Abstract
Finding innovative eco-friendly agents for pest control may be aided by investigating the plant-derived extracts’ properties on economic pests. Therefore, the insecticidal, behavioral, biological and biochemical effects of Magnolia grandiflora (Magnoliaceae) leaf water and methanol extracts, Schinus terebinthifolius (Anacardiaceae) wood methanol extract, and Salix babylonica (Salicaceae) leaf methanol extract in comparison with a reference insecticide novaluron against S. littoralis were evaluated. The extracts were analyzed by High-Performance Liquid Chromatography (HPLC). The most abundant phenolic compounds were 4-hydroxybenzoic acid (7.16 mg/mL) and ferulic acid (6.34 mg/mL) in M. grandiflora leaf water extract; catechol (13.05 mg/mL), ferulic acid (11.87 mg/mL), and chlorogenic acid (10.33 mg/mL) in M. grandiflora leaf methanol extract; ferulic acid (14.81 mg/mL), caffeic acid (5.61 mg/mL), and gallic acid (5.07 mg/mL) In the S. terebinthifolius extract; cinnamic acid (11.36 mg/mL), and protocatechuic acid (10.33 mg/mL) In the methanol extract from S. babylonica extract. S. terebinthifolius extract had a highly toxic effect against second larvae after 96 h and eggs with LC50 values of 0.89 and 0.94 mg/L, respectively. Despite M. grandiflora extracts didn’t show any toxicity against S. littoralis stages, they had an attractant effect on fourth- and second larvae, with feeding deterrence values of − 2.7% and − 6.7%, respectively, at 10 mg/L. S. terebinthifolius extract significantly reduced the percentage of pupation, adult emergence, hatchability, and fecundity, with values of 60.2%, 56.7%, 35.3%, and 105.4 eggs/female, respectively. Novaluron and S. terebinthifolius extract drastically inhibited the activities of α-amylase and total proteases to 1.16 and 0.52, and 1.47 and 0.65 ΔOD/mg protein/min, respectively. In the semi-field experiment, the residual toxicity of tested extracts on S. littoralis gradually decreased over time compared to novaluron. These findings indicate that extract from S. terebinthifolius is a promising insecticidal agent against S. littoralis.
Similar content being viewed by others
Introduction
The cotton leafworm, Spodoptera littoralis (Boisd) (Lepidoptera: Noctuidae), is the major destructive pest of several agricultural crops including cotton, eggplant, tomato, and some ornamental products in Africa, Mediterranean Europe and Middle Eastern countries. More than 100 host attacked by this pest species, which causes yield losses of 50%, related to its larval foliage consumption activity1. The management of insect pests is one of the main challenges for agricultural researchers, and it’s difficultly increases with pest resistance and cross-resistance to chemical insecticides2. The negative impacts of pesticides on human health and environment have prompted more research on alternative control strategies for the integrated management of native and invasive pests3,4,5,6,7. To produce high-quality, pest-free crops without endangering the environment, researchers have focused on finding alternative, effective, and environmentally friendly control methods8. Botanicals are plant-derived materials that can be used as major components in integrated pest management (IPM) to control insect pests9,10,11,12 and reduce the use of synthetic insecticides. Thus, plant extracts are viable alternatives as they can regulate pest insect populations by affecting biological and behavioral parameters13,14.
Among the plant families that regulate insect pest populations, the previously tested behavioral and insecticidal properties of Magnoliaceae, Salicaceae, and Anacardiaceae were analyzed in the present study. Many studies have been performed on Magnolia species for their biphenolic phytochemicals magnolol and honokiol, which possess diverse pharmacological properties. Moreover, Magnolia extracts and their bioactive chemicals have been evaluated for potential insecticidal activity15,16,17. Different solvent extracts of M. salicifolia Maxim showed good to moderate larvicidal effects on fourth larvae of Aedes aegypti18.
Willows (Salix spp., family Salicaceae) are deciduous trees or shrubs well known for their medicinal effects. Many ancient civilizations used extracts of willow bark and willow leaf because of their analgesic, antipyretic, and anti-inflammatory properties19,20. Many studies have documented the presence of bioactive secondary compounds, such as polyphenols, terpenoids, and most importantly, salicylate compounds, in these plants21,22,23,24,25, which play a critical role not only as a part of their defense mechanisms and signaling molecules, but also as therapeutic agents (especially salicin)26,27. Plants synthesize salicylic acid (SA) through two pathways: the isochorismate pathway (IC) and the phenylalanine ammonia-lyase (PAL) pathway28. Willow is a well-known source of SA, which induces systemic resistance against several plant diseases29. Aqueous extracts of willow reduced Fusarium wilt in tomato seedlings by decreasing the level of lipid peroxidation30. The bark extract of the common willow has been approved by the EU pesticide regulations for agricultural applications as a basic substance with fungicidal properties31,32.
Schinus terebinthifolia (Anacardiaceae), commonly known as Brazilian pepper, has received particular attention owing to its nutritional, ornamental, and health-promoting properties, which can be attributed to a plethora of bioactive components, particularly phenols, tannins, flavonoids, saponins, alkaloids, and sterols33,34,35. Schinus terebinthifolius has insecticidal properties against Stegomyia aegypti36, Anopheles gambiae, A. arabiensis, and Culex quinquefasciatus37, S. littoralis38, and whitefly, Bemisia tabaci39. The essential oils of S. terebinthifolius fruits can also be used in the control of S. littoralis and Phthorimaea operculella, in association with IPM practices40.
Chitin synthesis inhibitors are insect growth regulators that affect insect chitin biosynthesis41,42,43. Novaluron is a benzoylphenylurea insecticide that interferes with developmental processes in immature insects, including abortive molting44,45,46. Novaluron ingested by adults can often be transferred transovarially to eggs, thereby reducing populations of economically important insect pests45,47. Moreover, it has low toxicity to mammals and several important natural enemies48.
Determining how digestive enzymes react to various inhibitors is a promising method to control phytophagous insects. There is limited published information on the inhibitors of S. littoralis digestive enzymes49,50.
The present study was conducted to evaluate the insecticidal activity of M. grandiflora, S. terebinthifolius, and S. babylonica extracts against different stages of S. littoralis (Boisd.). Furthermore, this study aimed to investigate the repellent and biological effects of these extracts on S. littoralis under laboratory or semi-field conditions; to determine the biochemical properties of extracts (e.g., in vitro inhibition of α-amylase and total protease activities); and to provide recommendations for using these plant-derived extracts in IPM programs to control this major pest.
Materials and methods
Insect rearing
This study has complied with relevant institutional, national, and international guidelines and legislation. This study does not contain any studies with human participants or animals performed by any of the authors. A laboratory strain of S. littoralis was reared on castor bean leaves, Ricinus communis L., under constant conditions of 27 ± 2 °C and 65 ± 5% relative humidity (RH), in the Insect Physiology Laboratory, Department of Applied Entomology and Zoology, Faculty of Agriculture, Alexandria University, Egypt. Moths were provided with Nerium oleander L. leaves for egg laying. Moreover, as the field strain, egg masses of S. littoralis were collected from cotton fields at El-Beheira Governorate, Egypt, and maintained in the laboratory under the aforementioned conditions.
