Abstract
Multiple trauma patients with severe chest trauma are at increased risk for tracheostomy. While the risk factors associated with the need for tracheostomy are well established in the general critical care population, they have not yet been validated in a cohort of patients suffering severe thoracic trauma. This retrospective cohort study analysed data on patients aged 18 years or older who were admitted to one of the six participating academic level I trauma centres with multiple injuries, including severe thoracic trauma (AISThorax ≥ 3) between 2010 and 2014. A multivariable binary regression was used to identify predictor variables for tracheostomy and to develop the Tracheostomy in Thoracic Trauma Prediction Score (T3P-Score). The study included 1019 adult thoracic trauma patients, of whom 165 underwent tracheostomy during their intensive care unit (ICU) stay. Prehospital endotracheal intubation (adjusted OR [AOR]: 2.494, 95% CI [1.412; 4.405]), diagnosis of pneumonia during the ICU stay (AOR: 4.374, 95% CI [2.503; 7.642]), duration of mechanical ventilation (AOR: 1.008/hours of intubation, 95% CI [1.006; 1.009]), and an AISHead ≥ 3 (AOR 1.840, 95% CI [1.039; 3.261]) were independent risk factors for tracheostomy. Patients with sepsis had a lower risk of tracheostomy than patients without sepsis (AOR 0.486, 95% CI [0.253; 0.935]). The T3P-Score had high predictive validity for tracheostomy (ROCAUC = 0.938, 95% CI [0.920, 0.956]; Nagelkerke’s R2 was 0.601). The T3P-Score’s specificity was 0.68, and the sensitivity was 0.96. The severity of thoracic trauma did not predict the need for tracheostomy. Follow-up studies should validate the T3P-Score in external data sets and study the reasons for the reluctant use of tracheostomy in patients with severe thoracic trauma and subsequent sepsis.
Trial registration: The study was applied for and registered a priori with the respective ethics committees.
Similar content being viewed by others
Introduction
Multiple trauma patients with relevant thoracic trauma are at increased risk for prolonged mechanical ventilation compared to patients without severe thoracic injuries1. Therefore, it is of particular importance to determine which independent influencing factors in this vulnerable patient group increase the risk of prolonged ventilation and the likelihood of requiring tracheostomy. Tracheostomies are routinely performed in intensive care unit (ICU) patients requiring prolonged mechanical ventilation to prevent endotracheal tube-associated complications, such as ulceration, vocal cord paralysis, and laryngotracheal stenosis2,3. At the same time, tracheostomy is considered a relatively safe procedure, with a residual risk of severe complications, such as tension pneumothorax or injury to the aortic arch. The extent to which a tracheostomy can positively influence the course of a critically ill patient has been the subject of controversial discussions2. Tracheostomy improves oral hygiene, patient communication, facilitates weaning, and improves patient comfort when compared to endotracheal intubation3.
To date, few RCTs have contributed to evidence-based recommendations on which patients should receive tracheostomy and when. No RCTs on vulnerable subgroups (e.g. thoracic trauma patients) are available2. Due to the low number of studies, the recommendations of expert committees are used to decide on tracheostomies4. In the German S2k guideline of “prolonged weaning” by the German Respiratory Society, it is recommended to discuss the performance of a tracheostomy in patients after “clinical estimation of prolonged weaning” with concomitant inability of non-invasive ventilation 4–7 days after intubation. In this context, clinical estimation of prolonged weaning is defined as more than three spontaneous breathing attempts (SBA) or > 7 days after the last SBA5. Moreover, the Brazilian recommendations of mechanical ventilation 2013 highlight the early clinical estimation of prolonged weaning in three specific risk groups (severe polytrauma, high spinal cord injury, and severe traumatic brain injury [TBI]) without further specifications on when and how a tracheostomy should be performed6. The estimation of a prolonged weaning is still difficult, even for experts, as shown by high-quality RCTs, which challenges the current practice of "clinical estimation of prolonged weaning"7. Overall, there are currently primarily expert opinions and there is an urgent need for more evidence in this field. For this reason, we studied a typical risk group for prolonged mechanical ventilation: severely injured patients with severe thoracic trauma. The results were used to develop a tracheostomy prediction score for thoracic trauma patients, which can be used for the early identification of patients who have a high probability that a tracheostomy will be necessary in the further ICU stay to support clinical decision making and for research purposes. Therefore, we performed a retrospective data analysis using a comprehensive thoracic trauma database. A multivariable logistic regression analysis was performed to achieve the above-described objective.
