zenodo82.
References
Alroy, J. A multispecies overkill simulation of the end-pleistocene megafaunal mass extinction. Science 292, 1893–1896 (2001).
Contreras, D. Correlation is not enough: Building better arguments in the archaeology of human-environment interactions. in The Archaeology of Human-Environment Interactions (Routledge/Taylor and Francis Group, 2017).
Koch, P. L. & Barnosky, A. D. Late quaternary extinctions: State of the debate. Annu. Rev. Ecol. Evol. Syst. 37, 215–250 (2006).
McCool, W. C. et al. Climate change-induced population pressure drives high rates of lethal violence in the Prehispanic central Andes. Proc. Natl. Acad. Sci. 119, e2117556119 (2022).
Morin, E. Reassessing Paleolithic Subsistence: The Neandertal and Modern Human Foragers of Saint-Césaire (Cambridge University Press, 2012). https://doi.org/10.1017/CBO9781139150972.
Pushkina, D. & Raia, P. Human influence on distribution and extinctions of the late Pleistocene Eurasian megafauna. J. Hum. Evol. 54, 769–782 (2008).
Wilson, K. M. et al. Climate and demography drive 7000 years of dietary change in the Central Andes. Sci. Rep. 12, 2026 (2022).
Starkovich, B. M. & Ntinou, M. Climate change, human population growth, or both? Upper Paleolithic subsistence shifts in southern Greece. Quat. Int. 428, 17–32 (2017).
Starkovich, B. M. Optimal foraging, dietary change, and site use during the Paleolithic at Klissoura Cave 1 (southern Greece). J. Archaeol. Sci. 52, 39–55 (2014).
Grayson, D. K. & Delpech, F. changing diet breadth in the early upper palaeolithic of southwestern France. J. Archaeol. Sci. 25, 1119–1129 (1998).
Grayson, D. K. & Delpech, F. Specialized early upper palaeolithic hunters in southwestern France?. J. Archaeol. Sci. 29, 1439–1449 (2002).
Orbach, M., Amos, L. & Yeshurun, R. Human prey choice refutes “Levantine overkill”: Comment on Dembitzer et al. (2022). Quat. Sci. Rev. 2022, 107468. https://doi.org/10.1016/j.quascirev.2022.107468 (2022).
Cooper, A. et al. Abrupt warming events drove Late Pleistocene Holarctic megafaunal turnover. Science 349, 602–606 (2015).
Lorenzen, E. D. et al. Species-specific responses of Late Quaternary megafauna to climate and humans. Nature 479, 359–364 (2011).
Magyari, E. K. et al. Mammal extinction facilitated biome shift and human population change during the last glacial termination in East-Central Europe. Sci. Rep. 12, 6796 (2022).
Sommer, R. S. et al. When the pond turtle followed the reindeer: Effect of the last extreme global warming event on the timing of faunal change in Northern Europe. Glob. Change Biol. 17, 2049–2053 (2011).
Sommer, R. S., Kalbe, J., Ekström, J., Benecke, N. & Liljegren, R. Range dynamics of the reindeer in Europe during the last 25,000 years. J. Biogeogr. 41, 298–306 (2014).
Barnosky, A. D., Koch, P. L., Feranec, R. S., Wing, S. L. & Shabel, A. B. Assessing the causes of late pleistocene extinctions on the continents. Science 306, 70–75 (2004).
Haynes, G. Extinctions in North America’s late glacial landscapes. Quat. Int. 285, 89–98 (2013).
Sandom, C., Faurby, S., Sandel, B. & Svenning, J. C. Global late quaternary megafauna extinctions linked to humans, not climate change. Proc. R. Soc. B Biol. Sci. 281, 20133254 (2014).
Dembitzer, J., Barkai, R., Ben-Dor, M. & Meiri, S. Levantine overkill: 1.5 million years of hunting down the body size distribution. Quat. Sci. Rev. 276, 107316 (2022).
Broughton, J. M. & Weitzel, E. M. Population reconstructions for humans and megafauna suggest mixed causes for North American Pleistocene extinctions. Nat. Commun. 9, 5441 (2018).
Codding, B. F. & Bird, D. W. Behavioral ecology and the future of archaeological science. J. Archaeol. Sci. 56, 9–20 (2015).
Bird, D. W. & O’Connell, J. F. Behavioral ecology and archaeology. J. Archaeol. Res. 14, 143–188 (2006).
Faurby, S. & Svenning, J.-C. Historic and prehistoric human-driven extinctions have reshaped global mammal diversity patterns. Divers. Distrib. 21, 1155–1166 (2015).
