Abstract
Using stable carbon and nitrogen isotope analysis (δ13C and δ15N) to assess trophic interactions in freshwater ecosystems is a well established method, providing insight into ecosystem functioning. However, the spatial and temporal variability of isotope values, driven by environmental fluctuation is poorly understood and can complicate interpretations. We investigated how the temporal variation of stable isotopes in consumers (fish, crayfish and macrozoobenthos) of a canyon-shaped oligotrophic reservoir is associated with environmental factors such as water temperature, transparency, flooded area, and water quality measures. Consumers and their putative food sources were sampled and analyzed for carbon and nitrogen stable isotopes annually, and environmental parameters were measured monthly from 2014 to 2016. Results revealed significant differences in δ13C and δ15N values in each consumer among studied years. Over the years, fish and crayfish expressed differences in δ13C between 3 and 5‰, whereas in zoobenthos differences were 12‰. Variability in δ15N was similar across all consumers (2–4‰). Moreover, results suggest that the flooded area of the reservoir was a major driver of δ13C stable isotope values variation in consumers, while variation in δ15N was not linked to any of the studied environmental factors. Bayesian mixing models further showed significant changes in the origin of detritivorous zoobenthos carbon sources (reversal shift from terrestrial detritus to algae origin) between years with low water level to years with the standard water level. Other species showed only slight differences in food source utilization among years. Our study highlights the importance of environmental factors as sources of variation in consumer’s stable isotope values which should be considered especially when studied ecosystem strongly fluctuate in some environmental factor.
Similar content being viewed by others
Introduction
Stable isotopes analyses (SIA) are widely used for determining consumer resource utilizations and interactions in a wide variety of ecostems1. It is applicable to a variety of aquatic organisms from all trophic levels2, providing insights into aquatic food webs1. Among other applications, SIA can be used to estimate the relative importance of consumed autochthonous and allochthonous sources of organic matter3, and can detect long-term feeding interactions4. Nevertheless, each method has its limitations5 and variability in consumer stable isotopes values due to external drivers (i.e. in environmental variables such as water quality, habitat availability, and surface runoff from catchment) may cause uncertainty for interpretation of SIA results.
Temporal and spatial variability in consumer stable isotopes values are well-known in aquatic ecosystems2,6,7 and might reflect changes in adjacent ecosystems8,9,10. Values of stable carbon and nitrogen isotopes (δ13C and δ15N) of consumers generally reflect dietary carbon and nitrogen source intakes from past weeks to few months, depending on species, size, physiological processes, food availability, and temperature2. However, there is a substantial lack of information, linking variability in both aquatic consumer δ13C and δ15N values to changes in environmental variables over time or between ecosystems. Thus, such lack of information might lead to biases in long term (multiple year sampling) data interpretation from given ecosystems. Therefore, studies disentangling environmental factors and stable isotopes values changes are urgently needed.
Several paleoclimate studies suggest that stable isotopes values might reflect past large-scale environmental changes (i.e. climatic changes11,12,13). Yet, there is a lack of information linking changes in consumer stable isotopes values from natural conditions to environmental variables in time and space over shorter time spans. This informational knowledge gap might limit or create a bias in consumer-resource data interpretation in rapidly changing aquatic ecosystems.
Generally, spatial heterogeneity of consumer stable isotopes values is expected from large aquatic ecosystems14, possibly originating from a wide variety of available food sources or specific feeding preferences 15,16. For instance, Devlin et al.8, Ruokonen et al.17, and Veselý et al.18 have shown that the food source utilization of consumers may vary along the depth gradient of a waterbody, causing intraspecific variation in stable isotope values at the population level.
Temporal variation can be divided into seasonal and annual variability19. The former is created by seasonally changing consumer resource utilization and therefore reflects changes in food source availability, isotopic basal source variation, as well as physiological processes in the consumer body18,20,21,22,23. Although seasonal variability in consumer stable isotope values can be extensive, the magnitude of annual variation is usually low, making these temporal changes less visible at higher trophic levels within a given aquatic food web18,24. Still, an extensive change in stable isotopes inter-annual variation might appear, usually reflecting strong changes in the given ecosystems.
