Abstract
The mixed hemimicelle-based solid phase extraction method using the coated sodium dodecyl sulfate by magnetic iron oxide nanoparticles as adsorbent was developed for extraction and determination of Sunitinib malate in real samples prior to determination by UV–Visible spectrophotometry. For the characterization of synthesized nanoparticles, Fourier transform infrared spectroscopy, and scanning electron microscopy was used. The influences of different factors affecting the extraction efficiency of Sunitinib malate, including the pH, the adsorbent amount, the volume and eluent type, the amount of the surfactant, the ionic strength, extraction, and desorption time, were investigated. At the optimized conditions, a good linearity with correlation coefficients of 0.998 and 0.999 was obtained over the concentration ranges of 1–22 and 1–19 µg/mL for water and urine samples, in order. The good recoveries of 97% and 99% and also, the limits of detection equal with 0.9, and 0.8 µg/mL for water and urine samples were enhanced, respectively. These results demonstrate that mixed hemimicelle solid phase extraction is a fast, efficient, economical and selective sample preparation method for the extraction and determination of Sunitinib malate in different water and urine sample solutions.
Similar content being viewed by others
Introduction
Sunitinib malate is a novel oral multi-targeted tyrosine kinase inhibitor with established efficacy in treating metastatic renal cell carcinoma and imatinib-resistant gastrointestinal stromal tumor1,2,3,4. Sunitinib malate prevents the growth of cancer cells by obstructing a group of closely linked tyrosine kinase receptors, containing vascular endothelial growth factor receptors platelet-derived growth factor receptor, FMS-related tyrosine-kinase 3 and stem cell factor receptor (FCF c-KIT) at nanomolar concentrations5,6,7,8,9. In addition, SM is approved for the therapy of kidney cancer, advanced renal cell carcinoma, and gastrointestinal stromal tumors10,11,12. Therefore, the determination of Sunitinib malate in water and also in biological fluids has found significance. To date, various analytical techniques have been introduced for the determination of Sunitinib malate, such as LC–MS/MS, HPLC, and high-performance liquid chromatography-UV methods13,14,15. The need for the complicated instruments, on the one hand, and being inexpensive and time-consuming, on the other hand, arouse us to utilize spectrophotometry measurements which presents not only quick analysis but also ease of operation and being economic16,17,18.
An accurate and sensitive procedure such as sample purification and preconcentration are essential prior to instrumental analysis19,20,21,22,23,24,25,26,27. Several pre-treatment methods have been employed for the extraction of Sunitinib malate, including LLE, SPE, and CPE28,29,30,31. Solid phase extraction has gained more research interest due to being simple, and also flexible in terms of selecting the desirable solid phase as adsorbent, which results in the maximum preconcentration factor, short extraction time and low cost according to the low usage of the organic solvents32,33.
More recently, MIONPs have been widely applied in analytical research as a flexible adsorbent for the extraction and determination of various chemical analytes34,35,36,37,38,39. The magnetic nanoparticles provide some advantages over the traditional sorbents, for instance, the high surface area, short diffusion route, high number of surface-active sites, and superparamagnetic features, which lead to the maximum extraction recovery, rapid extraction, and simplicity of extraction for samples with large volumes40,41,42,43. Nanoparticles are inconstant because of the high level of surface energy, which might end in the nanoparticles agglomeration and less yield of operation44,45. To inhibit this problem and improve the extraction efficiency, the surface of the nanoparticles is modified with different organic and, or inorganic materials46,47. Surfactants are such organic subcategory that is utilized in this research according to some advantages. First and foremost, SPE is based on the adsorption of surfactant on the surface of the adsorbent, which makes mixed (hemimicelles and, or admicelles) (MHSPE), act as a solid–liquid interface and could be utilized for the extraction and preconcentration of any types of samples from various matrices17,30,31,48. Secondly, the modification procedure is simple and could be performed during the extraction; accordingly, it is time-consuming48. In this called method, as MHSPE, the ionic surfactants such as SDS, CTAB and, etc., were adsorbed on the surface of the mineral oxides such as, silica, titanium dioxide, alumina, and iron oxides32,45. The combination of mixed hemimicelles with solid phase extraction and magnetic nanoparticles has plenty of advantages, such as high extraction yield, regeneration of sorbent, high breakthrough volume, easy analytes elution, and no clean-up steps31. Facile process, being fast and modifying at the same time of extraction, another motivational reason to select it as the pretreatment method for most drugs and pollutants10,33,40,47.