Malhi, Y. et al. Megafauna and ecosystem function from the Pleistocene to the Anthropocene. Proc. Natl. Acad. Sci. 113, 838–846 (2016).
Teng, S. N., Xu, C., Teng, L. & Svenning, J.-C. Long-term effects of cultural filtering on megafauna species distributions across China. Proc. Natl. Acad. Sci. 117, 486–493 (2020).
Wong, B. B. M. & Candolin, U. Behavioral responses to changing environments. Behav. Ecol. 26, 665–673 (2015).
Klein, R. G. & Steele, T. E. Archaeological shellfish size and later human evolution in Africa. Proc. Natl. Acad. Sci. 110, 10910–10915 (2013).
Munro, N. D. et al. The emergence of animal management in the Southern Levant. Sci. Rep. 8, 9279 (2018).
Munro, N. D., Lebenzon, R. & Sapir-Hen, L. Revisiting Late Pleistocene-Early Holocene mountain gazelle (Gazella gazella) body size change in the southern Levant: A case for anthropogenic impact. PLoS ONE 17, e0273024 (2022).
Stiner, M. C. Thirty years on the “broad spectrum revolution” and paleolithic demography. Proc. Natl. Acad. Sci. 98, 6993–6996 (2001).
Burke, A. et al. The archaeology of climate change: The case for cultural diversity. Proc. Natl. Acad. Sci. 118, e2108537118 (2021).
Hussain, S. T. & Riede, F. Paleoenvironmental humanities: Challenges and prospects of writing deep environmental histories. WIREs Clim. Change 11, e667 (2020).
Xu, C., Kohler, T. A., Lenton, T. M., Svenning, J.-C. & Scheffer, M. Future of the human climate niche. Proc. Natl. Acad. Sci. 117, 11350–11355 (2020).
Schmidt, I. et al. Approaching prehistoric demography: Proxies, scales and scope of the Cologne Protocol in European contexts. Philos. Trans. R. Soc. B 376, 20190714 (2021).
Yu, Y. et al. Climatic factors and human population changes in Eurasia between the Last Glacial Maximum and the early Holocene. Glob. Planet. Change 221, 104054 (2023).
Ordonez, A. & Riede, F. Changes in limiting factors for forager population dynamics in Europe across the last glacial-interglacial transition. Nat. Commun. 13, 5140 (2022).
MacDonald, G. M. et al. Pattern of extinction of the woolly mammoth in Beringia. Nat. Commun. 3, 893 (2012).
Stuart, A. J. & Lister, A. M. Extinction chronology of the woolly rhinoceros Coelodonta antiquitatis in the context of late quaternary megafaunal extinctions in northern Eurasia. Quat. Sci. Rev. 51, 1–17 (2012).
Gamble, C., Davies, W., Pettitt, P. & Richards, M. Climate change and evolving human diversity in Europe during the last glacial. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 359, 243–254 (2004).
Sommer, R. S. Late Pleistocene and holocene history of mammals in Europe. In Mammals of Europe: Past, present and future (eds Hackländer, K. & Zachos, F. E.) 83–98 (Springer, 2020).
Blockley, S. P. E. et al. The chronology of abrupt climate change and Late Upper Palaeolithic human adaptation in Europe. J. Quat. Sci. 21, 575–584 (2006).
Eren, M. I. Hunter-Gatherer Behavior: Human Response During the Younger Dryas (Left Coast Press, 2012).
Rasmussen, S. O. et al. A stratigraphic framework for abrupt climatic changes during the Last Glacial period based on three synchronized Greenland ice-core records: refining and extending the INTIMATE event stratigraphy. Quat. Sci. Rev. 106, 14–28 (2014).
Weber, M.-J., Grimm, S. B. & Baales, M. Between warm and cold: Impact of the Younger Dryas on human behavior in Central Europe. Quat. Int. 242, 277–301 (2011).
Fahlke, J. M. Austausch der Terrestrischen Säugetierfauna an der Pleistozän/Holozän-Grenze in Mitteleuropa (Universitäts-und Landesbibliothek Bonn, 2009).
Graham, R. W. & Grimm, E. C. Effects of global climate change on the patterns of terrestrial biological communities. Trends Ecol. Evol. 5, 289–292 (1990).
Kathleen Lyons, S. et al. Holocene shifts in the assembly of plant and animal communities implicate human impacts. Nature 529, 80–83 (2016).
Markova, A. K., van Kolfschoten, T. & Puzachenko, A. Evolution of European Ecosystems During Pleistocene-Holocene Transition (24–8 Kyr BP) (GEOS Press, 2019).