Test extracts
Extracts from Magnolia grandiflora leaves (Magnoliaceae), Schinus terebinthifolius wood (Anacardiaceae), and Salix babylonica leaves (Salicaceae) were used in this study. Novaluron (Equo® 10% EC; field rate, 60 mL/100 L water; Isagro Co., Italy) was used as a positive control for evaluating and comparing with the effectiveness of these extracts. All solvents and reagents used in experiments were analytical grade.
Extraction procedure
Plant materials from the three tree species (M. grandiflora leaves, S. terebinthifolius wood, and S. babylonica leaves) were collected from Alexandria, Egypt. The collection of plants have been done and identified at the Department of Forestry and Wood Technology, Faculty of Agriculture, Alexandria University, Alexandria, Egypt. All plant materials were air-dried at room temperature for approximately 10 days and then ground to a powder using a small laboratory mill. Approximately 50 g of Magnolia grandiflora leaves was soaked in n-hexane (100 mL) in a conical flask for 3 days and then filtered through Whatman no. 1 filter paper. The solvent was evaporated using a rotary evaporator, and the n-hexane oily extract was concentrated.
For the extracts that were analyzed by HPLC, water and methanol extracts from M. grandiflora leaves and methanol extracts of S. terebinthifolius wood and S. babylonica leaves were used. Approximately 50 g of each ground material was soaked in 150 mL of solvent (water or methanol) for one week, then filtered through filter paper (Whatman no. 1), and concentrated by evaporating the solvent under reducing pressure with a rotary evaporator51. For the water extract of M. grandiflora leaves, a few drops of methanol were used to prevent any fungal growth. The content of the extracts was measured as a percentage per mass of the air-dried raw materials.
HPLC analysis of extracts
For the phytochemical analysis, the phenolic compounds from extracts of M. grandiflora leaves, S. terebinthifolius wood, and S. babylonica leaves were identified by HPLC (Agilent 1100). The instrument was composed of binary LC pump, a UV/Vis detector, and C18 column (125 mm × 4.60 mm, 5-µm particle size)52.
Toxicity tests
Ovicidal activity
Freshly deposited egg masses from the laboratory strain of S. littoralis were collected and counted using a hand lens (10 ×). Six concentrations of each extract and novaluron (0.5, 1, 2, 5, 10, and 20 mg/L) were prepared. Oleander leaves containing approximately 100 eggs were dipped for 20 s in each concentration of the test compounds separately. Another set of egg masses (100 eggs) on the oleander leaves was dipped in water to represent the control. Each concentration and control was replicated thrice. Treated and untreated egg masses were left to dry and maintained at 27 ± 2 °C, 65 ± 5% RH. After the maximum hatching time, the unhatched eggs in each treatment were counted using a binocular.
Larvicidal activity
The efficacy of the tested plant extracts and novaluron against the newly molted second and fourth larvae of S. littoralis was evaluated using a standard leaf-dip method. Six concentrations of each extract and insecticide (0.5, 1, 2, 5, 10, and 20 mg/L) were prepared. Castor bean leaves, which were almost equal in size, were dipped in the tested concentrations for 10 s and then left to dry. A set of castor leaves was dipped in distilled water only as the control. Each treatment was replicated thrice (20 larvae per treatment). The larvae were allowed to feed on treated leaves, and the mortality percentages were recorded 48 and 96 h post-treatment.
Repellent effects of the tested materials
Feeding deterrence activity
The feeding deterrence effect of the M. grandiflora leaf water extract, S. terebinthifolius wood methanol extract, and S. babylonica leaf methanol extract and of novaluron insecticide against second and fourth larvae of S. littoralis was determined using the leaf disc method (no-choice test) 48 h post-treatment. Three concentrations of M. grandiflora extract (1, 5, and 10 mg/L), S. terebinthifolius and S. babylonica extracts (1, 2, and 5 mg/L), and novaluron (0.5, 1, and 2 mg/L) were used. These concentrations were chosen after the preliminary tests according to their effectiveness. The feeding deterrence index (FD %) was calculated using the following equation: FD % = [(C − T) / (C + T)] × 100; where C is the consumption of control discs and T is the consumption of treated discs53.
Anti-oviposition activity
A no-choice test was used to evaluate the effects of the tested extracts on the oviposition. The three above-mentioned concentrations of each plant extract and insecticide were used. Each pair (female and male) of newly emerged adults was placed in a glass jar with a ball of cotton dipped in a 10% sugar solution for feeding. Oleander leaves were treated with the test concentrations. The adults were left to feed, mate, and lay eggs on control and treated oleander leaves. Adults were removed two days after the beginning of egg laying, oleander leaves were carefully taken, and the number of eggs laid by each female was counted using a binocular. The anti-oviposition effect was calculated as follows54: Repellent index (RI %) = [(C − T) / (C + T)] × 100; where C is the number of eggs in the control, and T is the number of eggs in the treatment.
Biological aspects
Bioactivity of the tested plant extracts and the insecticide was assessed under laboratory conditions (27 ± 2 °C, 65 ± 5% RH). The castor bean leaves were immersed separately in the above-mentioned concentrations of tested compounds or in distilled water for control, dried at room temperature, and transferred to petri dishes (12 cm in diameter). One hundred neonates (0–24 h) S. littoralis larvae were placed in each petri dish. Castor bean leaves were replaced with newly treated leaves every 24 h. To establish the pupation percentage and observe malformations, the larvae were continuously monitored until they reached the pupal stage. The pupae were sexed and transferred to 1-L glass containers (10 males and 10 females per container) to assess the percentage of adult emergence, mean number of eggs per female (fecundity), and hatchability.
Biochemical assays
The in vitro inhibition of α-amylase and total protease activity were determined by incubating the prepared homogenate for 30 min at 37 °C with LC50 concentrations of the tested compounds prepared in distilled water containing the emulsifying agent (0.01% Triton-X 100). The control treatments were prepared by adding 0.01% Triton-X 100 without the tested compounds. Fourth larvae were then dissected, and midguts were excised, collected, and washed repeatedly with ice-cold saline solution (0.9% NaCl). The midguts were then homogenized in distilled water using a glass homogenizer surrounded with ice. The protein content was estimated by the method of Lowry et al.55 using bovine serum albumin as a standard protein to construct the standard curve.
Alpha-amylase activity assay
The homogenate was centrifuged at 15,000 rpm for 15 min at 4 °C using IEC-CRU 5000 cooling centrifuge. The α-Amylase activity was estimated spectrophotometerically56. Fifty microliters of supernatant was added to 2.3 mM 2-chloro 4-nitrophenyl-α-Dmaltotrioside (CNPG3), 350 mM NaCl, 6 mM calcium acetate, 600 mM potassium thiocyanate, and 100 mM Good’s buffer (pH 6). An assay mixture without enzyme was used as a blank. The change in absorption at 405 nm was monitored using a Sequoia-Turner Model 340 spectrophotometer. The α-amylase activity was calculated as ΔOD405/mg protein/min.
