Abstract
Dexmedetomidine is an alpha-2 adrenoreceptor agonist with anti-inflammatory and anti-delirogenic properties. Pathogenesis of postoperative delirium (POD) includes cholinergic dysfunction and deregulated inflammatory response to surgical trauma. Acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) are discussed as biomarkers for both POD and severity in acute inflammation. To show whether there is a link between blood cholinesterase activities and dexmedetomidine, we performed a secondary analysis of a randomised, double-blind, placebo-controlled trial that recently showed a lower incidence of POD in the dexmedetomidine group. Abdominal or cardiac surgical patients aged ≥ 60 years were randomised to receive dexmedetomidine or placebo intra- and postoperatively in addition to standard general anaesthesia. We analysed the course of perioperative cholinesterase activities of 56 patients, measured preoperatively and twice postoperatively. Dexmedetomidine resulted in no change in AChE activity and caused a rapid recovery of BChE activity after an initial decrease, while placebo showed a significant decrease in both cholinesterase activities. There were no significant between-group differences at any point in time. From these data it can be assumed that dexmedetomidine could alleviate POD via altering the cholinergic anti-inflammatory pathway (CAIP). We advocate for further investigations to show the direct connection between dexmedetomidine and cholinesterase activity.
Similar content being viewed by others
Introduction
Dexmedetomidine is a highly selective alpha-2-adrenoreceptor agonist with specific sympatholytic effects that is commonly used for sedation, analgesia, and anxiolysis in the intensive care unit1,2. Sedation is mediated without significant respiratory depression by decreasing brain activity in the locus coeruleus1. In the perioperative setting, dexmedetomidine has been shown to significantly reduce the need for hypnotics and opioids3,4,5. Common side effects include hypotension and bradycardia6. The recently published SPICE III trial showed that dexmedetomidine reduces 90-day mortality in elderly patients (over 65 years of age) compared with other sedation regimens, regardless of the reason for admission. In contrast, younger patients with nonoperative status had a high probability of increased 90-day mortality7. Furthermore, dexmedetomidine has anti-delirogenic8,9,10 and anti-inflammatory properties, the latter of which may attenuate an excessive inflammatory response after surgery11.
Dexmedetomidine has been shown to reduce the incidence of postoperative delirium (POD) when used during surgery or in the intensive care unit8,12,13. POD is characterized by disturbance of attention and awareness that starts acutely (e.g. after surgery) with a tendency to fluctuate, and at least one additional disturbance in cognition, and is associated with high mortality, ranging from 24 to 50%14,15. While the molecular mechanisms underlying the anti-deliriogenic potential of dexmedetomidine are still unknown, several anti-inflammatory mechanisms have already been described16,17,18,19,20,21,22,23,24, including the enhancement of the cholinergic anti-inflammatory pathway (CAIP)25,26,27,28. Indeed, one of the hypothesised molecular mechanisms of POD is the deregulation of the CAIP caused by cholinergic deficiency, leading to increased inflammation29,30,31,32,33.
The CAIP acts as an immunological reflex that links the nervous and immune systems. Consisting of the efferent part of the vagus nerve, the neurotransmitter acetylcholine (ACh), and the alpha 7 subunit of the nicotinic acetylcholine receptor (α7nAChR), the CAIP mediates the cholinergic regulation of inflammation. Following tissue damage or infection, it becomes activated by the release of pro-inflammatory cytokines and in turn controls the immune response via ACh, which binds to the α7nAChR on immune cells34,35,36. This ultimately leads to a dose-dependent inhibition of pro-inflammatory cytokine production (e.g., tumor necrosis factor alpha (TNFα), interleukin (IL)-1β and IL-6) by suppressing the activation of NF-kB particularly in the spleen37,38,39. In contrast, the synthesis and secretion of anti-inflammatory cytokines (e.g., IL-10) are maintained39.
In mice with LPS-induced inflammation, pre-emptive administration of dexmedetomidine attenuated NF-κB activation and the production of TNF-α, IL-6, and IL-1β at both the mRNA and protein levels in the spleen40. Consistent with these results, Kho et al. also found an effect of dexmedetomidine on cholinesterases in the spleen of rats41.
The acetylcholine hydrolysing enzymes acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) degrade ACh. AChE is found in all excitable tissues, in most erythrocytes, in immune cells, and in placental tissue and hydrolyzes ACh with a very high affinity. AChE is inhibited at high acetylcholine concentrations. BChE (also called as “pseudo” or “plasma” cholinesterase) is found ubiquitously throughout the body, particularly in the liver, blood serum, pancreas and central nervous system. In the brain, BChE is located mainly in glial and endothelial cells. It hydrolyses with a lower affinity ACh and non-choline esters, and is not inhibited by higher ACh concentrations42.
The regulation of AChE and BChE is still the subject of ongoing research. However, previous results from cell cultures and animal experiments indicate that during inflammation, the expression and activity in particular of AChE is modulated in a cell-specific manner by a direct interaction with the α7nAChR, but also by the binding of NF-κB to the AChE promoter gene43,44,45.