Therefore, it is important to reveal the reasons underlying stable isotopes inter-annual variation. Here, we analyzed annual changes in stable isotopes of carbon and nitrogen values of consumers and assessed drivers of variation in the canyon-shaped reservoir Nýrsko, Czech Republic. We hypothesized that (i) food sources are utilized by consumers differently over the years due to variations in environmental factors (temperature, transparency, flooded area, oxygen, pH, NH4+, chemical oxygen demand by manganese – CODMn, and Chlorophyl a). Thus, (ii) the values of stable isotopes differ among consumers across years and within years due to different functional traits born by the species. This study will connect environmental variables to consumer stable isotope values variation over three years. Such results are of interest for food web ecologists and paleobiologists using stable isotopes as a proxy of environmental changes.
Material and methods
Study site
Nýrsko reservoir is an oligotrophic water supply reservoir (A = 1.48 km2, max. depth = 34 m) in the West Bohemia region of the Czech Republic (49°15′27″N, 13°8′46″E). The left side of the canyon-shaped reservoir gradually slopes to the bottom, which is composed of fine particles and covered by macrophytes. The right side of the reservoir is steeper, with stony shores in its lower section and a mix of sand and stony spots in its middle section. Muddy bottoms form the inlet section of the reservoir on both shores. Seasonal water level fluctuation between 2014 and 2016 was marginal, not exceeding 0.5 m and the flooded area ranged between 1.27 and 1.31 km2. In 2015, there was a planned dam reconstruction: water level decreased by 1.5 m below the normal operating level in late April—with water level fluctuation reaching 0.7 m at maximum. The flooded area ranged between 1.24 and 1.25 km2.
Field sampling
All major biological components were sampled annually from 2014 to 2016 in the first week of August. The consistent sampling protocol of Veselý et al.18 was applied. We sampled the same species groups over the years. Fish were collected by angling and gillnetting; the full list of species can be found in Veselý et al.18. For this study, adult European perch Perca fluviatilis L. 1758 and adult roach Rutilus rutilus (L. 1758) were chosen due to their presence in sufficient numbers in each year of sampling. Adult noble crayfish Astacus astacus (L. 1758) were caught manually with handheld nets as well as by scuba diving and with baited traps along the shoreline in the late afternoon and collected the following morning. Bulk crustacean zooplankton samples were collected using a net (mesh size 250 µm) pulled vertically through the water column. Zoobenthos was collected down to 1 m depth using a hand net (mesh size 500 µm). Macrophytes, periphyton and terrestrial detritus were collected by hand from the shoreline. All samples were placed on dry ice immediately after collection and then transferred to the laboratory freezer (− 30 °C) until further processing for SIA of carbon (δ13C) and nitrogen (δ15N). A piece of white dorsal muscle tissue of fish and a piece of abdominal muscle tissue of crayfish were used for SIA as recommended by Stenroth et al.25. Samples of fish, zoobenthos, terrestrial detritus, and macrophytes were identified to species or genus level. Zoobenthos species were further assigned into functional groups (Table S1). In general, we sampled same species over the years among groups Environmental data were obtained from the Vltava River Authority (Table 1).
All handling with organisms were conducted according to the principles of the Institutional Animal Care and Use Committee (IACUC) of the University of South Bohemia, Faculty of Fisheries and Protection of Waters, Research Institute of Fish Culture and Hydrobiology, Vodňany, based on the EU harmonized animal welfare act of Czech Republic. The entire study were approved by Ethical Commitee (IACUC—Institutional Animal Care and Use Committee). The principles of laboratory animal care and the national laws 246/1992 and regulations on animal welfare were followed (Ref. number 22761/2009-17210). This study is reported in accordance with Arrive guidelines (https://arriveguidelines.org). The noble crayfish were sampled under the permit from the Šumava Protected Landscape Area (Ref. number NPS 03232/2011).
No specific permissions were required for working with plants. Nevertheless, All procedures were conducted according to Czech law and in accordance to the standard guidelines.
Stable isotope analyses
Before the analysis of stable isotopes, all samples were dried at 50 °C for 48 h to the constant weight and grounded to a fine homogenous powder. Approximately 0.6 mg of animal samples and 1.5 mg of plant and detritus samples were weighed (at the precision of 0.001 mg) and transferred into tin cups. Stable isotope analyses were performed at the University of Jyväskylä using a Carlo Erba Flash EA 1112 elemental analyser connected to Thermo Finnigan DELTAplus and Advantage continuous-flow isotope ratio mass spectrometer (Thermo Electron Corporation, Waltham, MA, USA).
