Abstract
Both short sleep duration (SSD) and long sleep duration (LSD) are associated with an increased risk of morbidity and mortality. Here, we aimed to assess the prevalence of sleep duration disturbances among adults in association with demographic, medication use, personal habits, and chronic diseases, while also considering the impact of hypnotic drug use. We performed a cross-sectional study of 9991 adult participants of the Rafsanjan Cohort Study (RCS), as part of the Prospective epidemiological research studies in Iran (PERSIAN). Multivariate logistic regression analyses were conducted to assess the association between short (< 6 h) and long (> 9 h) sleep duration with demographic and lifestyle parameters and common non-communicable diseases. Additionally, we performed stratified analysis to investigate the association of sleep duration with the abovementioned factors and diseases, in groups with and without hypnotic drug use. We found higher odds of SSD significantly associated with age (P < 0.001), BMI (P < 0.001), physical activity (P < 0.001), and depression (P = 0.023). LSD displayed a positive association with the female sex (P < 0.001), opium consumption (P < 0.001), and history of MI (P = 0.045), and a reverse connection with education (P = 0.007), physical activity (P < 0.001) and alcohol consumption (P = 0.027). Stratifying for the hypnotic drug use, our sensitivity analyses indicated that in hypnotic drug users, education (P = 0.034) and physical activity (P < 0.001) were associated with LSD, in this group, significantly increased odds ratio of LSD were associated with opium consumption (P = 0.046) and thyroid dysfunction (P = 0.037). Our findings demonstrated the demographic and lifestyle factors and diseases associated with long and short sleep duration in the population of the RCS. Additionally, after stratifying for hypnotic drug use, our results indicated that some diseases are only associated with abnormal sleep duration upon using hypnotic drugs.
Similar content being viewed by others
Introduction
Sleep as a systematic biological rhythm-based behavior has a significant role in the revival of daily mental and physiological abilities. Normal sleep contributes significantly to the maintenance of psychological and physical health and recovery from illnesses1. The consensus published by the American Academy of Sleep Medicine (AASM) and Sleep Research Society (SRS) in 2015 defined normal sleep duration (NSD) as sleeping more than 6 and less than 9 h per night2. During the last half-century, the duration of night-time sleep has decreased by around 1.5–2 h per night, which points to a significant increase in chronic sleep deprivation among adults3,4. For example, about 30% of adults in the United States sleep less than 6 h at night and struggle with sleep insufficiency5.
Multiple published studies have suggested an association between abnormal sleep duration and increased risk of metabolic syndrome6,7, diabetes mellitus8, cancer9, stroke, cardiovascular diseases, and all-cause mortality10. The suggested underlying mechanism explained for this association is the critical role of enough sleep in energy homeostasis in the body, regulating the balance between energy intake and consumption11. According to previous studies, obesity is one of the adverse health consequences of sleep deprivation12. Another underlying mechanism is attributed to a hormonal disbalance in the body, such as the reported decreased serum level of adiponectin and leptin upon acute or chronic sleep deprivation13. Additionally, the fatigue caused by abnormal sleep duration resulted in more energy intake and increased eating times, concurrent with lower physical activity and energy consumption, which consequently leads to obesity, cardiovascular problems, and other related diseases14.
Despite statements supporting an adverse effect of abnormal sleep duration on cardiometabolic risk factors and diseases, some contradictory reports, especially on long sleep duration (LSD), did not find a significant association between abnormal sleep duration and cardiovascular and metabolic diseases15,16,17,18, which warrant further study on this subject.
Pharmacological agents and non-pharmacological agents are prescribed as treatment options for chronic and acute insomnia. While some pharmacological agents, specifically benzodiazepines (BDZ) and non-benzodiazepines (non-BDZ), are recommended by the US National Institute of Health for the management of acute insomnia, their administration for chronic insomnia is under debate due to their side effects, such as physical dependence and withdrawal symptoms, returning insomnia, and long-term safety issues19. Hypnotic drug use itself is suggested to be associated with cardiovascular diseases and all-cause mortality by some studies20,21,22. Therefore, it is necessary for studies that investigate the link between abnormal sleep duration with chronic diseases to consider the impact of hypnotic drugs in their analysis. This critical factor is not addressed correctly in most previous studies.