Puzachenko, A. Y. Diversity of regional complexes of mammals in Europe at the boundary between Pleistocene and Holocene. Princ. Ecol. 33, 74–100 (2019).
Riede, F. & Pedersen, J. B. late glacial human dispersals in Northern Europe and disequilibrium dynamics. Hum. Ecol. 46, 621–632 (2018).
Stewart, J. R. The evolutionary consequence of the individualistic response to climate change. J. Evol. Biol. 22, 2363–2375 (2009).
Gamble, C., Davies, W., Pettitt, P., Hazelwood, L. & Richards, M. The archaeological and genetic foundations of the European population during the Late Glacial: Implications for ‘agricultural thinking’. Camb. Archaeol. J. 15, 193–223 (2005).
Grimm, S. B. Resilience and Reorganisation of Social Systems During the Weichselian Lateglacial in North-West Europe: An Evaluation of the Archaeological, Climatic, and Environmental Record (Schnell & Steiner GmbH, 2019).
Naudinot, N. et al. Between Atlantic and Mediterranean: Changes in technology during the Late Glacial in Western Europe and the climate hypothesis. Quat. Int. 428, 33–49 (2017).
Terberger, T., Barton, N. & Street, M. The late glacial reconsidered—recent progress and interpretations. Humans, environment and chronology of the Late Glacial of the North European Plain. in Proceedings of Workshop 14 of the 15th UISPP Congress, Lisbon, September 2006 vol. 6, 189–207 (2009).
Wygal, B. T. & Heidenreich, S. M. Deglaciation and human colonization of Northern Europe. J. World Prehistory 27, 111–144 (2014).
NASA JPL. NASA Shuttle Radar Topography Mission Global 1 arc second. (2013) https://doi.org/10.5067/MEASURES/SRTM/SRTMGL1.003.
Charnov, E. L. Optimal foraging: Attack strategy of a mantid. Am. Nat. 110, 141–151 (1976).
Stephens, D. W. & Charnov, E. L. Optimal foraging: Some simple stochastic models. Behav. Ecol. Sociobiol. 10, 251–263 (1982).
Stephens, D. W. & Krebs, J. R. Foraging Theory (Princeton University Press, 1986).
Karger, D. N., Nobis, M. P., Normand, S., Graham, C. H. & Zimmermann, N. E. CHELSA-TraCE21k – high-resolution (1 km) downscaled transient temperature and precipitation data since the Last Glacial Maximum. Clim. Past 19, 439-456 (2023).
Bird, D. et al. p3k14c, a synthetic global database of archaeological radiocarbon dates. Sci. Data 9, 27 (2022).
Kretschmer, I. Demographische Untersuchungen zu Bevölkerungsdichten, Mobilität und Landnutzungsmustern im späten Jungpaläolithikum. (2015).
Schmidt, I. & Zimmermann, A. CRC806-E1 Database on Final Palaeolithic sites of Europe V-2020_08_20. (2020) https://doi.org/10.5880/SFB806.51.
Vermeersch, P. M. Radiocarbon Palaeolithic Europe database: A regularly updated dataset of the radiometric data regarding the Palaeolithic of Europe, Siberia included. Data Brief 31, 105793 (2020).
Boyle, K. The zooarchaeology of complexity and specialization during the Upper Palaeolithic in Western Europe. Oxford Handb. Zooarchaeol. https://doi.org/10.1093/oxfordhb/9780199686476.013.2 (2017).
Shipley, B. Cause and Correlation in Biology: A User’s Guide to Path Analysis, Structural Equations and Causal Inference (Cambridge University Press, 2000). https://doi.org/10.1017/CBO9780511605949.
Binford, L. R. Constructing Frames of Reference: An Analytical Method for Archaeological Theory Building Using Hunter-Gatherer and Environmental Data Sets (University of California Press, 2019).
Kelly, R. L. The three sides of a biface. Am. Antiq. 53, 717–734 (1988).
Shea, J. J. Strategic modeling in pleistocene archeology, thank you, Irv!. Evol. Anthropol. Issues News Rev. 24, 99–100 (2015).
Broughton, J. M. Declines in mammalian foraging efficiency during the Late Holocene, San Francisco Bay, California. J. Anthropol. Archaeol. 13, 371–401 (1994).
Simms, S. R. Behavioral Ecology and Hunter-Gatherer Foraging: An Example from the Great Basin Vol. 381 (BAR, 1987).
Smith, E. A. Inujjuamiunt Foraging Strategies: Evolutionary Ecology of an Arctic Hunting Economy (Aldine de Gruyter, 1991).