Patients and methods
This study was conducted by the Trauma Section of the German Interdisciplinary Association for Intensive and Emergency Medicine (Deutsche Interdisziplinäre Vereinigung für Intensiv- und Notfallmedizin, DIVI). In December 2015, the section embarked on a retrospective, observational study of the quality of care in patients with thoracic trauma (AISThorax ≥ 3) who underwent mechanical ventilation (MV) from 2010 to 2014. This study was part of a larger research project with the aim of improving the treatment of multiple injured patients with thoracic trauma, which was previously described by Wutzler et al.8. Six German university hospitals (Aachen, Cologne, Frankfurt, Freiburg, Kiel, and Marburg) contributed patient data for analysis. All participating hospitals were academic level I trauma centres.
This study follows the guidelines of the revised UN Declaration of Helsinki in 1975 and its latest amendment in 2013 (64th General Assembly). The following approvals were provided by each institution’s ethical committee: Independent Ethics Committee of the University RWTH Aachen: EK 346/15, Ethics Commission of the University of Cologne 18/2016, Ethics Committee Office of the University of Frankfurt: 220/16, Ethics Committee of the University of Kiel: B 248/16, Ethics Committee of the University of Freiburg: 275/16, Ethics Committee of the University of Marburg: No ethics committee vote necessary for the retrospective analysis. Due to the study’s retrospective nature, informed consent from the study participants was waived in accordance with the ethical approval from Independent Ethics Committee of the University RWTH Aachen. The other above mentioned ethics committees and commissions confirmed this decision.
The reporting was in accordance with the recommendations of the TRIPOD statement (v2015)9.
Implementation of the clinical database
Details of the database10 used are given in Supplement 1.
Definitions and diagnosis criteria
Overall injury severity was calculated by the Injury Severity Score (ISS), as described by Baker et al.11. The Abbreviated Injury Scale (AIS, Version 2005/Update 2008, Association for the Advancement of Automotive Medicine, Barrington, IL) was used as a global system for injury coding and severity classification. The severity of injuries was recorded according to the AIS as 1 (minor), 2 (moderate), 3 (severe, not life-threatening), 4 (serious, life-threatening), 5 (critical, survival uncertain), and 6 (maximum, currently untreatable). The decision to perform a tracheostomy was made by interdisciplinary teams (intensivists, trauma surgeons, and neurosurgeons). Multiple organ failure was diagnosed at any time during the hospitalization according to the Sequential Organ Failure Assessment (SOFA), where 3 or 4 points for an organ was considered as organ failure12. Sepsis was based on the sepsis-3 definition13. The patients’ physical status was graded using the ASA classification system on admission14.
The Clinical Pulmonary Infection Score (CPIS) was used to assess the risk of pneumonia in ventilated patients15. A total of > 6 points was accepted as pneumonia. In non-ventilated patients, pneumonia was defined as the presence of a new progressive infiltrate accompanied by at least two of the following symptoms:
-
Purulent respiratory secretions
-
Body temperature ≥ 38 °C or ≤ 35 °C
-
Leucocytosis (white blood cell count of ≥ 10,000/mm3) or leucopoenia (white blood cell count of ≤ 4500/mm3, or more than 15% immature neutrophils)
Statistics
Continuous values are presented as mean, standard deviation (SD), and range [X, Y], where applicable. Differences in categorical and continuous variables were evaluated by a chi-square test and a Mann–Whitney U test, respectively. The significance level was set at α = 0.05 (two-sided p-value). All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS 28.0; IBM Inc., Armonk, NY, USA). Calibration curves were calculated using SAS 9.4 (TS Level 1M4, SAS Institute, Cary, NC, USA).