Vienna Pee Dee belemnite and atmospheric N2 were used as reference standards for carbon and nitrogen, respectively. To control instrument stability, northern pike Esox lucius L., 1758 muscle tissue and birch Betula pendula R. leaves of known isotopic compositions were run after every six samples. Results are expressed using the conventional δ notation as parts per thousand difference from the international standards. Analytical precision was < 0.1 ‰ for δ13C and < 0.3 ‰ for δ15N.
Statistical analyses
Effect of environmental factors on stable isotopes variation
Linear mixed effects models were employed to assess the role of environmental factors as a driver of carbon and nitrogen stable isotopes values fluctuation over the years. For each species or functional group, a separate analysis was performed. Given that the values of stable isotopes in the animal body are gathered over time, we used environmental data from the end of April to the end of July of each respective year. To omit the effects of monthly variability on stable isotopes value, we used month as a random effect. The final model was determined by sequential deletion of the least significant explanatory variables from the full model. Parameter significance was evaluated using F-tests from analysis of deviance. The final model included only parameters with significant p-values. Temperature, transparency, flooded area, oxygen, pH, NH4+, chemical oxygen demand by manganese (CODMn), and Chlorophyl a were used as explanatory variables of stable isotopes values changes (Table 1, Fig. S1). These parameters were chosen using correlation matrix where parameters with high correlation with used parameters were omitted (value 0.6 consider as threshold among parameters used in analyses). The significant parameters of the final model, a simple linear regression was applied to test the biological effect of given environmental variables. We compared the slopes of given variables among consumers using the lstrends function of the ‘lsmean’ package and ran generalized linear models to reveal differences in each consumer carbon and nitrogen stable isotopes over the years. For all statistical tests, p-values < 0.05 were considered significant. Analyses were performed in R-software26 (4.05).
Food source utilization
To quantify the contribution of the different food sources to the isotopic signature of each consumer or functional group of consumers a separate Bayesian mixing model27 with a specific number of putative sources was run over years in MixSiar-package28 in R26. In European perch (n = 21), a four-source model was run (omnivorous fish, crayfish, zoobenthos and crustacean zooplankton; Table S2). In roach (n = 20) and noble crayfish (n = 21), a five-source model was run (zoobenthos, crustacean zooplankton, macrophytes, algae and detritus; Table S3). For predatory zoobenthos (n = 20), a two-source model including crustacean zooplankton and zoobenthos was run (Table S4.). In detritivorous zoobenthos (n = 20), a three-source model was run (macrophytes, algae and detritus, Table S5).
Results
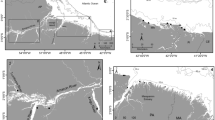
Consumer carbon stable isotope values varied across years, albeit the magnitude of variation depending on species and functional group (Fig. 1). In general, δ13C and δ15N values were similar in 2014 and 2016 although a significant change was noticed in 2015, when the carbon values were enriched across species and groups (Fig. 1B). The highest mean differences in carbon stable isotope values were found in predatory and detritivorous zoobenthos between 2014 and 2015, reaching 12.4 and 12.5‰, respectively. Consumers mean differences between 2014 and 2015 in European perch, roach, and noble crayfish were 2.44, 5.27, and 3.78‰, respectively (Fig. 1B). Annual variation in consumer δ15N was much less variation among years was much less pronounced and did not change more than 3‰ (Fig. 1A).
δ15N (A) and δ13C (B) values of consumers over time. Letters of significance denote differences over the years within species and functional groups. Perch = small italic letters, Roach = capital letters, noble crayfish = small bold letters, predatory zoobenthos = small letters, detritivorous zoobenthos = bold letters in italic.
Linear mixed effects models showed that environmental factors were not significantly related to changes of δ15N in any of the consumers over the years (p > 0.05). Controversely, δ13C stable isotope values of roach, noble crayfish, and detritivorous as well predatory zoobenthos were significantly associated with the size of the flooded area.
The extent of the flooded area differed across years, being most reduced in 2015. Neither δ13C nor δ15N values of the European perch were linked to environmental variables (Table 2). The other environmental variables did not differ over time (Fig. S1). Linear regressions between consumers δ13C and the flooded area revealed that with increasing extent of the flooded area, the carbon isotope values of consumers was more depleted (Fig. 2). Differences among slopes of linear regressions suggest specific response of given consumer to extent of the flooded area. Although roach and noble crayfish did not differ in their respective slopes (p = 0.87 and 0.57, respectively), significant differences were found between roach and predatory zoobenthos (p < 0.001 and p = 0.01, respectively), as well as between crayfish and predatory zoobenthos, as well as crayfish and detritivorous zoobenthos (p < 0.001 and p < 0.001, respectively).