Smith, E. A. et al. Anthropological applications of optimal foraging theory: A critical review [and comments and reply]. Curr. Anthropol. 24, 625–651 (1983).
Lupo, K. D. Evolutionary foraging models in zooarchaeological analysis: Recent applications and future challenges. J. Archaeol. Res. 15, 143–189 (2007).
Lupo, K. D., Schmitt, D. N. & Madsen, D. B. Size matters only sometimes: the energy-risk trade-offs of Holocene prey acquisition in the Bonneville basin, western USA. Archaeol. Anthropol. Sci. 12, 160 (2020).
Lupo, K. D. & Schmitt, D. N. When bigger is not better: The economics of hunting megafauna and its implications for Plio-Pleistocene hunter-gatherers. J. Anthropol. Archaeol. 44, 185–197 (2016).
Morin, E., Bird, D., Winterhalder, B. & Bliege Bird, R. Deconstructing hunting returns: Can we reconstruct and predict payoffs from pursuing prey?. J. Archaeol. Method Theory https://doi.org/10.1007/s10816-021-09526-6 (2021).
Ugan, A. & Simms, S. On prey mobility, prey rank, and foraging goals. Am. Antiq. 77, 179–185 (2012).
Yaworsky, P. M., Hussain, S. T. & Riede, F. Supplemental Information to Climate-driven habitat shifts of high-ranked prey species structure Late Upper Paleolithic hunting, Zenodo, https://doi.org/10.5281/zenodo.7687831 (2023).
Coope, G. R. & Elias, S. A. The environment of Upper Palaeolithic (Magdalenian and Azilian) hunters at Hauterive-Champréveyres, Neuchâtel, Switzerland, interpreted from coleopteran remains. J. Quat. Sci. 15, 157–175 (2000).
Jessen, C. A. et al. Early Maglemosian culture in the Preboreal landscape: Archaeology and vegetation from the earliest Mesolithic site in Denmark at Lundby Mose. Sjælland. Quat. Int. 378, 73–87 (2015).
Mortensen, M. F., Henriksen, P. S., Christensen, C., Petersen, P. V. & Olsen, J. Vegetation development in south-east Denmark during the Weichselian Late Glacial: palaeoenvironmental studies close to the Palaeolithic site of Hasselø. Dan. J. Archaeol. 3, 33–51 (2014).
Alvard, M. Intraspecific prey choice by Amazonian hunters [and comments and reply]. Curr. Anthropol. 36, 789–818 (1995).
Bird, D. W. & Bliege Bird, R. The ethnoarchaeology of Juvenile Foragers: Shellfishing strategies among Meriam children. J. Anthropol. Archaeol. 19, 461–476 (2000).
Bliege Bird, R. & Bird, D. W. Why women hunt. Curr. Anthropol. https://doi.org/10.1086/587700 (2008).
Hawkes, K., Hill, K. & O’Connell, J. F. Why hunters gather: Optimal foraging and the Ache of eastern Paraguay. Am. Ethnol. 9, 379–398 (1982).
O’Connell, J. F. & Hawkes, K. Alyawara plant use and optimal foraging theory. Hunt. Gatherer Foraging Strateg. Ethnogr. Archaeol. Anal. 1, 99–125 (1981).
O’Connell, J. F. & Hawkes, K. Food choice and foraging sites among the Alyawara. J. Anthropol. Res. 40, 504–535 (1984).
Broughton, J. M., Cannon, M. D., Bayham, F. E. & Byers, D. A. Prey body size and ranking in zooarchaeology: Theory, empirical evidence, and applications from the Northern Great Basin. Am. Antiq. 76, 403–428 (2011).
Byers, D. A. & Ugan, A. Should we expect large game specialization in the late Pleistocene? An optimal foraging perspective on early Paleoindian prey choice. J. Archaeol. Sci. 32, 1624–1640 (2005).
Grayson, D. K. The archaeological record of human impacts on animal populations. J. World Prehistory 68, 1–10 (2001).
Boivin, N. L. et al. Ecological consequences of human niche construction: Examining long-term anthropogenic shaping of global species distributions. Proc. Natl. Acad. Sci. 113, 6388–6396 (2016).
Sullivan, A. P., Bird, D. W. & Perry, G. H. Human behaviour as a long-term ecological driver of non-human evolution. Nat. Ecol. Evol. 1, 0065 (2017).
Bos, J. A. A. & Janssen, C. R. Local impact of palaeolithic man on the environment during the end of the Last Glacial in the Netherlands. J. Archaeol. Sci. 23, 731–739 (1996).