In this study, modified Fe3O4 nanoparticles with sodium dodecyl sulfate was formed mixed based solid-phase extraction method, which was successfully employed for extraction and determination of Sunitinib malate in urine and water samples by UV–Visible spectrophotometry technique. According to our literature survey, it is for the first time that the mixed hemimicelles solid phase extraction has been applied for the extraction and determination of Sunitinib malate in urine and water samples.
Results and discussion
Characterization of magnetic iron oxide nanoparticles
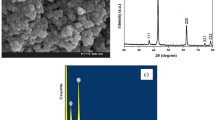
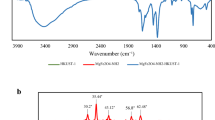
The SEM and IR were utilized to characterize the synthesized adsorbent, which modified with SDS. According to our previous work45, absorption bands appeared at 529, 477 and 3202 cm-1 confirm the true synthesis of Fe3O4 since they are related to the FeO, Fe2O3, and the surface OH group of the magnetic nanoparticles, respectively. After modification, new peaks, which stand for the S = O group and stretching mode of the aliphatic C-H groups of SDS were, emerged at 1252 and both 2928 & 2842 cm−1, respectively. These peaks demonstrated that the surface of MIONPs was successfully modified with SDS. Furthermore, SEM images (Fig. 1) show the uniform spherical shape of the modified magnetic nanoparticle with the size of about 39–59 nm.
Mixed hemimicelles-based solid-phase extraction optimization conditions
To maximize the extraction efficiency of Sunitinib malate, the influences of different factors such as the pH, the amounts of adsorbent, and surfactant, the ionic strength, eluent type and volume and extraction /desorption time were investigated and optimized. All the experiments were done three times and the averages of the results were applied for optimization. The UV–vis spectra of Sunitinib malate is shown in Fig. 2. All detections were performed at λmax = 425 nm.
Effect of pH
The most significant parameter affecting the formation of mixed-hemimicells based on the solid adsorbent is the pH of the sample solution49. The pH range of 3–10, which was adjusted by applying of 0.1 M HCl or NaOH solution, was investigated. As it is shown in Fig. 3, the highest absorption amount is related to pH 3, and a more increase in the pH from 3 to 10, leads to a reduction in the adsorption of SM. This can be related to the decrease in positive charge density on the MIONPs surface, on the other hand, the positively charged surface of MIONPs in acidic solutions was desirable for the adsorption of anionic surfactants like SDS. The point of zero charge of MIONPs is about 6.550. When the pH of the solution is above the isoelectric point of the MIONPs, the surface charge density of the MIONPs becomes less positive; hence, adsorption of SDS on nanoparticle surfaces is reduced due to the lack of the formation of mixed. Therefore, electrostatic attraction, between the negative head group of SDS as anionic surfactant and the positive surface of MIONPs is potent enough to produce hemimicelles. Despite the mentioned reason, the acidic solution sustains the positive charge of Sunitinib malate and provides the suitable electrostatic attraction with the negative head group of SDS, which is placed at the second layer of mixed hemimicelle. Accordingly, for the optimum value, pH = 3 was selected.
Effect of SDS amount
The SDS concentration effect, on the extraction efficiency of Sunitinib malate, was considered by adding different concentrations of SDS from 1 to 9 mg/mL to the sample solution. As shown in Fig. 4, in the absence of SDS, the Sunitinib malate hardly adsorbed on the surface of the adsorbent; on the other hand, the adsorption of SM on the surface of magnetic iron oxide nanoparticles is in strict need of modification. The notably increased adsorption is enhanced by the addition of 2 mg of surfactant, which is large enough for the formation of admicelles. By the movements toward smaller amounts of SDS the structure of admicelles changes to mixed hemimicelles and hemimicelles which are not proper for the adsorption of SM. Higher amounts of 2 mg are the beginning point of micelle formation which lead to the gradual decrease in adsorption of SM due to the redistribution of Sunitinib malate in the bulk solution. Therefore, 2 mg of SDS was chosen as the optimum value for the next experiments.