In the current study, we aimed to assess the abnormal sleep duration among adult participants of the Rafsanjan Cohort Study (RCS)23. In addition, we sought to determine the relationship between abnormal sleep duration with various non-communicable diseases, such as metabolic and cardiovascular problems, also demographic factors, and some lifestyle parameters such as physical activity and substance use (cigarette, alcohol, and opioid). The use of hypnotic treatments for abnormal sleep has been poorly studied regarding its impact on sleep duration-associated diseases and risk factors. To investigate how pharmaceutical treatment agents may impact sleep duration concerning its predisposing factors and its associated diseases, here we assessed these connections separately in individuals who used hypnotic drugs or not.
Methods
The current study was performed on 9991 participants of both genders aged 35–70 years old in the RCS23 which was a part of the Prospective Epidemiological Research Studies in Iran (PERSIAN)24. The RCS conducted in Rafsanjan city in the southeast of Iran was designed to recruit 10,000 participants from Rafsanjan’s urban and suburban areas. Recruitment was performed by a random selection approach via systematic clustering using household numbers. According to the PERSIAN Cohort Central Scientific Committee, the estimated sample size for RCS supports adequate statistical power23. Participation in the RCS was voluntary and upon signature of an informed consent form, and the confidentiality of the personal data of the participants was ensured by all necessary measures. Of all the participants, after excluding subjects with incomplete sleep habits questionnaire, 9981 entered our study. The protocol and questionnaires of this cross-sectional study were designed following the Persian cohort study protocols and under the supervision of the Iranian Ministry of Health and Medical Education (IMHME). In addition, they have been approved by the Ethics Committee of Rafsanjan University of Medical Sciences with the Ethical code of IR.RUMS.REC.1398.140, and all methods were carried out followed the relevant guidelines and regulations.
Data collection
Participants' demographic information was collected by a questionnaire including age, gender, wealth status index (WSI), education levels (The number of years the participant received education), etc. Other data collection included past medical history, anthropometry (height, waist circumference, hip circumference, waist circumference, weight, and BMI), physical activity (Metabolic equivalent of task: MET), medication use (past and present), and personal habits (smoking, opium, and alcohol consumption). All questionnaires prepared in the Farsi language were previously validated in the PERSIAN cohort study24,25.
MET is the daily physical activity of the participants, was weighted based on its relative metabolic cost, and MET-h/day for 24 h was derived in this way.
WSI was estimated by multiple correspondence analysis (MCA) of the economic variables. After this step, the subjects were categorized into four groups, including low class (≤ − 0.606), low-middle class (− 0.607–0.0349), middle-high class (0.035–1.169), and high class (≥1.170) based on the 25th, 50th and 90th percentiles.
Based on the Third Report of the National Cholesterol Education Program (NCEP-Adult Treatment Panel III), we defined dyslipidemia as LDL ≥ 130 mg/dL, or TC ≥ 200 mg/dL, or HDL ≤ 40 mg/dL in men, and 50 mg/dl in women or TG ≥ 150 mg/dL and or using lipid-lowering medications during the past 2 weeks26.
Sleep parameters assessment
Sleep habits of the population were assessed using several questions from the Pittsburgh questionnaire. In the present study, total sleep duration was a sum of sleep duration at night and daytime napping sleep hours. Entire sleep duration was classified into three groups: short sleep duration (SSD) (< 6 h), normal sleep duration (NSD) (6–9 h), and long sleep duration (LSD) (> 9 h)27. In the multivariable analysis, the 6–9 h category was selected as the reference. The frequency difference between the total number and some covariates was related to missing data.
Statistical analysis
Quantitative variables were described as mean ± standard deviation, and categorical variables as the frequency and percentage. Also, baseline characteristics and distribution of diseases of individuals were compared across the groups taking or non-taking hypnotic drugs by sleep duration using the chi-square test for categorical variables and the one-way ANOVA test for quantitative variables. The odds ratio (OR: with 95% CIs) of taking hypnotic drugs and non-taking hypnotic drugs based on sleep habits were evaluated by a multinomial logistic regression model and confounder's variables were identified using relevant epidemiological texts and based on subject matter knowledge. Potential confounding variables were sequentially entered into the model according to their hypothesized strengths of association with sleep duration. To reach this goal, confounding variables with a P-value < 0.25 were selected as confounders. All analyses were performed through State V.12. All P-values are two-sided, and P-values < 0.05 and 95% confidence intervals were considered statistically significant.