Schmidt, I., Gehlen, B. & Zimmermann, A. Population estimates for the Final Palaeolithic (14,000 to 11,600 years cal. BP) of Europe: Challenging evidence and methodological limitations. in On the Move: Mobility of people, objects and ideas during the European Upper Palaeolithic 221–238 (Société Préhistorique Française, 2019).
Baumann, C., Bocherens, H., Drucker, D. G. & Conard, N. J. Fox dietary ecology as a tracer of human impact on Pleistocene ecosystems. PLoS ONE 15, e0235692 (2020).
Bocherens, H. & Drucker, D. G. Isotopic insights on the ecological interactions between humans and woolly mammoths during the Middle and Upper Palaeolithic in Europe. in Human-Elephant Interactions: From Past to Present. 249–262 (Tübingen University Press, 2021).
Fernandez, P. & Legendre, S. Mortality curves for horses from the Middle Palaeolithic site of Bau de l’Aubesier (Vaucluse, France): Methodological, palaeo-ethnological, and palaeo-ecological approaches. J. Archaeol. Sci. 30, 1577–1598 (2003).
Wißing, C. et al. Stable isotopes reveal patterns of diet and mobility in the last Neandertals and first modern humans in Europe. Sci. Rep. 9, 4433 (2019).
Pape, R. & Löffler, J. Ecological dynamics in habitat selection of reindeer: an interplay of spatial scale, time, and individual animal’s choice. Polar Biol. 38, 1891–1903 (2015).
Fontana, L. The four seasons of reindeer: Non-migrating reindeer in the Dordogne region (France) between 30 and 18k? Data from the Middle and Upper Magdalenian at La Madeleine and methods of seasonality determination. J. Archaeol. Sci. Rep. 12, 346–362 (2017).
Nannini, N. et al. Marmot hunting during the Upper Palaeolithic: The specialized exploitation at Grotte di Pradis (Italian pre-Alps). Quat. Sci. Rev. 277, 107364 (2022).
Morin, E., Bliege Bird, R. & Bird, D. Mass procurement and prey rankings: insights from the European rabbit. Archaeol. Anthropol. Sci. 12, 262 (2020).
Álvarez-Fernández, E. Humans and marine resource interaction reappraised: Archaeofauna remains during the late Pleistocene and Holocene in Cantabrian Spain. J. Anthropol. Archaeol. 30, 327–343 (2011).
Cziesla, E. Robbenjagd in Brandenburg? Gedanken zur Verwendung großer Widerhakenspitzen. Ethnogr. Archaol. Z. 48, 1–48 (2007).
Heiri, O. et al. Validation of climate model-inferred regional temperature change for late-glacial Europe. Nat. Commun. 5, 4914 (2014).
Litt, T., Schmincke, H.-U. & Kromer, B. Environmental response to climatic and volcanic events in central Europe during the Weichselian Lateglacial. Quat. Sci. Rev. 22, 7–32 (2003).
Wetter, K. Menschheitsentwicklung: von der Eiszeit bis ins 21. Jahrhundert. (Theiss, 2012).
Street, M. The archaeology of the Pleistocene-Holocene transition in the Northern Rhineland, Germany. Quat. Int. 49–50, 45–67 (1998).
Nogués-Bravo, D., Rodríguez, J., Hortal, J., Batra, P. & Araújo, M. B. Climate change, humans, and the extinction of the Woolly Mammoth. PLOS Biol. 6, e79 (2008).
Barton, C. M. et al. In glacial environments beyond glacial terrains: Human eco-dynamics in late Pleistocene Mediterranean Iberia. Quat. Int. 318, 53–68 (2013).
Delpech, F. The range distributions of some Ungulate Mammals during the Dryas I (Recent Würm) in Aquitaine. Paleoecol. Implic. Geobios 23, 221–230 (1990).
Fontana, L. Reindeer Hunters of the Ice Age in Europe: Economy, Ecology, and the Annual Nomadic Cycle (Springer, 2022). https://doi.org/10.1007/978-3-031-06259-9.
Naudinot, N., Fagnart, J.-P., Langlais, M., Mevel, L. & Valentin, B. The last Lateglacial and Early Holocene societies in France (abridged version): Overview of thirty years of research. Gall. Préhist. https://doi.org/10.4000/galliap.1402 (2019).
Ivanovaitė, L., Serwatka, K., Hoggard, C. S., Sauer, F. & Riede, F. All these fantastic cultures? Research history and regionalization in the late palaeolithic tanged point cultures of Eastern Europe. Eur. J. Archaeol. 23, 162–185 (2020).