An association between cholinesterase enzyme activity and the occurrence of POD has been proposed by several studies, suggesting that lower activity even preoperatively puts patients at risk for POD46,47,48,49,50,51. However, given the differences in perioperative activity profiles of cholinesterases, this remains controversial. In addition, there is evidence for an indicative role of cholinesterases in systemic inflammation52,53,54. In this regard, especially BChE activity seems to be a promising candidate for clinical use, because it correlates negatively with conventional inflammatory markers such as C-reactive protein (CRP), with shorter time of latency and rapid determination by point-of-care measurement55,56,57,58.
Dexmedetomidine is a particularly interesting drug in this context, as it has an anti-inflammatory effect on the one hand and an anti-delirogenic potential on the other. Moreover, to the best of our knowledge, there have been no clinical reports of a possible effect of dexmedetomidine on cholinesterase activities.
Based on all these considerations, we hypothesise that dexmedetomidine has an influence on peripheral cholinesterase activities, potentially related to its anti-delirogenic and anti-inflammatory effects. To examine this hypothesis, we used data from the NEUPRODEX trial, a double-blind randomised controlled trial, which showed that perioperative administration of dexmedetomidine resulted in a significantly lower incidence of POD8. Therefore, this secondary analysis investigates whether the use of dexmedetomidine in addition to general anaesthesia alters the perioperative course of AChE and BChE activity.
Methods
Study design and population
This is a secondary analysis of the prospective, randomised, double-blind placebo-controlled NEUPRODEX trial that took place between July 2014 and July 20188. The primary outcome of this study was the postoperative incidence of delirium as measured by CAM-ICU (Confusion Assessment Method for the intensive care unit) or CAM (Confusion Assessment Method for normal wards) twice daily until the fifth postoperative day. The trial was approved by the regional ethics committee of Berlin, Germany (Landesamt für Gesundheit und Soziales, Approval Number 13/0491-EK 11) and was registered in the EU clinical trials register (2013–000,823-15) and the American National Institute of Health register (NCT02096068 on 26/03/2014). Inclusion and exclusion criteria are stated in our recent publication about the primary outcome as well as randomization process8. After written informed consent, the elderly patients were randomly assigned to dexmedetomidine or placebo group. The requirement for inclusion in this secondary analysis was the existence of a complete series of measurements of perioperative AChE and BChE activity. Patients with incomplete values were not included.
All patients received general anaesthesia induced with propofol and were maintained with either propofol or sevoflorane. All measures and procedures were performed in accordance with relevant guidelines and regulations. Our hospital pharmacy prepared syringes with dexmedetomidine or placebo, blinding both physicians and investigators. Starting ten minutes after induction, patients received either 0.7 µg/kg adjusted body weight (ABW)/h continuously or an equivalent volume of normal saline. The dose was reduced to 0.4 µg/kg ABW/h 30 min before the end of surgery. After arrival at the ICU, the dose was further reduced, but could be increased again to achieve a RASS score between 0 and − 1. During surgery, bradycardia as a side effect of dexmedetomidine was treated either by administration of orciprenaline or dose reduction of the syringe driver, containing either dexmedetomidine or placebo.
For analysis, we classified patients according to anticholinergic burden of their permanent medication taken at home. The Anticholinergic Drug Scale (ADS) developed by Carnahan and his colleagues59 with originally four items was dichotomized into 0 (no preoperative anticholinergic burden) and 1 (level of anticholinergic burden ≥ 1) to evaluate whether possible alterations of cholinesterase activity by these medications were balanced between groups.
Baseline measurements
The following baseline measurements were analysed: age, sex, Body Mass Index (BMI), American Society of Anaesthesiology Physical Score (ASA PS), quantity of preoperative long-term agents taken by the patient, polypharmacy defined as more than five preoperative long-term agents, Anti-cholinergic Drug Scale (ADS) of the long-term agents, type of surgery, incision-suture time, applied volume of placebo or dexmedetomidine.
Complementary to the baseline characteristics, opioid, anaesthetic, and red blood cell transfusion requirements were recorded from the intraoperative data as potential influencing factors on cholinesterase activities.
Cholinesterase activity
Cholinesterase activities were measured in all the whole blood samples drawn from arterial lines or venepuncture at three different points in time: preoperatively (on the evening before surgery or right before anaesthesia induction), 15 min after surgery, and the next morning after surgery at 8 a.m. (± 1 h). We analysed the samples with the portable point-of-care photometry test kit and machine “ChE check mobile” according to the instructions of the manufacture (SECURETEC, Neubiberg, Germany). This test kit applies a modified Ellmann reaction according to Worek et al. to determine the activity of AChE (normalized to haemoglobin U/g Hb) and BChE (U/l). The testing was performed immediately after collecting the blood sample.
In order to compare cholinesterase activities between delirium and no delirium, we first adjusted the delirium incidence for our observation period. Patients were classified as delirium positive if they were positively detected with the CAM/CAM-ICU at least once until the first postoperative day.
Statistical analysis
Baseline and intraoperative characteristics were expressed as median and lower and upper quartile, or frequencies with percentages. Descriptive statistics were computed for the dexmedetomidine group and the placebo group, heterogeneity between groups was assessed using Chi2 tests and Mann–Whitney U tests. Changes in cholinesterase activities over time within the group were assessed using Friedman rank analysis and the differences at each time point were compared using Mann–Whitney U tests. All testing was two-sided using a significance level of 0.05 to indicate statistical significance. Analyses were conducted with SPSS 25 [IBM SPSS Statistics® Version 25, IBM Germany, Ehningen, Germany] for Macintosh.