Effect of ionic strength
The ionic strength of the studied solution on extraction efficiency was evaluated by the utilization of NaCl with various concentrations of 1–10% (w /v). The results indicate that in the presence of salt, especially in extensive amounts, desorption of Sunitinib malate is diminished based on the competitive behaviour of SM and salt for the adsorption on the surface of adsorbent. Accordingly, no salt addition was utilized for the rest of experiments.
Effect of the MIONPs amount
The amount of the adsorbent definitely affects the mechanism of adsorption and extraction efficiency. In order to choose the required quantity of adsorbent (MIONPs) for the extraction of Sunitinib malate, different concentrations of MIONPs suspension were used in the range of 0.08–0.42 mg/mL. Nanoparticles have a higher surface area than ordinary sorbents (micron-size particle sorbents), which result in high extraction capacity and fast extraction dynamics. Therefore, satisfactory results can be obtained with fewer amounts of sorbents. As shown in Fig. 5, the extraction recovery of Sunitinib malate increased with increasing sorbent amounts from 80 up to 320 mg/mL of Fe3O4 nanoparticles, due to the increased accessible adsorption sites. The more addition of the adsorbent diminished the adsorption of SM, due to the dramatic reversal in admicelle formation, which ended in mixed and, or hemimicelles structures. Therefore, 320 mg/mL of the magnetic adsorbent amount was selected as the optimum.
The influence of kind and the volume of the eluent
To enhance the maximum release of SM, different organic solvents such as acetonitrile, ethanol, methanol, ethylene glycol and water were studied. As shown in Fig. 6a, the highest extraction recovery of Sunitinib malate was obtained by the use of methanol which conforms to the necessity of the utilization of organic solvent for the disruption of admicelles, structure. Furthermore, the highest solubility of SM in methanol in comparison with others might be the reason for the highest absorbance. Figure 6b exhibits the effect of different volumes of methanol in the range from 2 to 8 mL on the extraction efficiency of Sunitinib malate. By incrementing the volume to 3 mL, the extraction efficiency increased, and the diminution was observed for the higher amounts 3 to 8 ml. It would attribute to the analytes dilution in the high volumes of the eluent. Hence, for the optimum eluent volume, 3 mL of methanol was selected. Moreover, our primary solution, which extracted was acidic, and therefore, 3 ml of the basic solution (methanol: water; 94:6 v/v) demonstrated the best extraction efficiency, and was selected as the desirable extractor.
The impact of time on both extraction and back extraction
Extraction/back extraction time is defined as the needed time to bring both solution and adsorbent into contact in order to enhance the complete transfer of analyte to adsorbent/extractor solvent and in solid phase extraction is measured as the time for the shaking by the application of a shaker45. This extraction/ back extraction time must be long enough to make solution/adsorbent approximately bare of analyte. In this study, the effect of extraction and back extraction times on the extraction efficiency of Sunitinib malate were investigated in the range of 1–5 and 1–9 min, respectively. The results showed that the highest extraction efficiency was obtained in the shortest tested time as extraction time (1 min). After 1 min, the adsorption remained constant with less reduction. Also, the maximum desorption was observed at 7 min. The desorption's were incomplete in the times shorter than 7 min and then decreased. The rapid dynamic process and high surface area of MIONPs along with homogeneous distribution of the nano-sorbent throughout the sample and could be the possible reasons for gaining a rapid extraction process. In a word, MIONPs-based MHSPE is a method with the capability of a fast separation procedure which leads to a short analysis time. Therefore, 1 and 7 min were selected as the optimized adsorption and desorption time.
Analytical performance
In order to validate the proposed method, at the optimized experimental conditions, some figures of merits such as the limit of detection and quantification, the linear range of calibration curve, correlation of determinations (R2), and the accuracy and precision of the method were examined. The analytical performances of the presented method are presented in Table 1. The calibration curve was linear in the range from 1 to 22 and 1 to 19 µg/mL with determination coefficients of 0.998 and 0.0999 for water and urine samples, respectively. The LOD and LOQ were defined according to the IUPAC recommendation as follows: LOD = 3.3 (SD/S) and LOQ = 10 (SD/S) where SD is the standard deviation of the blank and S is the slope of calibration curve. The LODs were 0.9 and 0.8 µg/mL and also the LOQs were 2.9 and 2.6 µg/mL for determination of Sunitinib malate in water and urine samples, respectively. Moreover, to determine the relative standard deviation (%) of the analytical method five replicates were done for each concentration. The method accuracy was investigated by the measurement of relative error which is 100 multiplied by the resulting subtraction of founded and added concentration of SM in spiked samples divided to added concentrations. As the results reveal in Table 1, the proposed method exhibited good linearity, appropriate precision and low LOD and LOQ for the determination of the analyte.