Total protease activity assay
The homogenate was centrifuged at 4000 rpm for 15 min at 4 °C in an IEC-CRU 5000 cooling centrifuge. The supernatant was used to estimate total proteolytic activity. Total protease activity was measured57,58 using azocasein as a substrate. The homogenate was incubated in a total volume 60 μL of assay buffer (100 mM Tris–HCl, pH 8) for 20 min at 37 °C before addition of 200 μL of 2% azocasein (w/v in assay buffer). After 180 min at 37 °C, the reaction was stopped by addition of 300 μL cold 10% trichloroacetic acid (TCA). The reaction mixture was centrifuged at 3000 rpm for 10 min in an IEC-CRU 5000 cooling centrifuge. Then, 10 μL NaOH (10 N) were added to the reaction mixture to neutralize excess acidity, and the absorbance was measured at 440 nm using a Sequoia-Turner Model 340 spectrophotometer. An assay mixture without homogenate was used as a blank. The total protease activity was calculated as ΔOD440/mg protein/min.
Semi-field experiment
The residual toxicity of M. grandiflora, S. terebinthifolius, and S. babylonica extracts in comparison with novaluron against the field strain of fourth S. littoralis larvae was tested according to Raslan59 and El-Sheikh and Aamir60. Cotton seeds (Gossypium barbadense Linnaeus var. Giza 92) were sown in 50 plastic pots (30-cm in diameter) in the greenhouse of Cotton Pesticides Evaluation Department, Plant Protection Research Station, Alexandria, Egypt. Thirty days after emergence, the tested extracts and insecticide were applied as a foliar spray at a field rate (60 mL/100 L water) and a half field rate (30 mL/100 L water) using a hand-held sprayer with 1-L capacity until the leaves were saturated, and left to dry. The untreated plants were sprayed with tap water only. Cotton leaves from treated and untreated plants were randomly collected in perforated bags after 2 h of application and then 1, 2, 3, 4, and 7 days after the application, and transferred to the laboratory. Two cotton leaves from each sample were introduced to 20 newly molted fourth larvae of S. littoralis in Petri dish (12 cm in diameter) containing filter paper. Five replicates were performed for each treatment group. The Petri dishes were kept under laboratory conditions, at 27 ± 2 °C and 65 ± 5 RH %. The number of dead larvae was recorded, and mortality percentages were calculated 48 h after feeding.
Statistical analysis
The LC50 and LC95 values for the toxicity tests were calculated using the Biostat ver. (2.1) software61 for probit analysis. Data were compared by one-way analysis of variance (ANOVA) followed by Tukey’s studentized test when significant differences were found at P < 0.0562.
Results
Chemical composition of the extracts
The extract contents from the studied plants were in M. grandiflora leaf water extract (8.12%), M. grandiflora leaf methanol extract (12.15%), S. terebinthifolius wood methanol extract (16.14%), and in S. babylonica leaf methanol extract (15.24%).
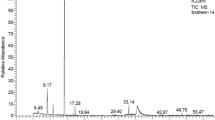
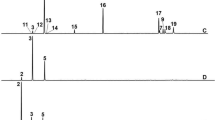
According to the HPLC analysis (Fig. 1 and Table 1), M. grandiflora leaf water extract (Fig. 1A) contained as main phenolic compounds 4-hydroxybenzoic acid (7.16 mg/mL), cinnamic acid (4.96 mg/mL), and ferulic acid (6.34 mg/mL), while the methanol extract (Fig. 1B) had catechol (13.05 mg/mL), ferulic acid (11.87 mg/mL), chlorogenic acid (10.33 mg/mL), and cinnamic acid (7.65 mg/mL). In the S. terebinthifolius wood extract (Fig. 1C), the phenolic compounds ferulic acid (14.81 mg/mL), caffeic acid (5.61 mg/mL), gallic acid (5.07 mg/mL), and chlorogenic acid (4.80 mg/mL) were abundant. In the methanol extract from S. babylonica leaves, (Fig. 1D) the phenolic compounds cinnamic acid (11.36 mg/mL), protocatechuic acid (10.33 mg/mL), ferulic acid (8.12 mg/mL), pyrogallol (8.05 mg/mL), and salicylic acid (6.44 mg/mL) were abundant.
In the current study, the n-hexane extract of M. grandiflora leaves, which was analyzed by GC–MS had the following main compounds: palmitic acid, oleic acid, undecane, palmitoleic acid, (1-propyloctyl) benzene, (1-methyldecyl)-benzene, (1-ethylnonyl) benzene, (1-propylnonyl) benzene, stearic acid, (1-pentylhexyl) benzene, (1-ethyldecyl) benzene, and linoleic acid at 7.28%, 7.22%, 5.37%, 4.66%, 4.63%, 4.21%, 3.88%, 3.86%, 3.46%, 3.36%, 3.3%, and 3%, respectively63.
Insecticidal effect of the tested extracts against S. littoralis stages
The insecticidal effects of the tested extracts were evaluated at different stages in S. littoralis. The toxic effects are shown as LC50s values in Table 2. In general, the toxicity of the tested materials increased with time after treatment. Moreover, the instar larvae were the most sensitive stage to all tested materials.
Ovicidal effect
The three extracts of M. grandiflora did not show any toxicity against S. littoralis eggs, but those of S. terebinthifolius and S. babylonica showed toxic effects. The highly toxic effect was shown by novaluron on eggs (LC50 = 0.62 mg/L). The S. terebinthifolius extract also exhibited high toxicity against S. littoralis eggs (LC50 = 0.94 mg/L) (Table 2).
Larvicidal effect
S. terebinthifolius and S. babylonica showed toxic effects against second and fourth larvae after 48 and 96 h. Novaluron showed high toxicity against second larvae after 96 h (LC50 = 0.55 mg/L). Among the tested extracts, that of S. terebinthifolius had high toxic effect against second larvae after 96 h (LC50 = 0.89 mg/L). Compared with novaluron (positive control), the S. terebinthifolius wood methanol extract exhibited high toxicity against S. littoralis at different stages (Table 2).
As the three extracts of M. grandiflora did not show any toxicity against S. littoralis stages, the water extract was only used in subsequent behavioral, biological, and semi-field experiments to investigate whether it had any other effects on S. littoralis. Moreover, its use in IPM programs with different modes of action apart from toxicity was also tested.
Repellent effects of the tested extracts
Phytophagous insects such as S. littoralis usually visit plants for either feeding or laying eggs. Identification of effective repellent or attractant agents for the early detection and suppression of S. littoralis populations is critical for managing this pest and reducing crop loss.