Institutional review board statement
The trial was approved by the regional ethics committee of Berlin, Germany (Landesamt für Gesundheit und Soziales, Approval Number 13/0491-EK 11) and was registered in the EU clinical trials register (2013-000823-15) and the American National Institute of Health register (NCT02096068).
Results
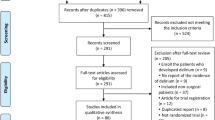
We screened 484 patients for eligibility that were scheduled to receive either elective coronary artery bypass graft surgery with reduced left ventricular ejection fraction ≤ 30%, or major abdominal surgery. 63 patients could be included according to inclusion and exclusion criteria. Three patients withdrew from enrolment as explained in our previous publication, yielding 60 patients. After merging preoperative cholinesterase measurements from the day before surgery with surgery day values, 56 cases (median age 69 years, 29% female) of complete cholinesterase activity measurements could be included in the analyses, while four cases had to be excluded because of missing cholinesterase values (see flow chart, Fig. 1).
Baseline characteristics did not differ between placebo and dexmedetomidine group
Baseline characteristics of the patients included in the present study differed slightly from our previously reported trial in the same population because four patients had to be excluded from analysis of cholinesterase activity, as stated in the methods8. Table 1 depicts the following baseline measurements. Of 56 patients included, 30 received standard general anaesthesia and 26 dexmedetomidine in addition. Median age did not differ significantly between the placebo group (69 years) and the dexmedetomidine group (71.5 years). Female patients were a minority in both groups, but the frequencies of female patients were not significantly different between placebo (30%) and dexmedetomidine (26.9%). There was also no significant difference between placebo and dexmedetomidine regarding Body Mass Index (median 27.1 m2/kg). About half of the population in both groups were patients with severe disease which limits activity (ASA PS III) or severe disease posing a constant threat to life (ASA PS IV). Most of the surgeries in both groups were abdominal (median 78.6%), only 21.4% were cardiac surgeries. Because preoperative medication can influence the cholinergic system, we analysed baseline values of the quantity of preoperative agents, the frequency of polypharmacy (intake of more than five different agents per day) and the number of cholinergic agents. Polypharmacy was frequent (55.4%) and 14.3% had at least one item on the ADS, with no significant differences between the two groups. Importantly, the applied volume of substance containing the agent in the dexmedetomidine group and the volume of placebo substance did not differ. Of interest, the baseline parameter with the lowest, yet not significant P-value was the incision-suture time. By tendency, it was faster in the dexmedetomidine (3.58 h) than in the placebo group (4.45 h).
Intraoperative opioid, anaesthetic, and transfusion requirements are comparable in both groups
In addition to baseline characteristics, we evaluated intraoperative opioid, anaesthetic and transfusion requirements, as these factors may potentially influence cholinesterase activity. There was no difference found between the two groups in regard to these measurements (Table 2).
AChE and BChE activities decrease postoperatively in placebo treated patients, but remain stable in dexmedetomidine treated patients
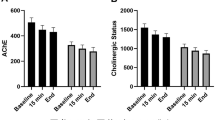
Enzyme activity of AChE in the placebo group changed significantly over the course of the three points in time preoperatively, directly after surgery and on postoperative day one, with an overall decline in activity (Fig. 2). In contrast, in the dexmedetomidine group, AChE activity did not change significantly in the course of these three points in time, despite a tendency of upregulated enzyme activity. The course of BChE activity showed a significant change in the placebo-treated patients and indicated a postoperative decline. In the dexmedetomidine group, BChE values decreased directly after surgery and increased again on postoperative day one. Nonetheless, this change in enzyme activity was significant over all three points in time.
Activities of cholinesterases. (A) Activities of acetylcholinesterase (AChE) (B) Activities of butyrylcholinesterase (BChE) before (preOP), 15 min after (postOP day 0) and one day after surgery (postOP day 1) compared between patients receiving standard general anaesthesia (Placebo, blue, n = 30) or general anaesthesia combined with perioperative dexmedetomidine (Dex, red, n = 26). The Boxplots show median and IQR (lower and upper hinges). P-values compare values of all three points in time within each group, using the Friedman rank analysis.
Regarding between-group comparisons, neither preoperative baseline values nor postoperative values of AChE and BChE activity showed any significant difference between dexmedetomidine and placebo treated patients (Table 3).
Cholinesterase activities in patients with or without delirium
AChE activities tends to decrease postoperatively in patients with delirium more, but remain stable in patients without delirium, while BChE activities decrease postoperatively in both, patients with and without delirium (Fig. 3).
Activities of cholinesterases. (A) Activities of acetylcholinesterase (AChE) (B) Activities of butyrylcholinesterase (BChE) before (preOP), 15 min after (postOP day 0) and one day after surgery (postOP day 1) compared between patients without delirium (no delirium, blue, n = 42) or patients with delirium (delirium, red, n = 14). The Boxplots show median and IQR (lower and upper hinges). P-values compare values of all three points in time within each group, using the Friedman rank analysis.
Discussion
The present study shows for the first time in human subjects that dexmedetomidine combined with general anaesthesia leads to a stable perioperative AChE activity in blood of patients with major abdominal or cardiac surgery. In contrast, general anaesthesia alone leads to a significant decrease in enzyme activity.