Model A: Multivariable binary logistic regression analysis
The binary logistic regression model was fit using the dichotomous variable “tracheostomy” as the dependent endpoint. Univariable logistic regression analyses of multiple variables were conducted to identify independent variables for inclusion in the multivariable logistic regression model (Table 2). The variables included age (years), sex (m/f), ASA classification (1/2/3/4/5), AISThorax categories, AISHead ≥ 3 (y/n), ISS categories (9–15/16–31/32–75), prehospital endotracheal intubation (y/n), duration of mechanical ventilation (hours), aspiration (y/n), pneumonia (y/n), multiple organ failure (y/n), and sepsis (y/n). The significance level for considering variables from the univariable analyses for the multivariable analysis was set at α = 0.05. The statistical significance of each regression coefficient was tested using the Wald test. The significance level for the interpretation in the multivariable regression analysis was set at α = 0.05. The goodness of fit was measured using ROCAUC, the Hosmer–Lemeshow test (X2), and the omnibus test. A calibration curve was produced which contrasts tracheostomy probabilities observed in the data with those estimated from the T3P-Score logistic regression model. Observed probabilities were smoothed by LOESS (k = 0.75) as recommended by Austin et al.16. The overall performance of the model was evaluated using Nagelkerke’s R2. The internal validation of the results was performed using bootstrapping (1000 replications, bias-corrected, and accelerated [BCa]), as recommended by Steyerberg et al.17. The collinearity analysis was performed by evaluating the tolerance and the variance inflation factor (VIF). Odds ratios (OR) and adjusted odds ratios (AOR) were reported with the associated 95% confidence interval (CI).
Model B: Tracheostomy in Thoracic Trauma Prediction Score (T3P-Score)
We repeated the multivariable logistic regression with the aim of developing the T3P-Score. The variable "duration of MV" was transformed into “duration of MV groups” and quantified using categorical regression (0–67/68–180/181–299/300–440/441–1258) for higher robustness. The variables AISHead ≥ 3 (y/n), duration of MV groups (0–67/68–180/181–299/300–440/441–1258 in hours), prehospital endotracheal intubation (y/n), pneumonia (y/n), and sepsis (y/n) were included in the score due to their performance in the model A (p < 0.05). Quality assurance of the score (goodness of fit, collinearity analysis, performance, and validation) was performed as previously described. The predicted probability can be calculated according to the general principles of logistic regression18.
Ethics approval and consent to participate
The following approvals were provided by each institution's ethical committee: Independent Ethics Committee of the University RWTH Aachen: EK 346/15, Ethics Commission of the University of Cologne 18/2016, Ethics Committee Office of the University of Frankfurt: 220/16, Ethics Committee of the University of Kiel: B 248/16, Ethics Committee of the University of Freiburg: 275/16, Ethics Committee of the University of Marburg: No ethics committee vote necessary for the retrospective analysis.
Informed consent
All the study protocol was performed in accordance with the relevant guidelines and regulations.
Results
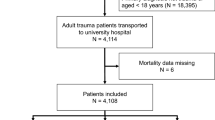
A total of 1019 thoracic trauma patients were considered for comparison (Fig. 1). The mean age was 48.4 years (SD: 18.8 [18, 94]), and 76.0% were male. The cohort included 165 (16.2%) who underwent tracheostomies, and 854 (83.8%) who did not undergo tracheostomies. The detailed characteristics of the study patients are presented in Table 1.
Trauma patients who underwent tracheostomies were older, had a higher mean ISS, and prehospital endotracheal intubation was more frequent compared to patients who did not undergo tracheostomies (Table 1). The mean time to tracheostomy (TTT) was 9.9 days. The relationship between the variables and tracheostomy is shown in Table 2. Patients’ characteristics that were associated with the performance of a tracheostomy were AISThorax categories, AISHead ≥ 3, ISS groups, prehospital endotracheal intubation, duration of mechanical ventilation, aspiration, pneumonia, MOF, and sepsis (Table 2). Pneumonia represented the strongest risk factor in the univariable analysis.
Model A: Multivariable logistic regression analysis
The AISThorax categories, AISHead ≥ 3, ISS groups, prehospital endotracheal intubation, duration of MV, aspiration, pneumonia, multiple organ failure, and sepsis were identified by univariable regression for consideration in our multivariable analysis. Table 3 shows the AOR for these risk factors.
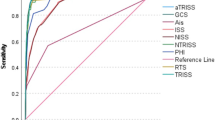
AISHead ≥ 3, prehospital endotracheal intubation, duration of, and pneumonia were independent variables increasing the risk of tracheostomy (Table 3). The presence of sepsis led to less frequent performance of tracheostomy. The predictive validity of the model was high (ROCAUC = 0.941, 95% CI [0.925, 0.958], Table 3 and Fig. 2). The overall performance of Model A was 0.602 (Nagelkerke’s R2).