Feeding deterrence activity
The tested extracts were investigated for their feeding deterrent or attractant activity against second and fourth larvae of S. littoralis. All the tested materials showed feeding deterrence (FD %) values above the negative control, except for M. grandiflora extract, which was a feeding attractant for second and fourth larvae (Fig. 2). The fourth larvae were generally more affected by the tested compounds than the second larvae. Novaluron had a higher feeding repellent activity against S. littoralis larvae than all tested extracts. S. terebinthifolius wood methanol extract had a significantly higher feeding deterrence activity than the other extracts (FD % = 21.9% and 18.4% for fourth and second larvae, respectively, at 5 mg/mL). In contrast, M. grandiflora extract showed an attractant effect that decreased with increasing concentration (FD% = − 9.6% and − 6.6% for fourth and second larvae, respectively, at 10 mg/L).
Anti-oviposition activity
Insect oviposition is an important step in reproduction and in determining the size of a population. Therefore, deterrence of oviposition by a pest insect can decrease population size and assist in its management. The oviposition behavior of some phytophagous insects is altered by volatile products of host and non-host species. The deterrent or attractant activity of the tested materials against oviposition is shown as the repellent index (RI%) in Fig. 2. All tested compounds showed anti-oviposition activity, except for the M. grandiflora leaf water extract, which had RI% = − 8%, -6.7% and -2.8% at 1, 5 and 10 mg/L, respectively.
The impact of the tested compounds on some biological aspects
The results on pupation, adult emergence, fecundity (mean number of eggs/female), and hatchability (%) of the resulting eggs of the treated S. littoralis larvae are shown in Table 3. Adult growth disruption and abnormalities are shown in Fig. 3 (A–G). All tested materials affected the assessed biological aspects at all concentrations, except for the M. grandiflora extract, which affected larvae only at the highest concentration (10 mg/L). The biological effects of S. terebinthifolius were significantly reducing the percentages of pupation, adult emergence, hatchability, and fecundity (60.2%, 56.7%, 35.3%, and 105.4% eggs/female, respectively, at 5 mg/L).
Malformations of S. littoralis adults affected by applications on larval stage. Control: normal adult (A). Adults resulting from larvae treated with 1 and 2 mg/L novaluron (B & C), with short, undeveloped legs and wings. Adults resulting from larvae treated with 2 and 5 mg/L S. terebinthifolius (D), which were unable to remove the old exoskeleton (exuvium) and (E) with abnormal and conjoined wings. Adults resulting from larvae treated with 2 and 5 mg/L S. babylonica (F), with head and mouthparts not completely molted, (G) with shrunken, folded, and undeveloped wings and preserved pupal head and mouthparts.
Alpha-amylase and total protease activities of S. littoralis fourth instar larvae
The significant inhibitory effects of LC50 S. terebinthifolius, S. babylonica, and novaluron on α-amylase and total proteases were determined in vitro (Fig. 4). Novaluron had the highest inhibition effect, with the activities of α-amylase and total proteases reduced to 1.16 and 0.52 ΔOD/mg protein/min, respectively, followed by the S. terebinthifolius extract, with activities reduced to 1.47 and 0.65 ΔOD/mg protein/min, respectively (compared with 2.34 and 1.05 ΔOD/mg protein/min in the control, respectively). The S. terebinthifolius extract reduced the activity of S. littoralis digestive enzymes.
Residual toxicity of tested compounds against S. littoralis field strain
A semi-field experiment was conducted to evaluate the residual efficacy of M. grandiflora, S. terebinthifolius, S. babylonica, and novaluron against the fourth S. littoralis larvae from the field strain. The highest mortality percentages of S. littoralis larvae were 100%, 100%, 95%, and 70% after 2 h of application with novaluron, S. terebinthifolius, S. babylonica, and M. grandiflora, respectively. The mortality percentages decreased gradually over time to become 40.0%, 20.0%, 10.0%, and 0%, respectively, after 7 days of spraying at the field rate (60 mL/100 L water). While, the mortality percentages were 30%, 15%, 5%, and 0% at the half field rate (30 mL/100 L water) (Table 4).
Discussion
Insect pest management has always been and will remain a constant challenge for agricultural researchers and producers alike. There is an urgent need to replace pesticides with alternative control methods that are effective, inexpensive, and environmentally-friendly, therefore plant-derived products have received much attention in recent years due to drawbacks associated with unwise use of synthetic insecticides8.
Novaluron, which had already been shown to be highly toxic against S. littoralis eggs64, also showed a great ovicidal activity against S. littoralis eggs compared to the control. The strong insecticidal activity of S. terebinthifoiuls against S. littoralis has been previously reported40, as well as against Anopheles gambiae, A. arabiensis, and Culex quinquefasciatus37. S. terebinthifolius also showed high toxicity against two whitefly species, Bemisia tabaci, and Trialeurodes ricini39, Aphis nerii Boyer de Fonscolombe65 and Plutella xylostella (Lepidoptera: Plutellidae)66.
In contrast, M. grandiflora extract was herein found to have no toxic effect on S. littoralis stages, similar to the findings for Magnolia citrata essential oil, which had weak insecticidal activity against S. littoralis larvae compared with the positive control permethrin38. These results also agree with those of Vásquez-Morales and Flores-Estévez67, who found that the seed and sarcotesta extracts of Magnolia schiedeana (Magnoliaceae) only showed insecticidal activity against Anastrepha ludens adults, whereas the extracts of leaves, flowers, bark, and follicles showed no significant biological activity. Moreover, Ali et al.68 observed that the leaf, flower, and seed essential oils of M. grandiflora at the highest dose of 125 mg/L resulted in only 20%, 0%, and 50% mortality of Aedes aegypti, respectively.
Furthermore, M. citrata oil has been reported to exhibit weak toxicity against first instar larvae and adult female A. aegypti69. Methanol extract of S. babylonica leaves showed strong toxicity against S. littoralis stages, which is consistent with the results of Hasaballah et al.70, who showed that the toxic effects of methanol and ethanol extracts of Salix safsaf could compete with the synthetic insecticide deltamethrin as a natural insecticide in the control of the housefly Musca domestica. Added to the antifungal effect of salicin, the major compound of willow extract, other metabolites may increase the potency of willow extracts71.
The attractant effect of Magnolia was first reported by Pavela38, who reported the attractant activity of M. citrata essential oil on S. littoralis. The oil from M. citrata leaves has a moderately strong attractant effect on the sterile male medfly Ceratitis capitata69. In contrast, essential oils from five different parts of M. grandiflora showed biting deterrence against Ae. aegypti68. Schinus terebinthifolius produced a significantly high feeding deterrence activity in second and fourth instar larvae. The ethanolic extracts of S. terebinthifolius were effective antifeedants for third instar larvae of Plutella xylostella (Lepidoptera: Plutellidae)72. Treatment with S. terebinthifolius extract prolonged the larval phase, allowing a higher food intake before reaching the pupal stage; this probably resulted from the effect of one or several deterrent factors, resulting in nutritional imbalance and damage to the insect life cycle73. Willow oil generated maximum attraction (28.79%) in Bemisia tabaci, contrary to the repellent effect of willow oil on S. littoralis74.