This is in line with findings of Nemoto and colleagues who studied the effect of intravenous propofol, midazolam and dexmedetomidine on the extracellular concentration of acetylcholine in the cerebral cortex of rats60. Propofol and midazolam led to a decrease in acetylcholine release, whereas they found no significant change after administration of dexmedetomidine. In contrast, dexmedetomidine-mediated activation of α2-adrenoceptors in animal models of neuropathic pain enhances analgesia by increasing ACh release in the spinal cord, whereas stimulation of α2-adrenoceptors results in inhibition of acetylcholine release under normal circumstances61. Kho et al. investigated the effect of dexmedetomidine on acetylcholine expression and AChE activity in the cortex, hippocampus, and spleen in rats with LPS-induced inflammation. In the spleen, dexmedetomidine exerts an activating effect on AChE and prevents an LPS-induced decrease in AChE activity. Additionally, the dexmedetomidine-mediated increase in AChE did not correlate with a decrease in ACh, nor did it coincide with an increase in apoptosis markers, indicating that AChE may also have played other roles than ACh hydrolysis41. In fact, Soreq and colleagues pointed out, that AChE also has “non-classical” effects, such as neuritogensis, synaptogenesis as well as haematopoiesis and thrombopoiesis62. In a small clinical study of 48 patients who had undergone major pancreatic surgery with or without splenectomy, the postoperative decrease in BChE activity was attenuated in patients with splenectomy compared to patients with a preserved spleen63. This highlights the integral role of the spleen in the CAIP and is consistent with the experimental work of Hoover and colleagues. They found that cholinergic function in the spleen is enhanced in sepsis by mechanisms of gene expression and reduction in cholinesterase activity, which facilitates cholinergic transmission64. Furthermore, they hypothesised that because choline is also a selective agonist for α7nAChRs and because the spleen lacks high-affinity choline transporters, the resulting prolonged presence of extracellular choline enhances cholinergic signalling in the spleen65.
We observed that patients treated with dexmedetomidine had stable AChE and rapidly recovering BChE activity, whereas they showed a lower incidence of POD in the primary study8. This may be related to the anti-inflammatory and immunomodulatory properties of dexmedetomidine. Deregulation of the CAIP caused by cholinergic deficiency leads to increased inflammation with neuroinflammation, which in turn promotes the development of POD31,32,33.
AChE plays a key role in cholinergic transmission by hydrolysing ACh at maximum rate, thereby maintaining not only cholinergic transmission but also the recycling process of ACh synthesis, where the absence of one component can lead to cholinergic dysfunction. Early animal studies demonstrated a central decrease in ACh synthesis and release in old age66,67. The present analysis focuses on elderly surgical patients with predominantly abdominal or high-risk cardiac surgery who are particularly affected by severe surgical trauma and long surgical duration with correspondingly high anaesthetic and opioid requirements. These factors influencing cholinergic transmitter balance, coincide with a predisposed population of high susceptibility to postoperative complications and may promote the development of POD. Furthermore, in the postoperative course of the CESARO study, a lower recovery of AChE activity was observed in the older subjects than in the younger ones. The authors argue that this effect is related to a decrease in regulatory capacity of the cholinergic system with age51.
BChE acitivity continuously decreased in placebo treated patients, whereas in dexmedetomidine treated patients, there was an increase on the first postoperative day compared to directly postoperative. On the one hand, this might be related to the fact that BChE activity is more susceptible to confounding factors, such as the outcome of hemodilutional cardiopulmonary bypass or malignancy42. On the other hand, BChE activity also responds rapidly to inflammation with a decrease in activity55. Characteristically for a negative acute phase protein, BChE activity has repeatedly been shown to be a prognostic biomarker for disease severity in acute inflammatory conditions such as sepsis, trauma, and burns55,56,57,58,68. Thereby, a clear pattern of BChE activity was observed: After an initial decrease in BChE activity, a sustained low level of activity was associated with higher morbidity and mortality, whereas an increase in activity during the course of the disease was considered as a sign of recovery and a better outcome56,57. However, because in the present study cholinesterases were measured only up to the first postoperative day, it can be merely speculated how BChE would have developed at later points in time in patients treated with dexmedetomidine.
Regarding the underlying physiological mechanism, Xiang and colleagues demonstrated that intraperitoneal administration of dexmedetomidine in mice augmented the electric activity of the cervical vagus nerve27. Furthermore, dexmedetomidine led to reduced levels of systemic inflammatory cytokines (TNF-α, IL-1β, IL-6). Interestingly, the anti-inflammatory effect of dexmedetomidine was also abolished by the administration of an antagonist at the α7nAChR, suggesting that dexmedetomidine has an influence on the CAIP. In similarly designed animal models, dexmedetomidine also reduced pro-inflammatory cytokine levels directly in the brain of rats69 and protected against cognitive dysfunction following systemic inflammation20,70. Moreover, Ohta and his colleagues investigated the anti-inflammatory effect of dexmedetomidine in patients with sepsis71. Patients treated with dexmedetomidine in addition to standard intensive care had significantly lower levels of the inflammatory markers CRP and PCT.