Differences in food source utilization were found in all consumers, with the most prominent different seen in 2015 when compared with 2014 and 2016. Changes in food source utilization were consumer-specific (Tables 3, 4, 5 and 6). In 2015, European perch utilized less zoobenthos which was replaced by crustacean zooplankton (Table 3), but used other food sources rather equally over the 3 years. Although zoobenthos was the most important food source for roaches during the study years (Table 4), the roach increased macrophyte utilization during low water levels in 2015. Noble crayfish decreased its utilization of zoobenthos and relied more on crustacean zooplankton and detritus as food sources in 2015 compared to other years (Table 5). Predatory zoobenthos increased zoobenthos and decreased crustacean zooplankton use in 2015 compared to the remaining years (Table 6). In detritivorous zoobenthos, a clear increase in algae use was observed in 2015 (Table 6).
Discussion
This study provides substantial field evidence that long-term water level drops function as a significant driver for the variation in stable isotope values of several consumer groups, exemplary identified in the case of the studied Nýrsko reservoir. This has severe implications for studies dealing with ecosystems with water level fluctuation. Specifically, without knowledge of magnitude of water level fluctuation, comparison with similar systems or multiple year observation might be problematic. In addition, neither hypothesis 1 (food sources are utilized by consumers differently over the years due to variations in environmental factors) nor hypothesis 2 (the values of stable isotopes differ among consumers across years and within years) can be rejected.
The water level of reservoirs fluctuates more frequently than in natural lakes19,29, with water level decreases reaching several meters inevitably causing major stress for littoral biota30. We presume that in the Nýrsko reservoir, due to major water level drop in 2015, the organic matter from the shallow part of the reservoir, remained almost unreachable to detritivorous zoobenthos. Such stress can result in changes of trophic subsidy from terrestrial (detritus) to aquatic ecosystem (benthic algae) derived carbon δ13C in detrivorous zoobenthos. In such cases, detritivorous zoobenthos is obligated to use food sources from deeper zones (i.e. benthic algae), which was emphasized in a clear change from detritus to algae as primary food source (Tb. 6). This change in food source utilization probably caused the observed variation of δ13C values of zoobenthos (mean algae and detritivorous zoobenthos δ13C value in 2015 was − 14.08 ± 3.26 and 10.55 ± 1.16, respectively), shifting from depleted to enriched values in δ13C. This substantial alteration was lifted to higher trophic levels via the trophic chain, likely decreasing the trophic position of noble crayfish by almost two levels downwards. Similarly, to a series of other studies31,32,33, water level drop was found to significantly decrease the density of benthic macroinvertebrates in 2015. Changes observed in the Nýrsko reservoir highlight the instability of such systems and reflect the importance of connecting environmental factors (water level fluctuation in the case of this study) with biomarkers such as bulk stable isotopes. Moreover, observations over multiple years and sites are needed to elucidate the key effects of specific environmental drivers on consumer-specific values. Such results will lead to significantly lower biases in food source utilization models across space or time.
Changes on the lower trophic level were particularly prominent, being known to express staggering trophic fluctuations in accordance with i.e. nutrient influx or depletion19. Indeed, zoobenthos was an important food source for consumers in the Nýrsko reservoir, where strong changes in its δ13C value in 2015 affected other consumer values. Of course, zoobenthos was not the only food source of consumers as they prey upon many sources. Thus, the values of a given consumer consists of the biomass ratio of given food sources2 and concomitantly, a consumer’s response to basal stable isotope changes might vary. Importantly, other physiological factors such as growth rate, varying trophic discrimination factors among consumers and prey, or the time needed by consumers to assimilate new values from a given diet should be taken into account2,34,35. Hence, our Bayesian mixing model results suggest that consumers (European perch, roach, and noble crayfish) responded differently to changes in basal food sources, which can be possibly given by their bodies’ different physiological processes and the ecological roles of consumers in the reservoir’s food web.