Novaluron at 2 mg/L had the highest RI% (68.8%), followed by S. terebinthifolius extract at 5 mg/L (30.9%). Aly and Ali64 showed that novaluron had the highest oviposition deterrence value (23%) in S. littoralis females. Ethanolic extracts of S. terebinthifolius suppressed oviposition in P. xylostella adults72.
Novaluron also causes S. littoralis growth disruption and abnormalities, selectively targeting immature insect stages by inhibiting chitin formation and causing abnormal endocuticular deposition abortive molting75. Novaluron had the highest sterility value (68.9%) for S. littoralis64. The ethanolic extracts of S. terebinthifolius negatively affected all the evaluated biological parameters of P. xylostella, increasing the duration of the larval stage, which led to reduced pupal mass and oviposition period76. Treatment with S. terebinthifolius extract resulted in the lowest pupal mass and a greatest prolongation of the larval stage compared with other treatments73. Phytochemical studies on S. terebinthifolius have isolated tannins that inactivate digestive enzymes of insects, hampering their digestion, which in turn affects their growth and survival73,77. The S. terebinthifolius extract possibly reduced pupal survival by impairing their ability to feed as a result of larval sensitivity to the secondary compounds present in the plant extracts78.
It was previously explained that tannins act by inactivating the digestive enzymes in the leaves of S. terebinthifolius, generating a tannin-protein complex that is difficult to digest and affects the growth and survival of insects73. A reduction in food digestibility was observed in the tannin fractions of S. terebinthifolius by Spodoptera frugiperda larvae (Smith 1797) (Lepidoptera: Noctuidae)79.
Various studies have demonstrated that plant phenolic metabolites negatively affect insect feeding behavior, growth, development, and reproduction, and they may have lethal effects on specific insects80,81. Furthermore, α-amylase and protease activities of S. littoralis were decreased in the midgut after feeding on an artificial diet containing caffeic acid82. Ferulic acid, the most abundant phenolic compound from extracts of M. grandiflora leaves and S. terebinthifolius wood decreased adult emergence, delayed the developmental period and reduced the nutritional indices of Spodoptera litura (Fabricius) larvae83. The larval growth, survival, adult emergence, pupal weight, and different nutritional indices of S. litura (Fab.) were adversely affected by the various concentrations of purified phenolic compounds like chlorogenic acid84. The effect of some phenolic acids (chlorogenic, caffeic, and ferulic) on the growth, development and midgut enzyme activities of S. litura larvae was studied through diet incorporation assay and can be utilized in insect control programs85. The two cinnamic acid derivatives were found to show higher levels of insecticidal, larvicidal and larval growth inhibition activities against Tribolium castaneum86. The lethal effect of chlorogenic acid on Mythimna separata (Walker) (Lepidoptera: Noctuidae) and that a sublethal concentration harmed larval growth and development87.
Conclusion
The extract of S. terebinthifolius can be used to efficiently manage S. littoralis, as they had various modes of action, such as toxicity, repellence, growth regulation, reduction of fecundity, and inhibition of digestive enzymes activity. Therefore, they can be considered suitable alternatives and can be incorporated into IPM systems for S. littoralis.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Garrido-Jurado, I., Montes-Moreno, D., Sanz-Barrionuevo, P. & Quesada-Moraga, E. Delving into the causes and effects of entomopathogenic endophytic metarhizium brunneum foliar application-related mortality in Spodoptera littoralis Larvae. Insects 11, 429. https://doi.org/10.3390/insects11070429 (2020).
Mosallanejad, H. & Smagghe, G. Biochemical mechanisms of methoxyfenozide resistance in the cotton leafworm Spodoptera littoralis. Pest Manag. Sci. 65, 732–736. https://doi.org/10.1002/ps.1753 (2009).
Jepson, P. C., Murray, K., Bach, O., Bonilla, M. A. & Neumeister, L. Selection of pesticides to reduce human and environmental health risks: A global guideline and minimum pesticides list. The Lancet Planetary Health 4, e56–e63. https://doi.org/10.1016/S2542-5196(19)30266-9 (2020).
Lengai, G. M. W., Muthomi, J. W. & Mbega, E. R. Phytochemical activity and role of botanical pesticides in pest management for sustainable agricultural crop production. Sci. Afr. 7, e00239. https://doi.org/10.1016/j.sciaf.2019.e00239 (2020).
Rani, L. et al. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 283, 124657. https://doi.org/10.1016/j.jclepro.2020.124657 (2021).
Samsidar, A., Siddiquee, S. & Shaarani, S. M. A review of extraction, analytical and advanced methods for determination of pesticides in environment and foodstuffs. Trends Food Sci. Technol. 71, 188–201. https://doi.org/10.1016/j.tifs.2017.11.011 (2018).
van den Berg, H. et al. Pesticide lifecycle management in agriculture and public health: Where are the gaps?. Sci. Total Environ. 742, 140598. https://doi.org/10.1016/j.scitotenv.2020.140598 (2020).
Khan, S. et al. Insecticidal activity of plant-derived extracts against different economically important pest insects. Phytoparasitica 45, 113–124. https://doi.org/10.1007/s12600-017-0569-y (2017).
Tawfeek, M. E., Ali, H. M., Akrami, M. & Salem, M. Z. M. Potential Insecticidal Activity of Four Essential Oils against the Rice Weevil, Sitophilus oryzae (L.)(Coleoptera: Curculionidae). BioResources 16, 7767–7783. https://doi.org/10.15376/biores.16.4.7767-7783 (2021).
Mansour, S., Bakr, R., Mohamed, R. & Hasaneen, N. Larvicidal activity of some botanical extracts, commercial insecticides and their binary mixtures against the housefly, Musca domestica L.. The Open Toxinol. J. 3, 1–13. https://doi.org/10.2174/1875414701104010001 (2011).
Singh, A., Bhardwaj, R. & Singh, I. K. in Biofertilizers for Sustainable Agriculture and Environment (eds Bhoopander Giri, Ram Prasad, Qiang-Sheng Wu, & Ajit Varma) 413–433 (Springer, 2019).
Souto, A. L. et al. Plant-derived pesticides as an alternative to pest management and sustainable agricultural production: Prospects, applications and challenges. Molecules 26, 4835. https://doi.org/10.3390/molecules26164835 (2021).
Jeon, J.-H., Kim, Y.-K., Lee, S.-G., Lee, G.-H. & Lee, H.-S. Insecticidal activities of a Diospyros kaki root-isolated constituent and its derivatives against Nilaparvata lugens and Laodelphax striatellus. J. Asia-Pacific Entomol. 14, 449–453. https://doi.org/10.1016/j.aspen.2011.07.005 (2011).