Although the few publications on the role of cholinesterases in POD yield inconsistent findings, they highlight the close relationship between the cholinergic system, perioperative inflammation and POD. In a cohort observational study of patients undergoing arthroplasty surgery, Cerejeira and colleagues found a correlation between low preoperative esterase activity and a change in blood CRP concentration47. Strikingly, this correlation was only found in patients with delirium but not in patients without POD. Additionally, a study by Adam and colleagues of patients undergoing cardiac surgery also found that POD correlated with decreased AChE activity before and after surgery, confirming that pre-existing altered cholinergic function may be a risk marker for POD46. Consistent with these reports, a recently published prospective observational study investigated the relationship between POD and cholinesterases in elderly non-cardiac surgery patients48. Both cholinesterases were found to be significantly lower postoperatively in patients with POD compared to those without POD. The authors of that study speculate that postoperatively decreased AChE and BChE activity in POD patients might be a response to already decreased acetylcholine levels before surgery. However, we cannot confirm this with our analysis, as the preoperative values in both groups are within the reference range. Nevertheless, it is quite interesting that the baseline values of both cholinesterase activities were lower in the dexmedetomidine than in the placebo group, although this is not statistically significant. Given the studies suggesting that low cholinesterase activity preoperatively increases the risk of POD46,47,48,49,50,51, one could speculate whether, without treatment with dexmedetomidine, the incidence of delirium might have been even higher in this group than in the placebo group.
In contrast to our results, a study in cardiac surgery patients showed no difference in postoperative measurements of AChE and BChE between patients with and without POD72. In addition, Mueller and her colleagues reported upregulated AChE in patients with POD and considered this as a compensatory mechanism for cholinergic deficit to provide new metabolites for acetylcholine synthesis51.
Strengths and limitations
This secondary analysis was conducted using a randomised, double-blind, placebo-controlled trial. To our knowledge, this is the first clinical trial to investigate the influence of intra- and postoperatively administered dexmedetomidine on the perioperative course of AChE and BChE. Overall, the results of the present study strengthen the role of the cholinergic system in delirium pathogenesis and at the same time suggest a regulatory effect of dexmedetomidine on the cholinergic system.
The present study did not show a significant difference in postoperative changes of AChE activity between dexmedetomidine and placebo treated patients. This may be due to a relatively small population size. Administrative and technical limitations led to incomplete follow-up measurements in some subjects. Moreover, the trial was powered for the primary outcome of POD incidence on the one hand, on the other hand, baseline values of both groups showed large interquartile ranges pointing towards high variation. We used a portable point-of-care photometer, which was originally designed for the analysis of ChE inhibition in organophosphate poisoning.
Cholinesterase activity is subject to a circadian rhythm73. This is a matter of ongoing research, also in the context of drugs for treatment of Alzheimer disease and optimal timing for their intake74. In our study, preoperative values were measured either on the evening before surgery or in the morning before surgery.
Therefore, preoperative values are likely influenced by circadian fluctuation. In our opinion, strict timing of AChE and BChE measurements could yield more homogenous results and significant between-group differences. Considering the existing difference between the groups in terms of their baseline esterase activities (AChE: 44.80 vs. 40.5 and BChE: 3088 vs. 2512), we have to assume that the preoperative values were probably influenced by circadian fluctuations. In our opinion, strict timing of AChE and BChE measurements could lead to more homogeneous baseline activity values and significant differences in activity progression between the groups.
A further subdivision of the groups into patients who actually developed delirium or remained delirium-free was not made due to the already very small group sizes (a total of 14 with delirium, 3 of which were in the dexmedetomidine group). However, such an analysis would probably be helpful with a larger population to obtain more informative results. There is also limited interpretation of cholinesterase activities in the groups with and without delirium up to the first postoperative day (Fig. 3), as postoperative delirium is the very disease that dexmedetomidine is supposed to ameliorate.
It should also be considered that our patients were recruited from two distinct populations: major abdominal surgeries and coronary artery bypass graft surgeries. One could suppose that these populations are distinct from one another, for instance in regard to perioperative blood loss and subsequent transfusions which is usually higher in cardiac surgeries. It has already been demonstrated by John and her colleagues that these factors alter the course of perioperative cholinesterases in patients who underwent cardiac surgery75.
As pro-inflammatory markers such as CRP were not recorded in the primary database, we could not analyse the relationship between cholinesterase activity, inflammatory markers and dexmedetomidine.
There is only limited literature about the regulation of cholinesterases, which makes it challenging to examine homogenous populations. A further limitation results from the lack of comparable studies on the effect of dexmedetomidine on cholinesterases. Accordingly, many reference studies have a different experimental design and comparability to our study is limited.
Therefore, we regard this secondary analysis rather as hypothesis-generating and we advocate for further investigations that are statistically powered for this question and that examine homogenous populations.
Conclusion
The present secondary analysis of a randomised controlled trial points towards a possible association between the perioperative use of dexmedetomidine and the regulation of cholinesterase activities. Patients treated with dexmedetomidine had stable perioperative AChE activity levels and the initial postoperative decline in BChE activity recovered rapidly, whereas placebo-treated patients showed a steady postoperative decline in both enzyme activities. Since neuroinflammation plays a pivotal role in the pathogenesis of POD, our data makes it tempting to speculate that dexmedetomidine may augment the CAIP by acting on cholinesterase activity, thereby alleviating POD.