Ethical approval
The Ethics Committee of Rafsanjan University of Medical Sciences approved this study (Ethical codes: ID: IR.RUMS.REC. 1398.140). Written informed consent was obtained from the participants. The participant's data were kept confidential and only accessible to the study investigators.
Result
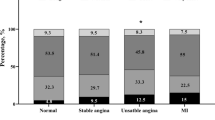
The demographic characteristics of the participants are presented in Table 1. Among 9981 participants, 1105 (11.07%) were hypnotic drug users (HDU), and 8876 (88.93%) were non-hypnotic drug users (NHDU). LSD (> 9 h) was significantly more common in women in the NHDU group (P < 0.001), but no significant difference was observed in the frequency of SSD or LSD between men and women in the HDU group (Fig. 1). In the total population and NHDU group, LSD (> 9 h) had a significantly higher mean of age and lower mean of WSI (P < 0.001), and also SSD (< 6 h) had a significantly higher mean of BMI (P < 0.001). In the total population, HDU and NHDU groups, LSD (> 9 h) had a significantly lower mean of education, physical activity, and alcohol consumption (P < 0.05), and also NSD (6–9 h) had a significantly lower mean of waist circumference (P < 0.001).
Table 2 presents the frequency of non-communicable diseases by sleep duration in HDU and NHDU groups. In the total population and NHDU group, the prevalence of diabetes (P < < 0.001), Thyroid disease (P = 0.045), MI (P = 0.001), CHD (P = 0.002), depression, and hypertension (P < 0.001) were higher in people with LSD (> 9 h). Also, in the HDU group, the prevalence of Thyroid disease (P = 0.011) was higher in LSD (> 9 h) (Table 2).
Furthermore, the odds ratios of selected variables related to HDU and NHDU groups in different sleep duration groups are reported in Table 3. According to the results of backward logistic regression analysis, the odds of SSD (< 6 h) increased by increasing the age (OR:1.02 (95% CI 1.015–1.03), P < 0.001), BMI (OR:1.03 (95% CI 1.02–1.04), P < 0.001), physical activity (OR: 1.03 (95% CI 1.02–1.04), P < 0.001) and depression (OR: 1.17 (95% CI 1.02–1.33), P = 0.023) in total population compared to the reference group. We observed that in the total population, people with MI had increased odds of LSD (OR: 1.66 (95% CI 1.01–2.72), P = 0.045). Alcohol consumption also showed a protective effect on LSD in all individuals (OR: 0.59 (95% CI 0.37–0.94), P = 0.027) (Table 3).
In the NHDU group, age (OR:1.02 (95% CI 1.02–1.03), P < 0.001), education (OR: 1.02 (95% CI 1.01–1.04), P = 0.006), BMI (OR: 1.03 (95% CI 1.02–1.05), P < 0.001), physical activity (OR: 1.03 (95% CI 1.02–1.04), P < 0.001), and alcohol consumption (OR: 1.28 (95% CI 1.04–1.58), P = 0.022) were related to increased odds of SSD (Table 3). In the total population and HDU group, depression showed a significantly increased OR for SSD (total OR: 1.17 (95% CI 1.02–1.33), P = 0.023, HDU group OR: 1.45 (95% CI 1.01–2.09), P = 0.049). In the total population, the odds of LSD were significantly higher in women (OR: 3.03 (95% CI 2.18–4.2), P < 0.001) compared to men, and this higher ratio was also observed in the NHDU group (OR: 3.43 (95% CI 2.39–4.91), P < 0.001). In the HDU group, the odds of over LSD (> 9 h) increased in people with thyroid disease (OR: 1.96 (95% CI 1.04–3.67), P = 0.037) (Table 3).