Moustafa, M. A. M. et al. Insecticidal activity of lemongrass essential oil as an eco-friendly agent against the black Cutworm Agrotis ipsilon (Lepidoptera: Noctuidae). Insects 12, 737. https://doi.org/10.3390/insects12080737 (2021).
Ho, K.-Y., Tsai, C.-C., Chen, C.-P., Huang, J.-S. & Lin, C.-C. Antimicrobial activity of honokiol and magnolol isolated from Magnolia officinalis. Phytother. Res. 15, 139–141. https://doi.org/10.1002/ptr.736 (2001).
Sarrica, A., Kirika, N., Romeo, M., Salmona, M. & Diomede, L. Safety and toxicology of Magnolol and Honokiol. Planta Med 84, 1151–1164. https://doi.org/10.1055/a-0642-1966 (2018).
Zhao, X. et al. Extracts of Magnolia species-induced prevention of diabetic complications: A brief review. Int. J. Mol. Sci. 17, 1629. https://doi.org/10.3390/ijms17101629 (2016).
Kelm, M. A., Nair, M. G. & Schutzki, R. A. Mosquitocidal compounds from Magnolia salicifolia. Int. J. Pharmacogn. 35, 84–90. https://doi.org/10.1076/phbi.35.2.84.13279 (1997).
Mahdi, J. G. Medicinal potential of willow: A chemical perspective of aspirin discovery. J. Saudi Chem. Soc. 14, 317–322. https://doi.org/10.1016/j.jscs.2010.04.010 (2010).
Noleto-Dias, C., Ward, J. L., Bellisai, A., Lomax, C. & Beale, M. H. Salicin-7-sulfate: A new salicinoid from willow and implications for herbal medicine. Fitoterapia 127, 166–172. https://doi.org/10.1016/j.fitote.2018.02.009 (2018).
El-Sayed, M. M., El-Hashash, M. M., Mohamed, H. R. & Abdel-Lateef, E.E.-S. Phytochemical investigation and in vitro antioxidant activity of different leaf extracts of Salix mucronata Thunb. J. Appl. Pharm. Sci 5, 080–085. https://doi.org/10.7324/JAPS.2015.501213 (2015).
El-Shazly, A., El-Sayed, A. & Fikrey, E. Bioactive secondary metabolites from Salix tetrasperma Roxb. Z Naturforsch C J Biosci 67, 353–359. https://doi.org/10.5560/znc.2012.67c0353 (2012).
Khan, M. I. R., Fatma, M., Per, T. S., Anjum, N. A. & Khan, N. A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 6, 462. https://doi.org/10.3389/fpls.2015.00462 (2015).
Ruuhola, T., Julkunen-Tiitto, R. & Vainiotalo, P. In Vitro degradation of willow Salicylates. J. Chem. Ecol. 29, 1083–1097. https://doi.org/10.1023/A:1023821304656 (2003).
Shara, M. & Stohs, S. J. Efficacy and safety of White Willow Bark (Salix alba) extracts. Phytother. Res. 29, 1112–1116. https://doi.org/10.1002/ptr.5377 (2015).
Alamgir, A. Therapeutic Use of Medicinal Plants and their Extracts (Springer, 2017).
Wiesneth, S., Aas, G., Heilmann, J. & Jürgenliemk, G. Investigation of the flavan-3-ol patterns in willow species during one growing-season. Phytochemistry 145, 26–39. https://doi.org/10.1016/j.phytochem.2017.10.001 (2018).
Mutlu-Durak, H. & Yildiz Kutman, B. Seed treatment with biostimulants extracted from Weeping Willow (Salix babylonica) enhances early maize growth. Plants 10, 1449. https://doi.org/10.3390/plants10071449 (2021).
Orellana, C. Aspirin protects against cancer of the upper aerodigestive tract. Lancet Oncol. 4, 200. https://doi.org/10.1016/S1470-2045(03)01054-4 (2003).
Farag, H. R. M., Abdou, Z. A., Salama, D. A., Ibrahim, M. A. R. & Sror, H. A. M. Effect of neem and willow aqueous extracts on fusarium wilt disease in tomato seedlings: Induction of antioxidant defensive enzymes. Ann. Agric. Sci. 56, 1–7. https://doi.org/10.1016/j.aoas.2011.05.007 (2011).
Deniau, M. et al. Willow extract (Salix cortex), a basic substance of agronomical interests. Int. J. Bio-resource Stress Manag. 10, 408–418. https://doi.org/10.23910/IJBSM/2019.10.4.2009 (2019).
Marchand, P. A. Basic substances under EC 1107/2009 phytochemical regulation: Experience with non-biocide and food products as biorationals. J. Plant Protect. Res. 56, 312–318 (2016).
Cavalher-Machado, S. C. et al. The anti-allergic activity of the acetate fraction of Schinus terebinthifolius leaves in IgE induced mice paw edema and pleurisy. Int. Immunopharmacol. 8, 1552–1560. https://doi.org/10.1016/j.intimp.2008.06.012 (2008).
de Oliveira, V. S. et al. Aroeira fruit (Schinus terebinthifolius Raddi) as a natural antioxidant: Chemical constituents, bioactive compounds and in vitro and in vivo antioxidant capacity. Food Chem. 315, 126274. https://doi.org/10.1016/j.foodchem.2020.126274 (2020).
Locali-Pereira, A. R., Lopes, N. A. & Nicoletti, V. R. Pink Pepper (Schinus terebinthifolius Raddi) from extracts to application: Truths about a fake pepper. Food Rev. Int. 5, 1–30. https://doi.org/10.1080/87559129.2022.2062767 (2022).
Silva, A. G. et al. The essential oil of Brazilian pepper, Schinus terebinthifolia Raddi in larval control of Stegomyia aegypti (Linnaeus, 1762). Parasit. Vectors 3, 79. https://doi.org/10.1186/1756-3305-3-79 (2010).
Kweka, E. J., Nyindo, M., Mosha, F. & Silva, A. G. Insecticidal activity of the essential oil from fruits and seeds of Schinus terebinthifolia Raddi against African malaria vectors. Parasit. Vectors 4, 129. https://doi.org/10.1186/1756-3305-4-129 (2011).
Pavela, R. Acute, synergistic and antagonistic effects of some aromatic compounds on the Spodoptera littoralis Boisd. (Lep., Noctuidae) larvae. Ind. Crops Prod. 60, 247–258. https://doi.org/10.1016/j.indcrop.2014.06.030 (2014).
Hussein, H. S., Salem, M. Z. M. & Soliman, A. M. Repellent, attractive, and insecticidal effects of essential oils from Schinus terebinthifolius fruits and Corymbia citriodora leaves on two whitefly species, Bemisia tabaci, and Trialeurodes ricini. Sci. Hortic. 216, 111–119. https://doi.org/10.1016/j.scienta.2017.01.004 (2017).
Ennigrou, A., Casabianca, H., Laarif, A., Hanchi, B. & Hosni, K. Maturation-related changes in phytochemicals and biological activities of the Brazilian pepper tree (Schinus terebinthifolius Raddi) fruits. S. Afr. J. Bot. 108, 407–415. https://doi.org/10.1016/j.sajb.2016.09.005 (2017).