Conclusion
Our study demonstrates the importance of environmental factors as a source of variation in the stable isotope values of consumers. Specifically, we found that water level fluctuation among years was the major driver of δ13C isotope value changes. Our results therefore suggest that such changes were caused by the inaccessibility of the littoral zone for basal consumers. Nonetheless, not all consumers’ δ13C values were related to water level fluctuations, as consumers with higher trophic positions were enriched to a lesser extent, while zoobenthos consumers and predators showed the largest variation in δ13C values. Specific responses of a given consumer or its functional group in terms of δ13C were related to variability in food source use and likely to differences in their physiological processes and ecology. Moreover, our results highlight the need to jointly test long-term isotopic data and environmental variables of a given aquatic ecosystem to confirm drivers of variability in stable isotope values.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Post, D. M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 83, 703–718 (2002).
Fry, B. Stable Isotope Ecology (Springer, 2007).
Boon, P. I. & Bunn, S. E. Variations in the stable isotope composition of aquatic plants and their implications for food web analysis. Aquat. Bot. 48, 99–108 (1994).
Kling, G. W., Fry, B. & O’Brien, W. J. Stable isotopes and planktonic trophic structure in arctic lakes. Ecology 73, 561–566 (1992).
Nielsen, J. M., Clare, E. L., Hayden, B., Brett, M. T. & Kratina, P. Diet tracing in ecology: Method comparison and selection. Methods Ecol. Evol. 9, 278–291 (2018).
Coulter, A. A., Swanson, H. K. & Goforth, R. R. Seasonal variation in resource overlap of invasive and native fishes revealed by stable isotopes. Biol. Invasions 21, 315–321 (2019).
Jung, A. S., Van Der Veer, H. W., Van Der Meer, M. T. & Philippart, C. J. Seasonal variation in the diet of estuarine bivalves. PLoS One 14, e0217003 (2019).
Devlin, S. P., Vander Zanden, M. J. & Vadeboncoeur, Y. Depth-specific variation in carbon isotopes demonstrates resource partitioning among the littoral zoobenthos. Freshw. Biol. 58, 2389–2400 (2013).
Possamai, B., Vieira, J. P., Grimm, A. M. & Garcia, A. M. Temporal variability (1997–2015) of trophic fish guilds and its relationships with El Niño events in a subtropical estuary. Estuar. Coast. Shelf Sci. 202, 145–154 (2018).
Syvaranta, J., Hamalainen, H. & Jones, R. I. Within-lake variability in carbon and nitrogen stable isotope signatures. Freshw. Biol. 51, 1090–1102 (2006).
Janbu, A. D., Paasche, Ø. & Talbot, M. R. Paleoclimate changes inferred from stable isotopes and magnetic properties of organic-rich lake sediments in Arctic Norway. J. Paleolimnol. 46, 29 (2011).
Leng, M. et al. Late quaternary palaeoenvironmental reconstruction from Lakes Ohrid and Prespa (Macedonia/Albania border) using stable isotopes. Biogeosciences 7, 3109–3122 (2010).
Jiang, Q., Shen, J., Liu, X., Zhang, E. & Xiao, X. A high-resolution climatic change since holocene inferred from multi-proxy of lake sediment in westerly area of China. Chin. Sci. Bull. 52, 1970–1979 (2007).
Finlay, J. C. & Kendall, C. Stable isotope tracing of temporal and spatial variability in organic matter sources to freshwater ecosystems. Stable Isot. Ecol. Environ. Sci. 2, 283–333 (2007).
Harvey, C. J. & Kitchell, J. F. A stable isotope evaluation of the structure and spatial heterogeneity of a Lake Superior food web. Can. J. Fish. Aquat. Sci. 57, 1395–1403 (2000).
Xu, D. et al. Spatial heterogeneity of food web structure in a large shallow eutrophic lake (Lake Taihu, China): Implications for eutrophication process and management. J. Freshw. Ecol. 34, 229–245 (2019).
Ruokonen, T., Kiljunen, M., Karjalainen, J. & Hämäläinen, H. Invasive crayfish increase habitat connectivity: A case study in a large boreal lake. Knowl. Manag. Aquat. Ecosyst. https://doi.org/10.1051/kmae/2013034 (2012).
Veselý, L. et al. The crayfish distribution, feeding plasticity, seasonal isotopic variation and trophic role across ontogeny and habitat in a canyon-shaped reservoir. Aquat. Ecol. 54, 1169–1183 (2020).
Kalff, J. Limnology: Inland Water Ecosystems Vol. 592 (Prentice Hall, 2002).