Riede, F., Hussain, S. T., Timmreck, C. & Svenning, J.-C. CLIOdynamic ARCHaeology: computational approaches to Final Palaeolithic/Early Mesolithic archaeology and climate change. Antiquity 94, e13 (2020).
Sauer, F. & Riede, F. A critical reassessment of cultural taxonomies in the Central European Late Palaeolithic. J. Archaeol. Method Theory 26, 155–184 (2019).
Langlais, M. et al. The evolution of Magdalenian societies in South-West France between 18,000 and 14,000 calBP: Changing environments, changing tool kits. Quat. Int. 272–273, 138–149 (2012).
Maier, A. The Central European Magdalenian: Regional Diversity and Internal Variability (Springer, 2015).
Montoya, C. Les traditions techniques à l’Epigravettien: analyses de séries du tardiglaciaire entre Alpes et Méditerranée. (Unpublished Doctoral Dissertation, Université de Provence (Aix-Marseille I), 2004).
Valentin, B. Jalons pour une paléohistoire des derniers chasseurs: XIVe-VIe millénaire avant J.-C. (Publications de la Sorbonne, 2008).
Bodu, P. & Valentin, B. Groupes à Federmesser ou Aziliens dans le sud et l’ouest du Bassin parisien: Propositions pour un nouveau modèle d’évolution. Bull. Soc. Préhist. Fr. 94, 341–348 (1997).
Burdukiewicz, J. M. Late Glacial hunter-gatherer reactions to the Younger Dryas cooling event in the southern and eastern Baltic regions of Europe. Quat. Int. 242, 302–312 (2011).
Schild, R. Terminal Paleolithic of the North European Plain: A review of lost chances, potential, and hopes. World Archaeol. 3, 193–274 (1984).
Street, M. et al. L’occupation du bassin de Neuwied (Rhénanie centrale, Allemagne) par les Magdaléniens et les groupes à Federmesser (aziliens). Bull. Soc. Préhist. Fr. 103, 753–780 (2006).
Tanged points cultures in Europe: read at the International Archaeological Symposium, Lublin, September, 13–16, 1993. (Maria Curie-Skłodowska Univ. Press, 1999).
Mevel, L., Weber, M.-J. & Berg-Hansen, I. M. The ahrensburgian revisited. techno-economic variability documented by several settlements from northernmost Germany (teltwisch, alt duvenstet, kleine nordende). in L’europe du nord-ouest autour de 9 600 cal. b.c. : quels changements ? 433–453 (Société Préhistorique Française, 2019).
Sobkowiak-Tabaka, I. & Winkler, K. The Ahrensburgian and the Swiderian in the area around the middle Oder River: reflections on similarities and differences. Quartär 1, 217–240. https://doi.org/10.7485/QU64_10 (2021).
Stefański, D. Tanged Point Technocomplex: Siberian, but what else? New findings from Kraków region, southern Poland. Quartär 1, 241–264. https://doi.org/10.7485/QU64_11 (2021).
Winkler, K. Ahrensburgien und Swiderien im mittleren Oderraum: technologische und typologische Untersuchungen an Silexartefakten der Jüngeren Dryaszeit. (Wachholtz, 2019).
Barton, N. & Roberts, A. The transition from the Younger Dryas to the Pre-Boreal in southern Britain: some new perspectives on the spatial patterning and chronology of long blade sites. in Préhistoire de l’Europe du Nord-Ouest : Mobilités, Climats et Identités Culturelles, 381–389 (Société Préhistorique Française, 2019).
Langlais, M. et al. The pleistocene-holocene transition in Southwestern France: A focus on the laborian. Quat. Int. 564, 37–47 (2020).
Naudinot, N. & Jacquier, J. Socio-economic organization of Final Paleolithic societies: New perspectives from an aggregation site in Western France. J. Anthropol. Archaeol. 35, 177–189 (2014).
Valentin, B., Weber, M.-J. & Bodu, P. Initialisation and progression of the core reduction process at Donnemarie-Dontilly (Seine-et-Marne, France), site of the Belloisian tradition: New interpretative key for comparisons with contemporaneous industries and ‘Federmesser-Gruppen’ assemblages. Bull. Soc. Préhist. Fr. 111, 659–678 (2014).
Kind, C.-J. The Mesolithic in southwest Germany. Preistoria Alp. 44, 137–145 (2009).
Kozłowski, S. K. Thinking mesolithic. (Oxbow Books; Distributed in the USA by David Brown, 2009).
Sørensen, M., Lübke, H. & Groß, D. The Early Mesolithic in Southern Scandinavia and Northern Germany. in Star Carr Volume I 305–329 (White Rose University Press, 2018).