In total subjects, HDU and NHDU groups, opium use was related to increased odds of LSD (OR: 2.15 (95% CI 1.59–2.92), P < 0.001, OR: 2.13 (95% CI 1.01–4.46), P = 0.046, and OR: 2.16 (95% CI 1.54–3.02), P < 0.001, respectively). Higher education also showed a protective effect on LSD in all individuals (OR: 0.96 (95% CI 0.94–0.99), P = 0.007), HDU group (OR: 0.93 (95% CI 0.87–0.99), P = 0.034) and NHDU groups (OR: 0.97 (95% CI 0.94–0.99), P = 0.037). Moreover, in all three groups with increasing physical activity, the odds of SSD (< 6 h) increased (P < 0.001), and the odds of LSD (> 9 h) decreased (P < 0.001) (Table 3).
Discussion
The findings of the present study showed that a higher odds ratio of SSD (< 6 h) is significantly associated with age, higher education level, BMI, physical activity, alcohol consumption, and depression. LSD (> 9 h), on the other hand, showed a positive association with age, female sex, opium consumption, and history of MI, and a reverse association with higher education, physical activity, and alcohol consumption.
For more accurate results, we performed a sensitivity analysis, stratifying for hypnotic drug use in our study population to assess whether hypnotic drug use may impact the association of sleep duration with risk factors and the related medical conditions. In this study, women slept more than men. These findings support objective and subjective reports documenting longer sleep duration among women28,29. Our sensitivity analysis showed that in the HDU group, LSD is not associated with the female sex suggesting the gender differential impact of hypnotic drugs. It has been demonstrated by previous studies that the frequency of sleep duration is sex-dependent, and they are more prevalent among females. The average total sleep time (TST) was significantly longer in women30. The results of the present study confirm that LSD (> 9 h) is three times more likely in women compared to men, and additionally indicate a sex differential impact of HDU in adults.
The close relationship between sleep duration and depression and anxiety is previously demonstrated31,32,33,34,35. Interestingly, here we found a significant 45% increased odds ratio of SSD (< 6 h) associated with depression and a 96% increased odds ratio of LSD (> 9 h) in Thyroid diseases, only in the HDU group, and these associations were not significant in the NHDU group. Considering this is a cross-sectional study, it is suggested that in the follow-up phase of the study, this association is further investigated.
We observed 28% higher odds of SSD (< 6 h) associated with alcohol consumption in the NHDU group. This finding is in line with recent reports that indicated worse sleeping patterns with alcohol drinking habits, such as having trouble staying asleep and frequent wakening during the night, shorter duration of sleep, and snoring36,37,38. Stratifying for the HDU group, we found a reverse relationship and a 41% decreased odds ratio of LSD in alcohol consumers. We suggest future studies that assess whether hypnotic drugs may alleviate the adverse sleep duration consequences of drinking habits in adults.
Controversial reports have been published regarding the association or lack of association between physical activity and odds of sleep disturbances, which may be explained by the type and duration of the physical activity measured in different studies, and the impact of variation in demographic and socioeconomic factors in other study populations39,40,41,42,43,44,45,46. Findings from our study showed physical activity to be slightly associated with LSD (< 6 h) (around 3% increased odds ratio) and inversely connected to approximately 20% lower odds ratio of LSD (> 9 h). This relationship was observed in the HDU group.
Our results showed that chronic opium use is associated with a more than doubled odds ratio of LSD similarly in HDU and NHDU groups. Abnormal sleep duration due to chronic substance use has been previously demonstrated47,48,49,50,51. In the present study, we further assessed whether hypnotic drug use might alleviate or exacerbate this association. Our statistical analyses do not support the significant impact of hypnotic drug use on the association of sleep duration and opium consumption.
A significant association is reported between SSD and BMI in some previous cross-sectional and longitudinal studies52,53. However, due to the contrasting results of previous studies that indicate a beneficial or harmful impact of LSD on obesity, the link between LSD and BMI is not well defined52. Our results confirm the association between SSD and higher BMI but did not support a positive relationship between LSD and obesity.
Previous studies have demonstrated contradictory results on the relationship between sleep duration and education levels. Some found LSD associated with higher education, and some did not find a significant association between them54,55,56. Our results showed a negative association between education levels and sleep duration in the RCS population, as more education was positively associated with SSD and negatively associated with LSD.