Real sample analysis
Under the optimized conditions, the mixed hemimicelles SPE method was applied for the extraction and determination of Sunitinib malate in spiked tap water and urine samples. Since Sunitinib malate was not detected in the real samples, 10 µg/mL of Sunitinib malate was added into the water and urine samples, and extraction was done based on the procedure. The analytical results are summarized in Table 2. It can be seen, that the enhanced recoveries are about 97% and 99% for water samples and urine samples, respectively, which indicate the suitability of MIONPs sorbents for selective extraction and determination of Sunitinib malate in real samples. Also, the relative standard deviations (RSDs %) for five replicate extractions were 0.67 and 0.41% and the gained relative errors were 3.1 and 0.04 for water and urine, respectively; indicating the proper precision and accuracy of the method. Also, the performance of the proposed method was compared to that of other reported methods for determination of Sunitinib Malate and the results are presented in Table 3.
Conclusion
The proposed mixed hemimicelles solid phase extraction was developed for extraction and determination of Sunitinib malate in tap water and urine samples prior to spectrophotometric determination. Sodium dodecyl sulfate was utilized for the modification of magnetic iron oxide nanoparticles and the construction of mixed hemimicelles. The proposed method provides various advantages like short analysis time, simplicity, low cost, high extraction efficiency, low detection limits and proper recoveries. This method presents satisfactory repeatability and accuracy for the extended dynamic linear ranges of 1–22 and 1–19 µg/ml for water and urine samples, respectively. Also, the satisfactory recoveries and precision of the proposed MHSPE method indicate that MIONPs sorbents have considerable potential for the extraction and determination of Sunitinib malate from biological fluids. Finally, this method was handled for designation of SM in water and urine samples with low detection limits and proper recoveries of 97 and 99, respectively. Hence, the proposed analysis method could be successfully applied for extraction and determination of drugs in real samples.
Experimental
Materials and instruments
All used reagents were of analytical grade and distilled water was used for making of all the aqueous solutions. The FeCl3.6H2O, FeCl2.4H2O, SDS, NaOH (99%), HCl (37%), aqueous ammonia (25 wt%), NaCl, methanol, ethanol, ethylene glycol, acetonitrile, dimethyl sulfoxide and Sunitinib malate were purchased from Merck (Darmstadt, Germany). The molecular structure of Sunitinib malate is presented in Fig. 7. A stock solution of the Sunitinib malate (100 mg/L) was prepared by dissolving proper amount of Sunitinib malate in dimethyl sulfoxide and methanol and stored at 4 °C. All the solutions were prepared by suitable diluting of the stock solution with distilled water. The Climo-Shaker ISF1-X (Kuhner AG, Switzerland) was used for shacking of the mixtures. A Jenway model 4510 (Stone, UK), pH meter with a glass electrode was applied for the pH measurements. All of spectrophotometric measurements of the solutions were done by a Analytic Jena SPECORD 250 UV–vis spectrophotometer (Germany). The UV detection of Sunitinib malate was performed at 425 nm. The FT-IR spectra of the prepared nanoparticles was carried out by a Shimadzu prestige-21 (Japan) FT-IR spectrophotometer in the range of 400–4000 cm-1. The structure of the synthesized nanoparticles was characterized by a scanning electron microscopy (SEM, LEO 1430VP).
Magnetic iron oxide nanoparticles preparation
The nanoparticles of Fe3O4 were synthesized with the help of a conventional co-precipitation method similar to our previous works45. The 4.3 g of FeCl2·4H2O and 11.68 g of FeCl3.6H2O were dissolved in 200 mL of deionized water under a nitrogen atmosphere at 85 °C. An ammonia solution (20 mL, 30% w/w) was added dropwise into the mixture with vigorous stirring (400 rpm) for 30 min. The orange color of the solution changed to black quickly. After cooling down to room temperature, the obtained MNPs precipitate was separated from the reaction mixture under the magnetic field. The magnetite precipitates were eluted twice with deionized water, and once with 0.02 mol/L sodium chloride.