Gijswijt, M. J., Deul, D. H. & de Jong, B. J. Inhibition of chitin synthesis by benzoyl-phenylurea insecticides, III. Similarity in action in Pieris brassicae (L.) with Polyoxin D. Pest. Biochem. Physiol. 12, 87–94. https://doi.org/10.1016/0048-3575(79)90098-1 (1979).
Hajjar, N. P. & Casida, J. E. Insecticidal benzoylphenyl ureas: Structure-activity relationships as chitin synthesis inhibitors. Science 200, 1499–1500. https://doi.org/10.1126/science.200.4349.1499 (1978).
Post, L. C., de Jong, B. J. & Vincent, W. R. 1-(2,6-disubstituted benzoyl)-3-phenylurea insecticides: Inhibitors of chitin synthesis. Pestic. Biochem. Physiol. 4, 473–483. https://doi.org/10.1016/0048-3575(74)90072-8 (1974).
Cutler, G. C. & Scott-Dupree, C. D. Novaluron: Prospects and limitations in insect pest management. Pest Technology 1, 38–46 (2007).
Kostyukovsky, M. & Trostanetsky, A. The effect of a new chitin synthesis inhibitor, novaluron, on various developmental stages of Tribolium castaneum (Herbst). J. Stored Prod. Res. 42, 136–148. https://doi.org/10.1016/j.jspr.2004.12.003 (2006).
Merzendorfer, H. Chitin synthesis inhibitors: Old molecules and new developments. Insect Sci. 20, 121–138. https://doi.org/10.1111/j.1744-7917.2012.01535.x (2013).
Joseph, V. S. Ingestion of novaluron elicits transovarial activity in Stephanitis pyrioides (Hemiptera: Tingidae). Insects 11, 216. https://doi.org/10.3390/insects11040216 (2020).
Soares, W. S. et al. Physiological selectivity of insecticides from different chemical groups and cuticle thickness of Protonectarina sylveirae (Saussure) and Brachygastra lecheguana (Latreille). Sociobiology 66, 358–366. https://doi.org/10.13102/sociobiology.v66i2.3478 (2019).
Abdelgaleil, S. A. M., Abou-Taleb, H. K., Al-Nagar, N. M. A. & Shawir, M. S. Antifeedant, growth regulatory and biochemical effects of terpenes and phenylpropenes on Spodoptera littoralis Boisduval. Int. J. Trop. Insect Sci. 40, 423–433. https://doi.org/10.1007/s42690-019-00093-8 (2020).
Hemmati, S. A., Shishehbor, P. & Stelinski, L. L. Life table parameters and digestive enzyme activity of Spodoptera littoralis (Boisd)(Lepidoptera: Noctuidae) on selected legume cultivars. Insects 13, 661. https://doi.org/10.3390/insects13070661 (2022).
Seidel, V. in Natural Products Isolation (eds Satyajit D. Sarker & Lutfun Nahar) 27–41 (Humana Press, 2012).
Hassan, H. S. et al. Natural plant extracts and microbial antagonists to control fungal pathogens and improve the productivity of Zucchini (Cucurbita pepo L.) in vitro and in greenhouse. Horticulturae 7, 470. https://doi.org/10.3390/horticulturae7110470 (2021).
Stefanazzi, N., Stadler, T. & Ferrero, A. Composition and toxic, repellent and feeding deterrent activity of essential oils against the stored-grain pests Tribolium castaneum (Coleoptera: Tenebrionidae) and Sitophilus oryzae (Coleoptera: Curculionidae). Pest Manag. Sci. 67, 639–646. https://doi.org/10.1002/ps.2102 (2011).
Pascual-Villalobos, M. J. & Robledo, A. Screening for anti-insect activity in Mediterranean plants. Ind. Crops Prod. 8, 183–194. https://doi.org/10.1016/S0926-6690(98)00002-8 (1998).
Lowry, O. H. Protein measurement with the folin phenol reagent. J. Biol. Chem. 193, 265–275 (1951).
Kaufman, R. A. & Tietz, N. W. Recent advances in measurement of amylase activity–a comparative study. Clin. Chem. 26, 846–853. https://doi.org/10.1093/clinchem/26.7.846 (1980).
Loseva, O. et al. Changes in protease activity and Cry3Aa toxin binding in the Colorado potato beetle: Implications for insect resistance to Bacillus thuringiensis toxins. Insect Biochem. Mol. Biol. 32, 567–577. https://doi.org/10.1016/S0965-1748(01)00137-0 (2002).
Mohan, M. & Gujar, G. T. Characterization and comparison of midgut proteases of Bacillus thuringiensis susceptible and resistant diamondback moth (Plutellidae: Lepidoptera). J. Invertebr. Pathol. 82, 1–11. https://doi.org/10.1016/S0022-2011(02)00194-5 (2003).
Raslan, S. A. A. Preliminary report on initial and residual mortality of the natural product, spinosad for controlling cotton leafworm egg masses in 2002 cotton season at Sharkia governorate, Egypt. 2nd International Conference, Plant Protection Research Institute, Cairo, Egypt, 21–24 December, 2002. Volume 1, 635–637. (2002).
El-Sheikh, E.-S.A. & Aamir, M. M. Comparative effectiveness and field persistence of insect growth regulators on a field strain of the cotton leafworm, Spodoptera littoralis, Boisd (Lepidoptera: Noctuidae). Crop Prot. 30, 645–650. https://doi.org/10.1016/j.cropro.2011.02.009 (2011).
Biostat. Biostat ver. (2.1). Computer program for probit analysis. 2011. (2011).
SAS. Statistical Analysis System (SAS). SAS/STAT User’s Guide. Version 8, 6th Edition, SAS institute Inc., Cary, North Carolina, U.S.A. 2002. (2002).
Salem, M. Z. M., Abo Elgat, W. A. A., Taha, A. S., Fares, Y. G. D. & Ali, H. M. Impact of three natural oily extracts as pulp additives on the mechanical, optical, and antifungal properties of paper sheets made from Eucalyptus camaldulensis and Meryta sinclairii Wood Branches. Materials 13, 1292. https://doi.org/10.3390/ma13061292 (2020).
Aly, M. F. K. & Ali, A. M. Impact of some essential plant oils and insect growth regulators on immature stages of Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae) in Egypt. J. Plant Prot. Pathol. 8, 561–570. https://doi.org/10.21608/jppp.2017.46852 (2017).
Hussein, H. S., Tawfeek, M. E. & Abdelgaleil, S. A. M. Chemical composition, aphicidal and antiacetylcholinesterase activities of essential oils against Aphis nerii Boyer de Fonscolombe (Hemiptera: Aphididae). J. Asia-Pacific Entomol. 24, 259–265. https://doi.org/10.1016/j.aspen.2021.02.001 (2021).