In the present study, it was shown that sleep duration decreases with age. Therefore, we may further emphasize the importance of considering the age group when assessing the relation of sleep duration with diseases and all-cause mortality. Since advanced age is itself associated with higher mortality, and this may affect the connection of sleep duration with mortality57,58,59. For example, a Swedish prospective cohort study on 43,863 individuals (64% women) found that both SSD and LSD are associated with mortality only among young individuals, and in participants older than 65, they did not find an association between abnormal sleep duration and mortality when considering the impact of age in their analysis60.
Previous observational studies have indicated both SSD and LSD are positively associated with MI and CVD, suggesting abnormal sleep duration is a potent risk factor for CVD61,62,63. Here, we found LSD to be associated with MI. However, our findings do not support a statistically significant relationship between SSD and MI. On the other hand, our study did not find a significant association between abnormal sleep duration and CHD.
Some previous reports demonstrated an association between both SSD and LSD with an increase in the risk of thyroid dysfunction, suggesting that abnormal sleep duration may exert a detrimental impact on thyroid function leading to an increased risk of subclinical thyroid problems64,65. The present study found significantly increased odds of thyroid dysfunction associated with LSD only among the NHDU group. Regarding SSD, our data does not indicate a significant relationship between sleep duration and thyroid diseases.
The strength points of the present study are the stratification for hypnotic drug use when analyzing the association of sleep duration with the related risk factors and diseases. Additionally, a comprehensive cohort study performed allowed us to assess the relationship between sleep duration with multiple illnesses (depression, MI, CHD, diabetes, etc.) and lifestyle factors such as opium use, alcohol consumption, and physical activity in multivariate regression models, based on the data obtained in the RCS cohort. Additionally, computer-assisted, server-based face-to-face interviews in the RCS cohort studies performed by trained healthcare experts have increased the quality and accuracy of data collection. However, a limitation of this study is that data on smoking, opium use, sleep duration, and medical history of diseases is based on self-reports of participants and are not based on clinical assessment and sleep laboratory data, which may have entered some levels of misclassification due to self-reporting and recall biases. Another limitation was the cross-sectional design of the study. In contrast, did not allow for deriving any causal inferences. Accordingly, this relationship will be reconsidered in the follow-up phase of this prospective study.
Conclusion
Overall, our results indicated demographic and lifestyle factors such as age, BMI, education, physical activity, alcohol consumption, and depression to be associated with SSD. Age, female sex, opium consumption, and history of MI displayed a significant association with higher odds of LSD, while education, physical activity, and alcohol consumption were associated with lower odds of LSD. Our sensitivity analyses showed a connection between thyroid dysfunction and depression with SSD only in hypnotic drug users, suggesting that more caution needs to be taken before hypnotic drug administration.
Data availability
The current study’s data are available at the PERSIAN Adult Cohort Study Center, Rafsanjan University of Medical Sciences, Iran. The data are not available publicly. However, upon reasonable request, the data can be obtained from the corresponding author (Fatemeh Ayoobi, ayoobi.fatemeh@gmail.com).
Abbreviations
- LSD:
-
Long sleep duration
- SSD:
-
Short sleep duration
- NSD:
-
Normal sleep duration
- RCS:
-
Rafsanjan cohort study
- PERSIAN:
-
Prospective epidemiological research studies in Iran
- BMI:
-
Body mass index
- MI:
-
Myocardial ischemia
- CHD:
-
Chronic heart diseases
- RLS:
-
Restless leg syndrome
- CVD:
-
Cardiovascular diseases
- HTN:
-
Hypertension
- BDZ:
-
Benzodiazepines
- MET:
-
Metabolic equivalent of task
- ORs:
-
Odds ratios
- WSI:
-
Wealth score index
- HDU:
-
Hypnotic drug users
- NHDU:
-
Non-hypnotic drug users
References
Lee, K. A. & Gay, C. L. Sleep in late pregnancy predicts length of labor and type of delivery. Am. J. Obstet. Gynecol. 191(6), 2041–2046 (2004).
Consensus Conference, P. et al. Recommended amount of sleep for a healthy adult: A joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. J. Clin. Sleep Med. 11(6), 591–592 (2015).
Hirshkowitz, M. et al. National Sleep Foundation’s sleep time duration recommendations: Methodology and results summary. Sleep Health 1(1), 40–43 (2015).
Ohayon, M. et al. National Sleep Foundation’s sleep quality recommendations: First report. Sleep Health 3(1), 6–19 (2017).