Model B: The T3P-Score
The final T3P-Score model included AISHead ≥ 3, prehospital endotracheal intubation, duration of MV groups, pneumonia, and sepsis (Table 4). The T3P-Score predicts the need for tracheostomy (ROCAUC = 0.938, 95% CI [0.920, 0.956]; Table 4 and Fig. 2). The overall performance of the model was 0.601 (Nagelkerke’s R2). The specificity and sensitivity were 0.96 and 0.68 (cut-off value: − 2.19), respectively. The calibration curve is displayed in Fig. 3.
Discussion
By combining the variables AISThorax categories, AISHead ≥ 3, ISS groups, prehospital endotracheal intubation, duration of MV, aspiration, pneumonia, multiple organ failure, and sepsis, we were able to achieve a high predictive value for Model A. Moreover, after recalibration and focusing on a reduced variable set, the developed T3P Score showed a high specificity and sensitivity to predict a tracheostomy during the ICU stay of severely injured patients with severe thoracic trauma. The inclusion of 1069 patients provided a reliable basis of data, and the goodness of fit procedures demonstrated the high performance of the score.
As expected, severe TBI was a strong adjusted risk factor influencing the need for tracheostomy. This is in accordance with the current literature, according to which an early tracheostomy is associated with a better outcome (especially secondary complications) in TBI patients19. Therefore, current recommendations favour early tracheostomy in these patients, although this recommendation has been increasingly challenged, as the result of several randomised controlled studies with contradictory results20. In this context, the TracMan multicentre randomised controlled study reported no beneficial effect of an early tracheostomy vs. late tracheostomy on mortality, duration of ICU stay, or the hospital length of stay in 909 ICU patients7. In contrast, another multicentre randomised controlled study by Terragni et al. could observe a reduction in the duration of MV and the ICU stay in 419 patients. For this reason, on the one hand, it is necessary to interpret the results in light of the investigated endpoint or outcome, and on the other hand, to conduct further RCTs with subsequent pooling of the study results in meta-analyses21.
The fact that prehospital intubation increases the risk of tracheostomy is not surprising. Current guidelines and teaching systems for the prehospital management of severely injured patients recommend a cautious approach with a recommendation for prehospital intubation in the presence of a GCS < 9, direct injury to the airway, severe maxillofacial fractures, head or neck injury, inhalation trauma, hypercarbia, or insufficient oxygenation. Accordingly, prehospital intubation is a surrogate for impaired neurological status, relevant ventilation failing, or direct trauma to the airway22,23. Studies of our group as well as studies of others could show that prehospital intubation is also a risk factor for the development of pneumonia during intensive care treatment8,24. The risk of occurrence of pneumonia is known to increase with the duration of mechanical ventilation, which was impressively demonstrated by the detection of a cut-off point at 102 h of MV in a former study by our research group8. In addition, various studies show a strong positive correlation between pneumonia and the duration of mechanical ventilation, whereby the order of causality could not be conclusively clarified until today25. Certainly, the distinction between ventilator-associated pneumonia and pneumonia due to trauma was and is impossible, therefore this was also not attempted by us. However, the duration of MV and the occurrence of pneumonia were adjusted predictors for tracheostomy in our study. Therefore, it is reasonable that prehospital intubation represents a risk factor for tracheostomy in our study.
The performance of tracheostomy is recommended for foreseeable mechanical ventilation ≥ 7 days, although the optimal timing remains controversial. This was shown by Adriolo et al. in an 2015 Cochrane review, and since then, no convincing attempts have been made by RCTs to fill this lack of evidence2. Therefore, current guidelines still recommend a tracheostomy 7–10 days after admission26, although the development of modern tubes has extended the safe time interval to 10 days until tube-associated complications occur27. This was also consistent with the observed mean TTT of 10 days in our study, demonstrating the implementation of these recommendations.