Preparation of real samples
The efficiency of this method was evaluated by the application of water and urine as real samples. Tap water samples were freshly prepared from our analytical laboratory (Ardabil, Iran). Water samples were just filtered with a Millipore filter before extraction. The urine samples were taken from a voluntary female. The collected urine samples were centrifuged at 2500 rpm for 20 min at room temperature to sediment white lipidic solid, then filtered with a 0.2 µm syringe filter. The spiked urine samples were diluted 1:10 using distilled water, in order to decrease the matrix effect.
Ethics declaration
The urine sample preparation method was carried out in accordance with relevant guidelines and regulations. The urine sample was taken from a voluntary female and informed consent of participating subject, was obtained. The researchers institute did not require approval for the use of urine in experiments in this instance.
Magnetic MHSPE procedure
The step-by-step procedure to accomplish MHSPE of Sunitinib malate, was as follows (Fig. 8): a 10 mL of the aqueous solution containing Sunitinib malate (10 µg/mL) was transferred into a 25 mL beaker. Next, an amount of 0.5 mL (30 mg/mL) of Fe3O4 NPs suspension and 0.2 mL of the SDS solution (2 mg/mL) were sequentially added. In order to complete the extraction process, the mixtures were shacked and permitted for 5 min. After that, the SDS-coated MIONPs were isolated from the solutions using an external magnet. After 2 min, the solutions became clear and supernatant solutions were decanted. The 5 mL of methanol was used for the elution of preconcentrated analyte from the isolated adsorbent. The concentration of Sunitinib malate was determined at a wavelength of 425 nm using a UV–Vis spectrophotometer.
Data availability
All data supporting the conclusions of this research article are included within the manuscript.
Abbreviations
- MHSPE:
-
Mixed hemimicelle-based solid phase extraction
- SDS:
-
Sodium dodecyl sulfate
- SDS-MIONPs:
-
Sodium dodecyl sulfate—magnetic iron oxide nanoparticles
- SM:
-
Sunitinib malate
- FT-IR:
-
Fourier transform infrared spectroscopy
- SEM:
-
Scanning electron microscopy
- LOD:
-
Limits of detection
- LOQ:
-
Limit of quantification
- TKI:
-
Tyrosine kinase inhibitor
- VEGFR:
-
Vascular endothelial growth factor receptors
- PDGF:
-
Platelet derived-growth factor receptor
- GIST:
-
Gastrointestinal stromal tumors
- LC–MS/MS:
-
Liquid chromatography-tandem mass spectrometry
- HPLC:
-
High-performance liquid chromatography
- LLE:
-
Liquid–liquid extraction
- SPE:
-
Solid-phase extraction
- CPE:
-
Cloud point extraction
- MIONPs:
-
Magnetic iron oxide nanoparticles
- CTAB:
-
Cetyltrimethyl ammonium bromide
- PZC:
-
Point of zero charge
- FeCl3·6H2O:
-
Ferric chloride hexahydrate
- FeCl2·4H2O:
-
Ferrous chloride tetrahydrate
- NaOH:
-
Sodium hydroxide
- HCl:
-
Hydrochloric acid
- NaCl:
-
Sodium chloride
References
Speed, B. et al. Pharmacokinetics, distribution, and metabolism of [14C] sunitinib in rats, monkeys, and humans. Drug Metab. Dispos. dmd. 111.042853 (2011).
He, L. et al. LC-ESI-MS/MS determination of simotinib, a novel epidermal growth factor receptor tyrosine kinase inhibitor: application to a pharmacokinetic study. J. Chromatogr. B. 947, 168–172 (2014).
Qiu, F., Bian, W., Li, J. & Ge, Z. Simultaneous determination of sunitinib and its two metabolites in plasma of Chinese patients with metastatic renal cell carcinoma by liquid chromatography–tandem mass spectrometry. Biomed. Chromatogr. 27, 615–621 (2013).
Zhou, Q. & Gallo, J. M. Quantification of sunitinib in mouse plasma, brain tumor and normal brain using liquid chromatography–electrospray ionization-tandem mass spectrometry and pharmacokinetic application. J. Pharm. Biomed. Anal. 51, 958–964 (2010).