Chong Y, Fryar CD, Gu Q. Prescription sleep aid use among adults: United States, 2005–2010: US Department of Health and Human Services, Centers for Disease Control and … (2013).
St-Onge, M.-P. et al. Sleep duration and quality: Impact on lifestyle behaviors and cardiometabolic health: A scientific statement from the American Heart Association. Circulation 134(18), e367–e386 (2016).
Xi, B., He, D., Zhang, M., Xue, J. & Zhou, D. Short sleep duration predicts risk of metabolic syndrome: A systematic review and meta-analysis. Sleep Med. Rev. 18(4), 293–297 (2014).
Shan, Z. et al. Sleep duration and risk of type 2 diabetes: A meta-analysis of prospective studies. Diabetes Care 38(3), 529–537 (2015).
Qin, Y., Zhou, Y., Zhang, X., Wei, X. & He, J. Sleep duration and breast cancer risk: A meta-analysis of observational studies. Int. J. Cancer 134(5), 1166–1173 (2014).
Yin, J. et al. Relationship of sleep duration with all-cause mortality and cardiovascular events: A systematic review and dose-response meta-analysis of prospective cohort studies. J. Am. Heart Assoc. 6(9), e005947 (2017).
Brinkman JE, Reddy V, Sharma S. Physiology, sleep (2018).
Ischander, M. M. & Lloyd, R. D. Jr. Severe paediatric obesity and sleep: A mutual interactive relationship!. J. Sleep Res. 30(3), e13162 (2021).
Okoli, A., Hanlon, E. C. & Brady, M. J. The relationship between sleep, obesity, and metabolic health in adolescents: A review. Curr. Opin. Endocr. Metab. Res. 17, 15–19 (2021).
Gates, M. et al. Impact of fatigue and insufficient sleep on physician and patient outcomes: A systematic review. BMJ Open 8(9), e021967 (2018).
Song, Q., Liu, X., Wang, X. & Wu, S. Age-and gender-specific associations between sleep duration and incident hypertension in a Chinese population: The Kailuan study. J. Hum. Hypertens. 30(8), 503–507 (2016).
Iftikhar, I. H. et al. Sleep duration and metabolic syndrome. An updated dose–risk metaanalysis. Ann. Am. Thorac. Soc. 12(9), 1364–72 (2015).
Hua, J., Jiang, H., Wang, H. & Fang, Q. Sleep duration and the risk of metabolic syndrome in adults: A systematic review and meta-analysis. Front. Neurol. 12, 127 (2021).
Wang, S. et al. Associations between sleep duration and cardiovascular diseases: A meta-review and meta-analysis of observational and Mendelian randomization studies. Front. Cardiovasc. Med. https://doi.org/10.3389/fcvm.2022.930000 (2022).
Bergemann, N. Hypnotics: Guidelines and Current References 2339–2377 (Springer, 2022).
Fogari, R. et al. Diazepam as an oral hypnotic increases nocturnal blood pressure in the elderly. Aging Clin. Exp. Res. 31(4), 463–468 (2019).
Manolis, T. A., Manolis, A. A., Apostolopoulos, E. J., Melita, H. & Manolis, A. S. Cardiovascular complications of sleep disorders: A better night’s sleep for a healthier heart/from bench to bedside. Curr. Vasc. Pharmacol. 19(2), 210–232 (2021).
Sogawa, R. et al. Sex-and age-specific all-cause mortality in insomnia with hypnotics: Findings from Japan multi-institutional Collaborative Cohort Study. Sleep Med. 100, 410–418 (2022).
Hakimi, H. et al. The profile of Rafsanjan cohort study. Eur. J. Epidemiol. 36(2), 243–252 (2021).
Postchi, H. et al. Prospective Epidemiological Research Studies in Iran, PERSIAN Cohort, Study Protocol 1st edn. (Mir Mah Publication, 2016).
Pourshams, A. et al. Cohort profile: The Golestan Cohort Study—a prospective study of oesophageal cancer in northern Iran. Int. J. Epidemiol. 39(1), 52–59 (2010).
Jamali, Z. et al. Prevalence of dyslipidemia and its association with opium consumption in the Rafsanjan cohort study. Sci. Rep. 12(1), 1–13 (2022).