Visentin, D. The Early Mesolithic in Northern Italy and Southern France: An Investigation into Sauveterrian Lithic Technological Systems (Archaeopress Publishing Ltd, 2018).
Barton, R. N. E., Jacobi, R. M., Stapert, D. & Street, M. J. The Late-glacial reoccupation of the British Isles and the Creswellian. J. Quat. Sci. 18, 631–643 (2003).
Weber, M.-J. From Technology to Tradition: Re-evaluating the Hamburgian-Magdalenian Relationship (Wachholtz, 2012).
Langlais, M. Les Sociétés Magdaléniennes de L’isthme pyrénéen. (Éd. du Comité des travaux historiques et scientifiques, 2010).
Weniger, G.-C. The magdalenian in Western Central Europe: Settlement pattern and regionality. J. World Prehistory 3, 323–372 (1989).
Álvarez-Alonso, D., Yravedra, J., Jordá Pardo, J. F. & Arrizabalaga, A. The Magdalenian sequence at Coímbre cave (Asturias, Northern Iberian Peninsula): Adaptive strategies of hunter–gatherer groups in montane environments. Quat. Int. 402, 100–111 (2016).
Laroulandie, V. Hunting fast-moving, low-turnover small game: The status of the snowy owl (Bubo scandiacus) in the Magdalenian. Quat. Int. 414, 174–197 (2016).
Bratlund, B. Hunting strategies in the Late Glacial of northern Europe: A survey of the faunal evidence. J. World Prehist. 10, 1–48 (1996).
Bietti, A. The late Upper Paleolithic in Italy: An overview. J. World Prehist. 4, 95–155 (1990).
Mussi, M. Earliest Italy: An Overview of the Italian Paleolithic and Mesolithic (Kluwer Academic/Plenum Publishers, 2001).
Laplace, G. Les subdivisions du leptolithique italien: Étude de typologie analytique. Bull. Paletnol. Ital. 73, 25–63 (1964).
Palmadi Cesnola, A. Il Paleolitico Inferiore e Medio in Italia. (2001).
Montoya, C. Apport de l’analyse technique à la compréhension de l’évolution des groupes humains épigravettiens d’Italie Nord Orientale: la production lithique de l’US 15–65 du Riparo Dalmeri. Preistoria Alp. 43, 191–208 (2008).
Perrin, T. The time of the last hunters: Chronocultural aspects of early holocene societies in the western Mediterranean. Open Archaeol. 9, 275 (2023).
Dalmeri, G., Ferrari, S. & Peresani, M. Rise and fall in the utilization of trapezoidal microliths during the Late Upper Palaeolithic in Europe - an overview from the Italian record. in Hunters in a Changing World. Environment and Archaeology of the Pleistocene-Holocene Transition (ca. 11000–9000 BC) in Northern Central Europe 1–9 (Marie Leidorf, 2004).
Bertolini, M., Cristiani, E., Modolo, M., Visentini, P. & Romandini, M. Late Epigravettian and Mesolithic foragers of the eastern Alpine region: Animal exploitation and ornamental strategies at Riparo Biarzo (Northern Italy). Quat. Int. 423, 73–91 (2016).
Riede, F. Climate and demography in early prehistory: Using calibrated 14 C dates as population proxies. Hum. Biol. 81, 309–337 (2009).
Baales, M. & Street, M. Hunter-gatherer behavior in a changing late glacial landscape: Allerød archaeology in the Central Rhineland, Germany. J. Anthropol. Res. 52, 281–316 (1996).
Crombé, P., Deeben, J. & Strydonck, M. V. Hunting in a changing environment: the transition from the Younger Dryas to the (Pre)boreal in Belgium and the southern Netherlands. Transit. Ruptures Contin. Préhist. 1, 583–604. https://doi.org/10.13140/2.1.2968.2244 (2010).
Fischer, A. Pioneers in deglaciated landscapes: The expansion and adaptation of late palaeolithic societies in Southern Scandinavia. Late Glacial North-West Eur. Hum. Adapt. Environ. Change End Pleistocene 100–121 (1991).
Fischer, A., Mortensen, M. F., Henriksen, P. S., Mathiassen, D. R. & Olsen, J. Dating the Trollesgave site and the Bromme culture: Chronological fix-points for the Lateglacial settlement of Southern Scandinavia. J. Archaeol. Sci. 40, 4663–4674 (2013).
Kozlowski, S. K. Mapping the Central/East European terminal palaeolithic/earliest mesolithic. Archaeol. Balt. 7, 29–35 (2006).