Interestingly, neither thoracic injury severity nor injury severity according to ISS represented independent risk factors after the adjustment. We can only speculate about the reason for this observation. Possible causes could have been interventions, such as surgical stabilization of the thoracic wall, as well as innovative non-invasive ventilation (NIV) techniques, which negated the independent influence of thoracic trauma as a predictor of tracheostomy. In this context, Duggal et al. were able to show in a systematic review that the use of NIV for thoracic trauma patients was able to reduce complication rates and the need for intubation in various studies28. Moreover, a Cochrane review as well as a meta-analysis by Coughlin et al. pooled the existing evidence that thoracic wall stabilization could shorten ventilation time and reduce the risk of intubation in flail chest patients29 and the non-flail chest study by the Chest Wall Injury Society, a multicentre RCT, was able to demonstrate comparable results for non-flail chest patients30. Thoracic wall stabilizations were not included in the database. Since this intervention was rarely performed, its potential influence remains speculative.
A remarkable observation in our study was the OR of 0.49 for sepsis diagnosis. In this context, it remains open whether sepsis patients were less frequently tracheostomized or whether tracheostomized patients less frequently developed sepsis (e.g., pneumonia-associated). Due to the limitations of the registry, the causality remains open. However, studies such as those by Nseir et al. demonstrated that tracheostomy reduced the risk of ventilator-associated pneumonia. Consequently, this could be a possible explanation for the lower sepsis rate in the subgroup31. Another explanation could be the higher mortality in the non-tracheotomized group, which consecutively may have led to a lower sepsis prevalence. Furthermore, the variable “sepsis” could have been a collider. We did not generate a directed acyclic graph a priori, so there remains a risk of collider bias. Follow-up studies should therefore urgently clarify whether tracheostomy can reduce sepsis risk compared with endotracheal intubation and may therefore represent a prophylactic intervention in severely injured patients with relevant thoracic trauma. Conversely, if restrained use of tracheostomy can be demonstrated in sepsis patients, restrained use may represent avoidance of another hit to the immune system32,33. It is possible that the decision to perform tracheostomies was made less frequently in patients in a cytokine storm and with adequate oxygenation to avoid further elevation of inflammatory cytokines through the use of mechanical ventilation34. Finally, this remains an open question in our study and needs to be investigated in follow-up research.
The calculated risk score based on a reduced variable set (Table 3) from our data allows a sufficient prognosis beyond that explained by baseline factors. Overall, the T3P-score shows a sensitivity of 0.68 and a specificity of 0.96, which is comparable to well-established scores (e.g., qSOFA35). According to our study, it remains open to what extent the score is applicable to populations other than the German population; therefore, it should be validated for use in additional populations.
Strengths and limitations
Our study has several limitations. Due to the retrospective nature of the study, drawing definitive conclusions about the clinical utility of the score is limited. Prospective evaluation of any clinical decision rule using the T3P-Score is warranted. Moreover, our study focused on adult patients and did not evaluate paediatric patients at risk for tracheostomy. Due to the middle-aged mean age in our study, the results have limited applicability to geriatric patients. Another limitation is the use of the inclusion criterion AISThorax. Although this is used regularly, in the future it could be replaced by alternative scoring systems (e.g. RibScore) due to improved discrimination. Sepsis and pneumonia are diseases that develop days after admission. For this reason, they are at risk of time bias. Their predictive value has to be evaluated in future studies. In contrast, we were able to investigate a large group of patients using data sets from six German level I trauma centres. We consider the resulting validity of the score to be high. Additionally, the bias that could arise from data from only one centre is excluded by the multicentre approach. Scores such as the T3P-Score, have been developed on the basis of national data. For this reason, the use of the score is recommended only after external validation in the respective population.
Conclusions
The T3P Score predicted the need for a tracheostomy when assessed among severely injured patients with severe thoracic trauma. The predictive validity was high, and further studies should investigate the scores in different populations.
Data availability
The data are available from the corresponding author upon request.