Ostadrahimi, A. et al. Does long sleep duration increase risk of metabolic syndrome in Azar cohort study population?. Health Promot. Perspect. 8(4), 290 (2018).
Jean-Louis, G., Kripke, D. F., Ancoli-Israel, S., Klauber, M. R. & Sepulveda, R. S. Sleep duration, illumination, and activity patterns in a population sample: Effects of gender and ethnicity. Biol. Psychiatry 47(10), 921–927 (2000).
Hoch, C. C. et al. Empirical note: Self-report versus recorded sleep in healthy seniors. Psychophysiology 24(3), 293–299 (1987).
Lindberg, E. et al. Sleep disturbances in a young adult population: Can gender differences be explained by differences in psychological status?. Sleep 20(6), 381–387 (1997).
Taylor, D. J., Lichstein, K. L., Durrence, H. H., Reidel, B. W. & Bush, A. J. Epidemiology of insomnia, depression, and anxiety. Sleep 28(11), 1457–1464 (2005).
Dong, L., Xie, Y. & Zou, X. Association between sleep duration and depression in US adults: A cross-sectional study. J. Affect. Disord. 296, 183–188 (2022).
Zhai, L., Zhang, H. & Zhang, D. Sleep duration and depression among adults: A meta-analysis of prospective studies. Depress. Anxiety 32(9), 664–670 (2015).
Li, Y. et al. Longitudinal association of sleep duration with depressive symptoms among middle-aged and older Chinese. Sci. Rep. 7(1), 1–7 (2017).
Ding, L. et al. The association of sleep duration and quality with depressive symptoms in older Chinese women. PLoS One 17(3), e0262331 (2022).
Britton, A., Fat, L. N. & Neligan, A. The association between alcohol consumption and sleep disorders among older people in the general population. Sci. Rep. 10(1), 1–7 (2020).
Chaput, J.-P., McNeil, J., Després, J.-P., Bouchard, C. & Tremblay, A. Short sleep duration is associated with greater alcohol consumption in adults. Appetite 59(3), 650–655 (2012).
Zheng, D. et al. Alcohol consumption and sleep quality: A community-based study. Public Health Nutr. 24(15), 4851–4858 (2021).
Kline, C. E. et al. Physical activity and sleep: An updated umbrella review of the 2018 Physical Activity Guidelines Advisory Committee report. Sleep Med. Rev. 58, 101489 (2021).
Vogel, O., Niederer, D., Wilke, J., El-Rajab, I. & Vogt, L. Habitual physical activity and sleep duration in institutionalized older adults. Front. Neurol. https://doi.org/10.3389/fneur.2021.706340 (2021).
Mochón-Benguigui, S., Carneiro-Barrera, A., Castillo, M. J. & Amaro-Gahete, F. J. Role of physical activity and fitness on sleep in sedentary middle-aged adults: The FIT-AGEING study. Sci. Rep. 11(1), 1–12 (2021).
Garfield, V., Llewellyn, C. H. & Kumari, M. The relationship between physical activity, sleep duration and depressive symptoms in older adults: The English Longitudinal Study of Ageing (ELSA). Prev. Med. Rep. 4, 512–516 (2016).
Tsunoda, K. et al. Prospective study of physical activity and sleep in middle-aged and older adults. Am. J. Prev. Med. 48(6), 662–673 (2015).
Štefan, L., Sporiš, G., Krističević, T. & Knjaz, D. Associations between sleep quality and its domains and insufficient physical activity in a large sample of Croatian young adults: A cross-sectional study. BMJ Open 8(7), e021902 (2018).
Chevance, G., Baretta, D., Romain, A. J., Godino, J. G. & Bernard, P. Day-to-day associations between sleep and physical activity: A set of person-specific analyses in adults with overweight and obesity. J. Behav. Med. 45(1), 14–27 (2022).
Zhu, G. et al. Objective sleep assessment in> 80,000 UK mid-life adults: Associations with sociodemographic characteristics, physical activity and caffeine. PLoS One 14(12), e0226220 (2019).
Madanifard, M., Mazaheri, M. & Bigdeli, I. Comparative investigation of sleep problems in opioid-dependent and normal subjects. J. Sleep Sci. 3(1–2), 25–29 (2018).