Data availability
The data presented in this study are available on reasonable request from the corresponding author. The data are not publicly available due German data privacy protection regulations for clinical trials according to the Federal Institute for Drugs and Medical Devices.
References
Lee, S. Dexmedetomidine: present and future directions. Korean J. Anesthesiol. 72(4), 323–330 (2019).
Nelson, L. E. et al. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology 98(2), 428–436 (2003).
Gurbet, A. et al. Intraoperative infusion of dexmedetomidine reduces perioperative analgesic requirements. Can. J. Anaesth. 53(7), 646–652 (2006).
Ngwenyama, N. E. et al. Effects of dexmedetomidine on propofol and remifentanil infusion rates during total intravenous anesthesia for spine surgery in adolescents. Paediatr. Anaesth. 18(12), 1190–1195 (2008).
Soliman, R. N. et al. Prospective, randomized study to assess the role of dexmedetomidine in patients with supratentorial tumors undergoing craniotomy under general anaesthesia. Middle East J. Anaesthesiol 21(3), 325–334 (2011).
Carollo, D. S., Nossaman, B. D. & Ramadhyani, U. Dexmedetomidine: a review of clinical applications. Curr. Opin. Anaesthesiol 21(4), 457–461 (2008).
Shehabi, Y. et al. Early sedation with dexmedetomidine in ventilated critically ill patients and heterogeneity of treatment effect in the SPICE III randomised controlled trial. Intensive Care Med. 47(4), 455–466 (2021).
van Norden, J., et al., The effect of peri-operative dexmedetomidine on the incidence of postoperative delirium in cardiac and non-cardiac surgical patients: A randomised, double-blind placebo-controlled trial. Anaesthesia, 2021. 76.
Su, X. et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: A randomised, double-blind, placebo-controlled trial. Lancet 388(10054), 1893–1902 (2016).
Wu, M. et al. Perioperative dexmedetomidine reduces delirium after cardiac surgery: A meta-analysis of randomized controlled trials. J. Clin. Anesth. 50, 33–42 (2018).
Wang, K. et al. Effects of dexmedetomidine on perioperative stress, inflammation, and immune function: Systematic review and meta-analysis. Br. J. Anaesth. 123(6), 777–794 (2019).
Chen, J., Yan, J. & Han, X. Dexmedetomidine may benefit cognitive function after laparoscopic cholecystectomy in elderly patients. Exp. Ther. Med. 5(2), 489–494 (2013).
Pasin, L. et al. Dexmedetomidine reduces the risk of delirium, agitation and confusion in critically Ill patients: A meta-analysis of randomized controlled trials. J. Cardiothorac. Vasc. Anesth. 28(6), 1459–1466 (2014).
Brouquet, A. et al. Impaired mobility, ASA status and administration of tramadol are risk factors for postoperative delirium in patients aged 75 years or more after major abdominal surgery. Ann. Surg. 251(4), 759–765 (2010).
Rudolph, J. L. et al. Impaired executive function is associated with delirium after coronary artery bypass graft surgery. J. Am. Geriatr. Soc. 54(6), 937–941 (2006).
Chen, C. & Qian, Y. Protective role of dexmedetomidine in unmethylated CpG-induced inflammation responses in BV2 microglia cells. Folia Neuropathol. 54(4), 382–391 (2016).
Chen, J.-H. et al. Activation of α2 adrenoceptor attenuates lipopolysaccharide-induced hepatic injury. Int. J. Clin. Exp. Pathol. 8(9), 10752–10759 (2015).
Kim, E. et al. Dexmedetomidine confers neuroprotection against transient global cerebral ischemia/reperfusion injury in rats by inhibiting inflammation through inactivation of the TLR-4/NF-κB pathway. Neurosci. Lett. 649, 20–27 (2017).
Li, B. et al. Anti-inflammatory effects of perioperative dexmedetomidine administered as an adjunct to general anesthesia: A meta-analysis. Sci. Rep. 5, 12342 (2015).
Mei, B., Li, J. & Zuo, Z. Dexmedetomidine attenuates sepsis-associated inflammation and encephalopathy via central α2A adrenoceptor. Brain Behav. Immunol. 91, 296–314 (2021).
Peng, M. et al. Dexmedetomidine attenuates lipopolysaccharide-induced proinflammatory response in primary microglia. J. Surg. Res. 179(1), e219–e225 (2013).
Qiu, Z. et al. Dexmedetomidine inhibits neuroinflammation by altering microglial M1/M2 polarization through MAPK/ERK pathway. Neurochem. Res. 45(2), 345–353 (2020).
Rong, H. et al. The effects of dexmedetomidine pretreatment on the pro- and anti-inflammation systems after spinal cord injury in rats. Brain Behav. Immunol. 64, 195–207 (2017).
Yang, Y. F. et al. Dexmedetomidine preconditioning for myocardial protection in ischaemia-reperfusion injury in rats by downregulation of the high mobility group box 1-toll-like receptor 4-nuclear factor κB signalling pathway. Clin. Exp. Pharmacol. Physiol. 44(3), 353–361 (2017).
Huang, D. Y. et al. Dexmedetomidine attenuates inflammation and pancreatic injury in a rat model of experimental severe acute pancreatitis via cholinergic anti-inflammatory pathway. Chin. Med. J. (Engl) 133(9), 1073–1079 (2020).