Abbreviations
- AIS:
-
Abbreviated injury scale
- AOR:
-
Adjusted odds ratios
- BCa:
-
Bias-corrected and accelerated
- CPR:
-
Cardiopulmonary resuscitation
- ECMO:
-
Extracorporeal membrane oxygenation
- GCS:
-
Glasgow coma scale
- ICU:
-
Intensive care unit
- ISS:
-
Injury severity score
- LOS:
-
Length of stay
- MOF:
-
Multiple organ failure
- MV:
-
Mechanical ventilation
- NIV:
-
Non-invasive ventilation
- NO:
-
Nitric oxide
- OR:
-
Odds ratio
- RCT:
-
Randomized controlled study
- RISC II:
-
Revised injury severity score II
- TBI:
-
Traumatic brain injury
- TR-DGU:
-
TraumaRegister DGU®
- TTT:
-
Time-to-tracheostomy
References
Bayer, J. et al. Thoracic trauma severity contributes to differences in intensive care therapy and mortality of severely injured patients: Analysis based on the TraumaRegister DGU(R). World J. Emerg. Surg. 12, 43 (2017).
Andriolo, B., Andriolo, R., Saconato, H., Atallah, Á. & Valente, O. Early versus late tracheostomy for critically ill patients. In Cochrane Database of Systematic Reviews (Wiley, 2015).
Mallick, A. & Bodenham, A. R. Tracheostomy in critically ill patients. Eur. J. Anaesthesiol. 27(8), 676–682 (2010).
Fichtner, F., Mörer, O., Laudi, S., Weber-Carstens, S. & Kaisers, U. S3-Leitlinie Invasive Beatmung und Einsatz extrakorporaler Verfahren bei akuter respiratorischer Insuffizienz. DIVI 4, 154–163 (2017).
Schonhofer, B. et al. Prolonged weaning—S2k-guideline published by the German Respiratory Society. Pneumologie 73(12), 723–814 (2019).
Barbas, C. S. et al. Brazilian recommendations of mechanical ventilation 2013. Part I. Revista Brasileira de terapia intensiva 26(2), 89–121 (2014).
Young, D., Harrison, D. A., Cuthbertson, B. H., Rowan, K. & TracMan, C. Effect of early vs late tracheostomy placement on survival in patients receiving mechanical ventilation: The TracMan randomized trial. JAMA 309(20), 2121–2129 (2013).
Wutzler, S. et al. Pneumonia in severely injured patients with thoracic trauma: Results of a retrospective observational multi-centre study. Scand. J. Trauma Resuscitation Emerg. Med. 27(1), 31 (2019).
Collins, G. S., Reitsma, J. B., Altman, D. G. & Moons, K. G. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. BMJ 350, g7594 (2015).
TraumaRegister, D. G. U. 20 years TraumaRegister DGU((R)): Development, aims and structure. Injury 45(Suppl 3), S6–S13 (2014).
Baker, S. P., O’Neill, B., Haddon, W. Jr. & Long, W. B. The injury severity score: A method for describing patients with multiple injuries and evaluating emergency care. J. Trauma 14(3), 187–196 (1974).
Vincent, J. L. et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 22(7), 707–710 (1996).
Bone, R. C. et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101(6), 1644–1655 (1992).
Saklad, M. Grading of patients for surgical procedures. In The Journal of the American Society of Anesthesiologists: 1941 281–284 (The American Society of Anesthesiologists, 1941).
Schurink, C. A. M. et al. Clinical pulmonary infection score for ventilator-associated pneumonia: Accuracy and inter-observer variability. Intensive Care Med. 30(2), 217–224 (2004).
Austin, P. C. & Steyerberg, E. W. Graphical assessment of internal and external calibration of logistic regression models by using loess smoothers. Stat. Med. 33(3), 517–535 (2014).
Steyerberg, E. W. et al. Internal validation of predictive models: Efficiency of some procedures for logistic regression analysis. J. Clin. Epidemiol. 54(8), 774–781 (2001).
Ranganathan, P., Pramesh, C. S. & Aggarwal, R. Common pitfalls in statistical analysis: Logistic regression. Perspect. Clin. Res. 8(3), 148–151 (2017).
de Franca, S. A., Tavares, W. M., Salinet, A. S. M., Paiva, W. S. & Teixeira, M. J. Early tracheostomy in severe traumatic brain injury patients: A meta-analysis and comparison with late tracheostomy. Crit. Care Med. 48(4), e325–e331 (2020).
Quinones-Ossa, G. A. et al. Current status of indications, timing, management, complications, and outcomes of tracheostomy in traumatic brain injury patients. J. Neurosci. Rural Pract. 11(2), 222–229 (2020).