Thannickal, T. C. et al. Opiates increase the number of hypocretin-producing cells in human and mouse brain and reverse cataplexy in a mouse model of narcolepsy. Sci. Transl. Med. 10(447), eaao4953 (2018).
Fadaei, M. et al. Comparison of sleep quality indices in patients with opium and methamphetamine addiction. J. Sleep Sci. 4(1–2), 17–23 (2019).
Valentino, R. J. & Volkow, N. D. Drugs, sleep, and the addicted brain. Neuropsychopharmacology 45(1), 3–5 (2020).
Dunn, K. E., Finan, P. H., Tompkins, D. A. & Strain, E. C. Frequency and correlates of sleep disturbance in methadone and buprenorphine-maintained patients. Addict. Behav. 76, 8–14 (2018).
Marshall, N. S., Glozier, N. & Grunstein, R. R. Is sleep duration related to obesity? A critical review of the epidemiological evidence. Sleep Med. Rev. 12(4), 289–298 (2008).
Xu, M. et al. Association between nocturnal sleep duration and obesity indicators among people with type 2 diabetes: A cross-sectional study in Ningbo, China. Diabetes Metab. Syndr. Obes. 15, 1357 (2022).
Sheehan, C., Zajacova, A., Connor, D. & Montez, J. K. State-level variation in the association between educational attainment and sleep. Popul. Res. Policy Rev. 41(3), 1137–1160 (2022).
Sosso, F. A. E., Holmes, S. D. & Weinstein, A. A. Influence of socioeconomic status on objective sleep measurement: A systematic review and meta-analysis of actigraphy studies. Sleep Health 7(4), 417–428 (2021).
Musshafen, L. A. et al. Associations between sleep and academic performance in US adolescents: A systematic review and meta-analysis. Sleep Med. 83, 71–82 (2021).
Cappuccio, F. P., D’Elia, L., Strazzullo, P. & Miller, M. A. Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep 33(5), 585–592 (2010).
Gallicchio, L. & Kalesan, B. Sleep duration and mortality: A systematic review and meta-analysis. J. Sleep Res. 18(2), 148–158 (2009).
da Silva, A. A. et al. Sleep duration and mortality in the elderly: A systematic review with meta-analysis. BMJ Open 6(2), e008119 (2016).
Åkerstedt, T. et al. Sleep duration, mortality and the influence of age. Eur. J. Epidemiol. 32(10), 881–891 (2017).
Daghlas, I. et al. Sleep duration and myocardial infarction. J. Am. Coll. Cardiol. 74(10), 1304–1314 (2019).
Sabanayagam, C. & Shankar, A. Sleep duration and cardiovascular disease: Results from the National Health Interview Survey. Sleep 33(8), 1037–1042 (2010).
Huang, Y.-M. et al. Sleep duration and risk of cardio-cerebrovascular disease: A dose-response meta-analysis of cohort studies comprising 3.8 million participants. Front. Cardiovasc. Med. https://doi.org/10.3389/fcvm.2022.907990 (2022).
Kim, W. et al. Association between sleep duration and subclinical thyroid dysfunction based on nationally representative data. J. Clin. Med. 8(11), 2010 (2019).
Luo, J., Sands, M., Wactawski-Wende, J., Song, Y. & Margolis, K. L. Sleep disturbance and incidence of thyroid cancer in postmenopausal women the Women’s Health Initiative. Am. J. Epidemiol. 177(1), 42–49 (2013).
Acknowledgements
The authors thank the people who participated in the study, the study-site personnel, and the Rafsanjan cohort center members in Rafsanjan, Iran. The Iranian Ministry of Health and Medical Education has contributed to the funding used in the PERSIAN Cohort through Grant no 700/534.
Author information
Authors and Affiliations
Contributions
N.J. and F.A. designed the study and supervised the project. A.V. and A.M. collected the data. F.A. prepared Tables 1, 2 and 3 and writes the results. P.K. performed the statistical analysis. Z.Jam., P.K. and F.A. wrote the main manuscript text. Z.Jal. revised the paper. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jalali, N., Khalili, P., Jamali, Z. et al. Sleep duration, hypnotic drug use, and risk factors: cross- sectional study. Sci Rep 13, 3459 (2023). https://doi.org/10.1038/s41598-023-30501-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-30501-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.