Kong, W. et al. Dexmedetomidine alleviates LPS-induced septic cardiomyopathy via the cholinergic anti-inflammatory pathway in mice. Am. J. Transl. Res. 9(11), 5040–5047 (2017).
Xiang, H. et al. Dexmedetomidine controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Inflammation 37(5), 1763–1770 (2014).
Zhu, Y. J. et al. Attenuation of neuroinflammation by dexmedetomidine is associated with activation of a cholinergic anti-inflammatory pathway in a rat tibial fracture model. Brain Res. 1644, 1–8 (2016).
Androsova, G. et al. Biomarkers of postoperative delirium and cognitive dysfunction. Front. Aging Neurosci. 7, 112 (2015).
Inouye, S. K. Delirium in older persons. N. Engl. J. Med. 354(11), 1157–1165 (2006).
Cerejeira, J. et al. The neuroinflammatory hypothesis of delirium. Acta Neuropathol. 119(6), 737–754 (2010).
Cerejeira, J., Lagarto, L. & Mukaetova-Ladinska, E. B. The immunology of delirium. NeuroImmunoModulation 21(2–3), 72–78 (2014).
van Gool, W. A., van de Beek, D. & Eikelenboom, P. Systemic infection and delirium: When cytokines and acetylcholine collide. Lancet 375(9716), 773–775 (2010).
Tracey, K. J. The inflammatory reflex. Nature 420(6917), 853–859 (2002).
Tracey, K. J. Reflex control of immunity. Nat. Rev. Immunol. 9(6), 418–428 (2009).
Tracey, K. J. Understanding immunity requires more than immunology. Nat. Immunol. 11(7), 561–564 (2010).
Huston, J. M. et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J. Exp. Med. 203(7), 1623–1628 (2006).
Huston, J. M. et al. Cholinergic neural signals to the spleen down-regulate leukocyte trafficking via CD11b. J. Immunol. 183(1), 552–559 (2009).
Borovikova, L. V. et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405(6785), 458–462 (2000).
Liu, Z. et al. The role of spleen in the treatment of experimental lipopolysaccharide-induced sepsis with dexmedetomidine. Springerplus 4, 800 (2015).
Kho, W. et al. Dexmedetomidine restores autophagic flux, modulates associated microRNAs and the cholinergic anti-inflammatory pathway upon LPS-treatment in rats. J. Neuroimmune Pharmacol. 5, 87 (2021).
Davis, L., Britten, J. J. & Morgan, M. Cholinesterase. Its significance in anaesthetic practice. Anaesthesia 52(3), 244–260 (1997).
de Oliveira, P. et al. Cell-specific regulation of acetylcholinesterase expression under inflammatory conditions. Clin. Hemorheol. Microcirc. 51(2), 129–137 (2012).
Liu, E. Y. L. et al. Interacting with α 7 nAChR is a new mechanism for AChE to enhance the inflammatory response in macrophages. Acta Pharm. Sin. B 10(10), 1926–1942 (2020).
Shaked, I. et al. MicroRNA-132 potentiates cholinergic anti-inflammatory signaling by targeting acetylcholinesterase. Immunity 31(6), 965–973 (2009).
Adam, E. H. et al. Cholinesterase alterations in delirium after cardiosurgery: A German monocentric prospective study. BMJ Open 10(1), e031212 (2020).
Cerejeira, J. et al. The cholinergic system and inflammation: Common pathways in delirium pathophysiology. J. Am. Geriatr. Soc. 60(4), 669–675 (2012).
Zhao, B., Ni, Y. & Tian, X. Low plasma cholinesterase activity is associated with postoperative delirium after noncardiac surgery in elderly patients: A prospective observational study. Psychosomatics 60(2), 190–196 (2019).
Lin, X. et al. Cerebrospinal fluid cholinergic biomarkers are associated with postoperative delirium in elderly patients undergoing Total hip/knee replacement: a prospective cohort study. BMC Anesthesiol. 20(1), 246 (2020).
Bosancic, Z. et al. Association of cholinesterase activities and POD in older adult abdominal surgical patients. BMC Anesthesiol. 22(1), 293 (2022).
Muller, A. et al. Relevance of peripheral cholinesterase activity on postoperative delirium in adult surgical patients (CESARO): A prospective observational cohort study. Eur. J. Anaesthesiol. 36(2), 114–122 (2019).
Zujalovic, B. et al. AChE-activity in critically ill patients with suspected septic encephalopathy: A prospective, single-centre study. BMC Anesthesiol. 20(1), 287 (2020).
Das, U. N. Acetylcholinesterase and butyrylcholinesterase as possible markers of low-grade systemic inflammation. Med. Sci. Monit. 13(12), 214–221 (2007).
Bahloul, M. et al. Value of serum cholinesterase activity in the diagnosis of septic shock due to bacterial infections. J. Intensive Care Med. 32(5), 346–352 (2017).
Zivkovic, A. R. et al. Reduced butyrylcholinesterase activity is an early indicator of trauma-induced acute systemic inflammatory response. J. Inflamm. Res. 9, 221–230 (2016).
Zivkovic, A. R. et al. A sustained reduction in serum cholinesterase enzyme activity predicts patient outcome following sepsis. Mediators Inflamm. 2018, 1942193 (2018).