Rodamer, M., Elsinghorst, P. W., Kinzig, M., Gütschow, M. & Sörgel, F. Development and validation of a liquid chromatography/tandem mass spectrometry procedure for the quantification of sunitinib (SU11248) and its active metabolite, N-desethyl sunitinib (SU12662), in human plasma: application to an explorative study. J. Chromatogr. B. 879, 695–706 (2011).
Oberoi, R. K., Mittapalli, R. K., Fisher, J. & Elmquist, W. F. Sunitinib LC–MS/MS assay in mouse plasma and brain tissue: application in CNS distribution studies. Chromatographia 76, 1657–1665 (2013).
Rizwana, I., Prakash, K. V. & Mohan, G. K. Analytical method development and validation for the estimation of sunitinib malate in bulk and its pharmaceutical dosage form using RP-HPLC. Indo. Am. J. Pharm. Res. 4, 561–565 (2014).
van Erp, N. P. et al. Pharmacogenetic pathway analysis for determination of sunitinib-induced toxicity. J. Clin. Oncol. 27, 4406–4412 (2009).
de Bruijn, P. et al. Bioanalytical method for the quantification of sunitinib and its n-desethyl metabolite SU12662 in human plasma by ultra performance liquid chromatography/tandem triple-quadrupole mass spectrometry. J. Pharm. Biomed. Anal. 51, 934–941 (2010).
Andriamanana, I., Gana, I., Duretz, B. & Hulin, A. Simultaneous analysis of anticancer agents bortezomib, imatinib, nilotinib, dasatinib, erlotinib, lapatinib, sorafenib, sunitinib and vandetanib in human plasma using LC/MS/MS. J. Chromatogr. B. 926, 83–91 (2013).
Ghazaghi, M., Mousavi, H. Z., Shirkhanloo, H. & Rashidi, A. Ultrasound assisted dispersive micro solid-phase extraction of four tyrosine kinase inhibitors from serum and cerebrospinal fluid by using magnetic nanoparticles coated with nickel-doped silica as an adsorbent. Microchim. Acta. 183, 2779–2789 (2016).
Musijowski, J., Piórkowska, E. & Rudzki, P. J. Determination of sunitinib in human plasma using liquid chromatography coupled with mass spectrometry. J. Sep. Sci. 37, 2652–2658 (2014).
Blanchet, B. et al. Development and validation of an HPLC-UV-visible method for sunitinib quantification in human plasma. Clin. Chim. Acta. 404, 134–139 (2009).
Lankheet, N. A. et al. Method development and validation for the quantification of dasatinib, erlotinib, gefitinib, imatinib, lapatinib, nilotinib, sorafenib and sunitinib in human plasma by liquid chromatography coupled with tandem mass spectrometry. Biomed. Chromatogr. 27, 466–476 (2013).
Minkin, P. et al. Quantification of sunitinib in human plasma by high-performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. B. 874, 84–88 (2008).
Bagheri, H., Zandi, O. & Aghakhani, A. Extraction of fluoxetine from aquatic and urine samples using sodium dodecyl sulfate-coated iron oxide magnetic nanoparticles followed by spectrofluorimetric determination. Anal. Chim. Acta. 692, 80–84 (2011).
Ershad, S. & Razmara, A. Extraction and preconcentration of indomethacin with magnetic nanoparticles adsorbent prior to its spectrophotometic determination. Int. J. PharmTech Res. 9, 55–61 (2016).
Kolaei, M., Dashtian, K., Rafiee, Z. & Ghaedi, M. Ultrasonic-assisted magnetic solid phase extraction of morphine in urine samples by new imprinted polymer-supported on MWCNT-Fe3O4-NPs: CENTRAL composite design optimization. Ultrason. sonochem. 33, 240–248 (2016).
Zhao, J., Liao, W. & Yang, Y. Magnetic solid-phase extraction for determination of sulpiride in human urine and blood using high-performance liquid chromatography. Biomed. Chromatogr. 29, 1871–1877 (2015).
Mirabi, A. & Aliakbari, N. Preparation of modified magnetic nanocomposites dithiooxamide/Fe3O4 for preconcentration and determination of trace amounts of cobalt ions in food and natural water samples. J. Chem. Health Risks. 6, 291–303 (2016).