Silva, P. R. C. et al. Schinus terebinthifolia leaf extract is a larvicidal, pupicidal, and oviposition deterring agent against Plutella xylostella. S. Afr. J. Bot. 127, 124–128. https://doi.org/10.1016/j.sajb.2019.08.054 (2019).
Vásquez-Morales, S. G. & Flores-Estévez, N. Bioprospecting of botanical insecticides: The case of ethanol extracts of Magnolia schiedeana Schltl. applied to a Tephritid, fruit fly Anastrepha ludens Loew. J. Entomol. Zool. Stud. 3, 71 (2015).
Ali, A. et al. Insecticidal and biting deterrent activities of Magnolia grandiflora essential oils and selected pure compounds against Aedes aegypti. Molecules 25, 1359. https://doi.org/10.3390/molecules25061359 (2020).
Luu-Dam, N. A., Tabanca, N., Estep, A. S., Nguyen, D. H. & Kendra, P. E. Insecticidal and attractant activities of Magnolia citrata leaf essential oil against two major pests from Diptera: Aedes aegypti (Culicidae) and Ceratitis capitata (Tephritidae). Molecules 26, 2311. https://doi.org/10.3390/molecules26082311 (2021).
Hasaballah, A., Selim, T., Tanani, M. & Nasr, E. Lethality and vitality efficiency of different extracts of Salix safsaf leaves against the house fly, Musca domestica L.(Diptera: Muscidae). Afr. Entomol. 29, 479–490. https://doi.org/10.4001/003.029.0479 (2021).
El-Shemy, H. A., Aboul-Enein, A. M., Aboul-Enein, K. M. & Fujita, K. Willow leaves’ extracts contain anti-tumor agents effective against three cell types. PLOS ONE 2, e178. https://doi.org/10.1371/journal.pone.0000178 (2007).
Couto, I. et al. Botanical extracts of the Brazilian savannah affect feeding and oviposition of Plutella xylostella (Linnaeus, 1758)(Lepidoptera: Plutellidae). J. Agric. Sci. (Toronto) 11, 322–333. https://doi.org/10.1007/s10343-020-00520-8 (2019).
Mello, M. O. & Silva-Filho, M. C. Plant-insect interactions: An evolutionary arms race between two distinct defense mechanisms. Braz. J. Plant. Physiol. 14, 71–81. https://doi.org/10.1590/S1677-04202002000200001 (2002).
El-Meniawi, F. A., El-Gayar, F. H., Rawash, I. A. & Hussein, H. S. The olfaction response of the cotton whitefly, Bemisia tabaci Gennadius (Homoptera: Aleyrodidae) to ten natural plant oils. Egy. J. Plant Pro Res. 1, 45–57 (2013).
Rouabhi, R., Djebar, H. & Djebar, M. Toxic effects of combined molecule from novaluron and diflubenzuron on paramecium caudatum. Am-Euras. J. Toxicol. Sci 1, 74–80 (2009).
Couto, I. F. S. et al. Changes in the biological characteristics of Plutella xylostella using ethanolic plant extracts. Gesunde Pflanzen 72, 383–391. https://doi.org/10.1007/s10343-020-00520-8 (2020).
Procópio, T. F. et al. Schinus terebinthifolius leaf extract causes midgut damage, interfering with survival and development of Aedes aegypti larvae. PLOS ONE 10, e0126612. https://doi.org/10.1371/journal.pone.0126612 (2015).
Sapindal, E., Ong, K. H. & King, P. J. H. Efficacy of Azadirachta excelsa vinegar against Plutella xylostella. Int. J. Pest Manag. 64, 39–44. https://doi.org/10.1080/09670874.2017.1293866 (2018).
Tirelli, A. A. et al. Efeito de frações tânicas sobre parâmetros biológicos e nutricionais de Spodoptera frugiperda (Lepidoptera: Noctuidae). Ciência e Agrotecnologia 34, 1417–1424. https://doi.org/10.1590/S1413-70542010000600009 (2010).
Divekar, P. A. et al. Plant secondary metabolites as defense tools against herbivores for sustainable crop protection. Int. J. Mol. Sci. 23, 2690. https://doi.org/10.3390/ijms23052690 (2022).
Mattar, V. T., Borioni, J. L., Hollmann, A. & Rodriguez, S. A. Insecticidal activity of the essential oil of Schinus areira against Rhipibruchus picturatus (F.) (Coleoptera: Bruchinae), and its inhibitory effects on acetylcholinesterase. Pest. Biochem. Physiol. 185, 105134. https://doi.org/10.1016/j.pestbp.2022.105134 (2022).
Nakhaie, B. M., Mikani, A. & Moharramipour, S. Effect of caffeic acid on feeding, α-amylase and protease activities and allatostatin—A content of Egyptian cotton leafworm, Spodoptera littoralis (Lepidoptera: Noctuidae). J. Pest. Sci. 43, 73–78. https://doi.org/10.1584/jpestics.D17-086 (2018).
Punia, A., Chauhan, N., Singh, R., Kaur, S. & Sohal, S. Growth disruptive effects of ferulic acid against Spodoptera litura (Fabricius) and its parasitoid Bracon hebetor (Say). Allelopath. J. 55, 79–92. https://doi.org/10.26651/allelo.j/2022-55-1-1372 (2022).
Gautam, S., Samiksha, C. S. S., Arora, S. & Sohal, S. K. Toxic effects of purified phenolic compounds from Acacia nilotica against common cutworm. Toxicon 203, 22–29. https://doi.org/10.1016/j.toxicon.2021.09.017 (2021).
Su, Q. et al. Effect of plant secondary metabolites on common cutworm, Spodoptera litura (Lepidoptera: Noctuidae). Entomol. Res. 48, 18–26. https://doi.org/10.1111/1748-5967.12238 (2018).
Buxton, T. et al. Insecticidal activities of cinnamic acid esters isolated from Ocimum gratissimum L. and Vitellaria paradoxa Gaertn leaves against Tribolium castaneum Hebst (Coleoptera: Tenebrionidae). Pest Manag. Sci. 76, 257–267. https://doi.org/10.1002/ps.5509 (2020).
Lin, D.-J. et al. The insecticidal effect of the botanical insecticide chlorogenic acid on Mythimna separata (Walker) is related to changes in MsCYP450 gene expression. Front. Plant Sci. 13, 1015095. https://doi.org/10.3389/fpls.2022 (2022).
Acknowledgements
The authors are grateful to Dr. Mamoun S. M. Abd El-Kareem, Atomic and Molecular Physics Unit, Experimental Nuclear Physics Department, Nuclear Research Centre, Egyptian Atomic Energy Authority, Inshas, Cairo for his assistance in the HPLC measurements.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors have equal contributions in collecting and writing the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hussein, H.S., Salem, M.Z.M., Soliman, A.M. et al. Comparative study of three plant-derived extracts as new management strategies against Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae). Sci Rep 13, 3542 (2023). https://doi.org/10.1038/s41598-023-30588-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-30588-x
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.