Zivkovic, A. R. et al. Reduced serum butyrylcholinesterase activity indicates severe systemic inflammation in critically Ill patients. Mediators Inflamm. 2015, 274607 (2015).
Zivkovic, A. R. et al. Bedside-measurement of serum cholinesterase activity predicts patient morbidity and length of the intensive care unit stay following major traumatic injury. Sci. Rep. 9(1), 10437 (2019).
Carnahan, R. M. et al. The anticholinergic drug scale as a measure of drug-related anticholinergic burden: Associations with serum anticholinergic activity. J. Clin. Pharmacol. 46(12), 1481–1486 (2006).
Nemoto, C. et al. Effects of dexmedetomidine, midazolam, and propofol on acetylcholine release in the rat cerebral cortex in vivo. J. Anesth. 27(5), 771–774 (2013).
Hayashida, K. & Eisenach, J. C. Spinal alpha 2-adrenoceptor-mediated analgesia in neuropathic pain reflects brain-derived nerve growth factor and changes in spinal cholinergic neuronal function. Anesthesiology 113(2), 406–412 (2010).
Soreq, H. & Seidman, S. Acetylcholinesterase-new roles for an old actor. Nat. Rev. Neurosci. 2(4), 294–302 (2001).
Zivkovic, A. et al. Reduced serum cholinesterase activity indicates splenic modulation of the sterile inflammation. J. Surg. Res. 220, 275–283 (2017).
Hoover, D. B. et al. Cholinergic leukocytes in sepsis and at the neuroimmune junction in the spleen. Int. Immunopharmacol. 81, 106359 (2020).
Hoover, D. B. Cholinergic modulation of the immune system presents new approaches for treating inflammation. Pharmacol. Ther. 179, 1–16 (2017).
Gibson, G. E. & Peterson, C. Aging decreases oxidative metabolism and the release and synthesis of acetylcholine. J. Neurochem. 37(4), 978–984 (1981).
Gibson, G. E., Peterson, C. & Jenden, D. J. Brain acetylcholine synthesis declines with senescence. Science 213(4508), 674–676 (1981).
Schmidt, K. et al. Point-of-care measured serum cholinesterase activity predicts patient outcome following severe burns. Burns 47(4), 863–872 (2021).
Paeschke, N. et al. Dexmedetomidine prevents lipopolysaccharide-induced microRNA expression in the adult rat brain. Int. J. Mol. Sci. 18(9), 81 (2017).
Yamanaka, D. et al. Preventive effects of dexmedetomidine on the development of cognitive dysfunction following systemic inflammation in aged rats. J. Anesth. 31(1), 25–35 (2017).
Ohta, Y. et al. Effect of dexmedetomidine on inflammation in patients with sepsis requiring mechanical ventilation: A sub-analysis of a multicenter randomized clinical trial. Crit. Care 24(1), 493 (2020).
John, M. et al. Acetylcholinesterase and butyrylcholinesterase in cardiosurgical patients with postoperative delirium. J. Intensive Care 5(1), 29 (2017).
Han, S. et al. Mixed-effect circadian rhythm model for human erythrocyte acetylcholinesterase activity—application to the proof of concept of cholinesterase inhibition by acorn extract in healthy subjects with galantamine as positive control. Eur. J. Clin. Pharmacol. 68(5), 599–605 (2012).
Wang, H. & Zhang, H. Reconsideration of anticholinesterase therapeutic strategies against Alzheimer’s disease. ACS Chem. Neurosci. 10(2), 852–862 (2019).
John, M. et al. Acetylcholinesterase and butyrylcholinesterase in cardiosurgical patients with postoperative delirium. J. Intensive Care 5, 29 (2017).
Acknowledgements
Assistance with the study: The authors thank A. Lütz, B. Weiß, A. Astrath, M. Kuhrmann, S. Heidgen, J. Thomas, F. Yürek, A. Wolf, T. Aslan, M. Isallari, S Rajput, S. Piper, J. Kruppa, and K. Scholtz. Maria Heinrich is a participant of the BIH Charité Digital Clinician Scientist Program funded by the Charité–Universitätsmedizin Berlin and the Berlin Institute of Health at Charité (BIH).
Funding
Open Access funding enabled and organized by Projekt DEAL. The NEUPRODEX study received funding from Orion Corporation (Espoo, Finland). The financial contributor had no influence in study design, data collection, analysis, decision to publish or preparation of the article. ChE measurement kits were funded by Dr Franz Köhler Chemie (DFKC) GmbH (Bensheim, Germany). The financial contributor had no role in study design, data collection, analysis, decision to publish or preparation of the article.
Author information
Authors and Affiliations
Contributions
This paper is based on the more comprehensive doctoral thesis of Y.J. Content and structure are following the doctoral thesis, highlighting the most important findings. Figures and tables were partially modified. The doctoral thesis will be published in German language on the open access repository of the Freie Universität Berlin (https://refubium.fu-berlin.de). So far there have not been publications in scientific journals with this subanalysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jacob, Y., Schneider, B., Spies, C. et al. In a secondary analysis from a randomised, double-blind placebo-controlled trial Dexmedetomidine blocks cholinergic dysregulation in delirium pathogenesis in patients with major surgery. Sci Rep 13, 3971 (2023). https://doi.org/10.1038/s41598-023-30756-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-30756-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.