Pourbasheer, E., Qasemi, F., Rouhi, M., Azari, Z. & Ganjali, M. R. Preconcentration and determination of 2-mercaptobenzimidazole by dispersive liquid–liquid microextraction and experimental design. J. Sep. Sci. 40, 2467–2473 (2017).
Razmi, R. et al. Preconcentration and determination of ceftazidime in real samples using dispersive liquid–liquid microextraction and high-performance liquid chromatography with the aid of experimental design. J. Sep. Sci. 39, 4116–4123 (2016).
Banaei, A., Vojoudi, H., Karimi, S., Bahar, S. & Pourbasheer, E. Synthesis and characterization of new modified silica coated magnetite nanoparticles with bisaldehyde as selective adsorbents of Ag(I) from aqueous samples. RSC Adv. 5, 83304–83313 (2015).
Rouhi, M., Pourbasheer, E. & Ganjali, M. R. Optimization of dispersive liquid–liquid microextraction combined with high performance liquid chromatography for the analysis of dipyridamole in water and urine samples. Monatsh Chem. 146, 1593–1601 (2015).
Pourbasheer, E., Sadafi, S., Ganjali, M. R. & Abbasghorbani, M. Dispersive liquid-liquid microextraction for preconcentration and determination of phenytoin in real samples using response surface methodology-high performance liquid chromatography. RSC Adv. 4, 62190–62196 (2014).
Borgul, P. et al. Oxidized thin aluminum films used as the polarized liquid-liquid interface support for norcocaine detection. Sens. Actuators B. Chem. 373, 132651 (2022).
Roosendaal, J. et al. Development and validation of LC–MS/MS methods for the quantification of the novel anticancer agent guadecitabine and its active metabolite β‑decitabine in human plasma, whole blood and urine. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1109, 132–141 (2019).
Parham, H. & Rahbar, N. Solid phase extraction–spectrophotometric determination of salicylic acid using magnetic iron oxide nanoparticles as extractor. J. Pharm. Biomed Anal. 50, 58–63 (2009).
Gholivand, M. B. & Torkashvand, M. Electrooxidation behavior of warfarin in Fe3O4 nanoparticles modified carbon paste electrode and its determination in real samples. Mater. Sci. Eng. C. 48, 235–242 (2015).
Du, J. et al. Mixed hemi/ad-micelle SDS-coated magnetic Fe2− xAlxO3 (x= 0.4) nanoparticles for the capture of gatifloxacin and prulifloxacin coupled with fluorimetric determination. Anal. Methods. 8, 2778–2785 (2016).
Bavili Tabrizi, A. & Dehghani Teymurlouie, N. Application of sodium dodecyl sulfate coated iron oxide magnetic nanoparticles for the extraction and spectrofluorimetric determination of propranolol in different biological samples. J. Mex. Chem. Soc. 60, 108–116 (2016).
Esmaeili‐Shahri, E. & Es'haghi, Z. Superparamagnetic Fe3O4@ SiO2 core–shell composite nanoparticles for the mixed hemimicelle solid‐phase extraction of benzodiazepines from hair and wastewater samples before high‐performance liquid chromatography analysis. J. Sep. Sci. 38, 4095–4104 (2015).
Amoli-Diva, M., Pourghazi, K. & Pourasadollah-Karani, H. Surfactant coated magnetic nanoparticle-based solid-phase extraction coupled with spectrophotometric detection for determination of ultra-trace amounts of indomethacin in biological fluids. Micro Nano Lett. 10, 135–139 (2015).
Tabrizi, A. B., Rashidi, M. R. & Ostadi, H. A nanoparticle-based solid-phase extraction procedure followed by spectrofluorimetry to determine carbaryl in different water samples. J. Braz. Chem. Soc. 25, 709–715 (2014).
Zandipak, R. & Taghavi, L. Synthesis of 2, 4-dinitrophenylhydrazine loaded sodium dodecyl sulfate-coated magnetite nanoparticles for adsorption of Hg (II) ions from an aqueous solution. Environ. Health Eng. Manage. J. 3, 183–189 (2016).
Ganjali, M. R., Pourbasheer, E. & Rezapour, M. Dextran capped Gd3+-doped Fe3O4 nanoparticles: electrochemical synthesis and characterization. Anal. Bioanal. Electrochem. 10, 394–403 (2018).