Migal, W. On preferential points of the Final Paleolithic in the Central European Lowland. in Studies in the Final Palaeolithic Settlement of the Great European Plain 185–200 (Institute of Archaeology and Ethnology, Polish Academy of Science, Cambridge 2007).
Jacquier, J., Langlais, M. & Naudinot, N. Late Laborian trapezoids: Function and origin of the first transverse projectile tips of Western Europe prehistory. Quat. Int. 564, 48–60 (2020).
Soto, A. et al. Epipalaeolithic assemblages in the Western Ebro Basin (Spain): The difficult identification of cultural entities. Quat. Int. 364, 144–152 (2015).
Groß, D. Welt und Umwelt frühmesolithischer Jäger und Sammler: Mensch-Umwelt-Interaktion im Frühholozän in der Nordmitteleuropäischen Tiefebene. (Ludwig, 2017).
Milner, N., Conneller, C., Taylor, B. & Milner, N. Star Carr Volume I: A persistent place in a changing world. (White Rose University Press, 2018).
Codding, B. F., Bliege Bird, R. & Bird, D. W. Provisioning offspring and others: Risk-energy trade-offs and gender differences in hunter–gatherer foraging strategies. Proc. R. Soc. B Biol. Sci. 278, 2502–2509 (2011).
Elston, R. G., Zeanah, D. W. & Codding, B. F. Living outside the box: An updated perspective on diet breadth and sexual division of labor in the Prearchaic Great Basin. Quat. Int. 352, 200–211 (2014).
Hawkes, K. Showing off: Tests of an hypothesis about men’s foraging goals. Ethol. Sociobiol. 12, 29–54 (1991).
Zhu, D., Galbraith, E. D., Reyes-García, V. & Ciais, P. Global hunter-gatherer population densities constrained by influence of seasonality on diet composition. Nat. Ecol. Evol. 5, 1536–1545 (2021).
Kass, J. M. et al. Wallace: A flexible platform for reproducible modeling of species niches and distributions built for community expansion. Methods Ecol. Evol. 9, 1151–1156 (2018).
GBIF.org. GBIF Home Page. GBIF Occurrence Search. https://www.gbif.org/occurrence/search (2022).
Yaworsky, P. M., Vernon, K. B., Spangler, J. D., Brewer, S. C. & Codding, B. F. Advancing predictive modeling in archaeology: An evaluation of regression and machine learning methods on the Grand Staircase-Escalante National Monument. PLOS ONE. 15. https://doi.org/10.1371/journal.pone.0239424 (2020).
Fick, S. E. & Hijmans, R. J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315 (2017).
Svenning, J.-C., Fløjgaard, C., Marske, K. A., Nógues-Bravo, D. & Normand, S. Applications of species distribution modeling to paleobiology. Quat. Sci. Rev. 30, 2930–2947 (2011).
Bevan, A. et al. rcarbon: Calibration and Analysis of Radiocarbon Dates. (2020).
Contreras, D. & Meadows, J. Summed radiocarbon calibrations as a population proxy: A critical evaluation using a realistic simulation approach. J. Archaeol. Sci. 52, 591–608 (2014).
Acknowledgements
We are obliged to Katherine Boyle for generously sharing her faunal dataset with us and thank Inga Kretschmer for making our life easier and providing us with easily workable radiometric information. Furthermore, we are grateful to all CLIOARCH group members, students, and guests for discussing and commenting on various aspects of research presented in this paper. We would also like to thank Brian Codding, K. Blake Vernon, and the University of Utah PhD Research group for feedback provided on an earlier version of this paper. Yaworsky, Hussain, and Riede acknowledge funding through the European Research Council (ERC) CoG project CLIOARCH (CLIOdynamic ARCHaeology: Computational approaches to Final Palaeolithic/earliest Mesolithic archaeology and climate change).
Funding
CLIOARCH is an ERC Consolidator Grant project and has received funding from the ERC under the European Union’s Horizon 2020 research and innovation program (Grant Agreement No. 817564).
Author information
Authors and Affiliations
Contributions
P.Y., S.H., and F.R. contributed to the design and performance of the research, as well as the analysis of the data and writing of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yaworsky, P.M., Hussain, S.T. & Riede, F. Climate-driven habitat shifts of high-ranked prey species structure Late Upper Paleolithic hunting. Sci Rep 13, 4238 (2023). https://doi.org/10.1038/s41598-023-31085-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-31085-x
This article is cited by
-
The Neanderthal niche space of Western Eurasia 145 ka to 30 ka ago
Scientific Reports (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.