Terragni, P. P. et al. Early vs late tracheotomy for prevention of pneumonia in mechanically ventilated adult ICU patients: A randomized controlled trial. JAMA 303(15), 1483–1489 (2010).
Galvagno, S. M. Jr., Nahmias, J. T. & Young, D. A. advanced trauma life support((R)) update 2019: Management and applications for adults and special populations. Anesthesiol. Clin. 37(1), 13–32 (2019).
Hilbert-Carius, P. et al. Care for severely injured persons: Update of the 2016 S3 guideline for the treatment of polytrauma and the severely injured. Anaesthesist 66(3), 195–206 (2017).
Karch, S. B., Lewis, T., Young, S., Hales, D. & Ho, C. H. Field intubation of trauma patients: Complications, indications, and outcomes. Am. J. Emerg. Med. 14(7), 617–619 (1996).
Kung, S. C. et al. Epidemiologic characteristics and outcomes of major trauma patients requiring prolonged mechanical ventilation. Medicine 96(52), e9487 (2017).
Hosokawa, K., Nishimura, M., Egi, M. & Vincent, J.-L. Timing of tracheotomy in ICU patients: A systematic review of randomized controlled trials. Crit. Care 19(1), 424 (2015).
Lin, W. C., Chen, C. W., Wang, J. D. & Tsai, L. M. Is tracheostomy a better choice than translaryngeal intubation for critically ill patients requiring mechanical ventilation for more than 14 days? A comparison of short-term outcomes. BMC Anesthesiol. 15, 181 (2015).
Duggal, A., Perez, P., Golan, E., Tremblay, L. & Sinuff, T. Safety and efficacy of noninvasive ventilation in patients with blunt chest trauma: A systematic review. Crit Care 17(4), R142 (2013).
Coughlin, T. A., Ng, J. W., Rollins, K. E., Forward, D. P. & Ollivere, B. J. Management of rib fractures in traumatic flail chest: A meta-analysis of randomised controlled trials. Bone Joint J. 98-B(8), 1119–1125 (2016).
Pieracci, F. M. et al. A multicenter, prospective, controlled clinical trial of surgical stabilization of rib fractures in patients with severe, nonflail fracture patterns (Chest Wall Injury Society NONFLAIL). J. Trauma Acute Care Surg. 88(2), 249–257 (2020).
Nseir, S. et al. Relationship between tracheotomy and ventilator-associated pneumonia: A case control study. Eur. Respir. J. 30(2), 314–320 (2007).
Stamatiou, R., Tsolaki, V., Hatziefthimiou, A., Zakynthinos, E. & Makris, D. Evaluation of airway inflammation in mechanically ventilated patients using cell count and protein concentration. Sci. Rep. 11(1), 19803 (2021).
Hardcastle, T. C., Muckart, D. J. J. & Maier, R. V. Ventilation in trauma patients: The first 24 h is different!. World J. Surg. 41(5), 1153–1158 (2017).
Ranieri, V. M. et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome A randomized controlled trial. JAMA 282(1), 54–61 (1999).
Adegbite, B. R. et al. A comparison of different scores for diagnosis and mortality prediction of adults with sepsis in Low-and-Middle-Income Countries: A systematic review and meta-analysis. EClinicalMedicine 42, 101184 (2021).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
The initiative to build the used database originates from the Trauma Section of the German Interdisciplinary Association of Intensive and Emergency Medicine. F.M.B., H.A., and F.H. designed the study. H.A. supervised the study. Data acquisition was done by S.W., P.S., T.L., M.F., M.M., M.W., J.B., M.C., A.S., I.M., and H.A. F.M.B. performed the statistical analysis. H.A. maintains the database. F.H. provided the resources. F.M.B. prepared the original draft. Review and editing was done by J.B., K.H., A.S., I.M., and F.H. All authors significantly contributed to the conduct of the study and had full access to the data. All authors have read and agreed to the published version of the manuscript. F.M.B., F.H., and H.A. were responsible for the decision to submit the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bläsius, F.M., Wutzler, S., Störmann, P. et al. Predicting tracheostomy in multiple injured patients with severe thoracic injury (AIS ≥ 3) with the new T3P-Score: a multivariable regression prediction analysis. Sci Rep 13, 3260 (2023). https://doi.org/10.1038/s41598-023-30461-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-30461-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.