Polačik, M., Harrod, C., Blažek, R. & Reichard, M. Trophic niche partitioning in communities of African annual fish: Evidence from stable isotopes. Hydrobiologia 721, 99–106 (2014).
Costalago, D., Navarro, J., Álvarez-Calleja, I. & Palomera, I. Ontogenetic and seasonal changes in the feeding habits and trophic levels of two small pelagic fish species. Mar. Ecol. Prog. Ser. 460, 169–181 (2012).
Matthews, B. & Mazumder, A. Consequences of large temporal variability of zooplankton δ15N for modeling fish trophic position and variation. Limnol. Oceanogr. 50, 1404–1414 (2005).
Taipale, S., Kankaala, P., Tiirola, M. & Jones, R. I. Whole-lake dissolved inorganic 13C additions reveal seasonal shifts in zooplankton diet. Ecology 89, 463–474 (2008).
Zohary, T., Erez, J., Gophen, M., Berman-Frank, I. & Stiller, M. Seasonality of stable carbon isotopes within the pelagic food web of Lake Kinneret. Limnol. Oceanogr. 39, 1030–1043 (1994).
Stenroth, P. et al. Stable isotopes as an indicator of diet in omnivorous crayfish (Pacifastacus leniusculus): The influence of tissue, sample treatment, and season. Can. J. Fish. Aquat. Sci. 63, 821–831 (2006).
R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/ (2021).
Moore, J. W. & Semmens, B. X. Incorporating uncertainty and prior information into stable isotope mixing models. Ecol. Lett. 11, 470–480 (2008).
Stock, B. C. & Semmens, B. X. Unifying error structures in commonly used biotracer mixing models. Ecology 97, 2562–2569 (2016).
Irz, P., Laurent, A., Messad, S., Pronier, O. & Argillier, C. Influence of site characteristics on fish community patterns in French reservoirs. Ecol. Freshw. Fish 11, 123–136 (2002).
Sutela, T., Aroviita, J. & Keto, A. Assessing ecological status of regulated lakes with littoral macrophyte, macroinvertebrate and fish assemblages. Ecol. Indic. 24, 185–192 (2013).
Hunt, P. & Jones, J. The effect of water level fluctuations on a littoral fauna. J. Fish Biol. 4, 385–394 (1972).
Kaster, J. & Jacobi, G. Benthic macroinvertebrates of a fluctuating reservoir. Freshw. Biol. 8, 283–290 (1978).
Kraft, K. The effect of unnatural water level fluctuations on benthic invertebrates in Voyageurs National Park. Research⁄Resources Management Report MWR-12. US Department of the Interior, National Park Service. International Falls, Minnesota (1988).
Glon, M., Larson, E. R. & Pangle, K. Comparison of 13C and 15N discrimination factors and turnover rates between congeneric crayfish Orconectes rusticus and O. virilis (Decapoda, Cambaridae). Hydrobiologia 768, 51–61 (2016).
Hesslein, R. H., Hallard, K. & Ramlal, P. Replacement of sulfur, carbon, and nitrogen in tissue of growing broad whitefish (Coregonus nasus) in response to a change in diet traced by δ34S, δ13C, and δ15N. Can. J. Fish. Aquat. Sci. 50, 2071–2076 (1993).
Acknowledgements
This study was supported by the Ministry of Education, Youth, and Sports of the Czech Republic (project CENAKVA II – LO1205 under the NPU I Programme) and by the Estonian University of Life Sciences (research project P190254PKKH to FE). This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 951963. We thank Mikko Kiljunen for his valuable comments. Additionally, we thank Jan Kubec and Martin Fořt for their help with the field sampling. In addition, we thank three anonymous reviewers for their valuable and constructive comments.
Author information
Authors and Affiliations
Contributions
L.V. analysed data and wrote the first draft of the manuscript. M.B.l., M.B.u., A.K. and L.V. collected samples. F.E., T.J.R. and L.V. proceeded samples. M.B.l., A.K., F.E., T.J.R., H.H., P.J.H., M.B.u. and M.J.K., provided comments on the manuscript. J.D. provided environmental data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Veselý, L., Ercoli, F., Ruokonen, T.J. et al. Strong temporal variation of consumer δ13C value in an oligotrophic reservoir is related to water level fluctuation. Sci Rep 13, 3642 (2023). https://doi.org/10.1038/s41598-023-30849-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-30849-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.