Asfaram, A., Ghaedi, M., Goudarzi, A., Soylak, M. & Mehdizadeh Langroodi, S. Magnetic nanoparticle based dispersive micro-solid-phase extraction for the determination of malachite green in water samples: optimized experimental design. New J. Chem. 39, 9813-9823 (2015).
Habila, M. A. et al. Mercaptobenzothiazole-functionalized magnetic carbon nanospheres of type Fe3O4@SiO2@C for the preconcentration of nickel, copper and lead prior to their determination by ICP-MS. Microchim. Acta 183, 2377–2384 (2016).
Habila, M. A., Alothman, Z. A., El-Toni, A. M., Labis, J. P. & Soylak, M. Synthesis and application of Fe3O4@SiO2@TiO2 for photocatalytic decomposition of organic matrix simultaneously with magnetic solid phase extraction of heavy metals prior to ICP-MS analysis. Talanta 154, 539–547 (2016).
Faraji, M., Yamini, Y. & Rezaee, M. Extraction of trace amounts of mercury with sodium dodecyle sulphate-coated magnetite nanoparticles and its determination by flow injection inductively coupled plasma-optical emission spectrometry. Talanta 81, 831–836 (2010).
Ranjbari, E., Hadjmohammadi, M. R., Kiekens, F. & De Wael, K. Mixed hemi/Ad-micelle sodium dodecyl sulfate-coated magnetic iron oxide nanoparticles for the efficient removal and trace determination of rhodamine-B and rhodamine-6G. Anal. chem. 87, 7894–7901 (2015).
Sharifabadi, M. K., Tehrani, M. S., Mehdinia, A., Azar, P. A. & Husain, S. W. Fast removal of citalopram drug from waste water using magnetic nanoparticles modified with sodium dodecyl sulfate followed by UV-spectrometry. J. Chem. Health Risks. 3 (2013).
Sobhanardakani, S., Zandipak, R. & Cheraghi, M. Synthesis of DNPH/SDS/Fe3O4 nanoparticles for removal of Cr (VI) ions from aqueous solution. Avicenna J. Environ. Health Eng. 3, e7789–e7798 (2016).
Mashhadizadeh, M. H. & Amoli-Diva, M. Atomic absorption spectrometric determination of Al3+ and Cr3+ after preconcentration and separation on 3-mercaptopropionic acid modified silica coated-Fe3O4 nanoparticles. J. Anal. At. Spectrom. 28, 251–258 (2013).
Azari, Z., Pourbasheer, E. & Beheshti, A. Mixed hemimicelles solid-phase extraction based on sodium dodecyl sulfate (SDS)-coated nano-magnets for the spectrophotometric determination of Fingolomid in biological fluids. Spectrochim. Acta, Part A 153, 599–604 (2016).
Banaei, A. et al. Synthesis of silica gel modified with 2, 2′-(hexane-1, 6-diylbis (oxy)) dibenzaldehyde as a new adsorbent for the removal of Reactive Yellow 84 and Reactive Blue 19 dyes from aqueous solutions: equilibrium and thermodynamic studies. Powder Technol. 319, 60–70 (2017).
Banaei, A. et al. Adsorption equilibrium and thermodynamics of anionic reactive dyes from aqueous solutions by using a new modified silica gel with 2, 2′-(pentane-1, 5-diylbis (oxy)) dibenzaldehyde. Chem. Eng. Res. Des. 123, 50–62 (2017).
Muthukumaran, C., Sivakumar, V. M. & Thirumarimurugan, M. Adsorption isotherms and kinetic studies of crystal violet dye removal from aqueous solution using surfactant modified magnetic nanoadsorbent. J. Taiwan Inst. Chem. Eng. 63, 354–362 (2016).
Beiraghi, A., Pourghazi, K. & Amoli-Diva, M. Mixed supramolecular hemimicelles aggregates and magnetic carrier technology for solid phase extraction of ibuprofen in environmental samples prior to its HPLC-UV determination. Chem. Eng. Sci. 108, 103–110 (2014).
Rajabi, A. A., Yamini, Y., Faraji, M. & Nourmohammadian, F. Modified magnetite nanoparticles with cetyltrimethylammonium bromide as superior adsorbent for rapid removal of the disperse dyes from wastewater of textile companies. Nanochem. Res. 1, 49–56 (2016).
Author information
Authors and Affiliations
Contributions
E.P. acted as the corresponding author, supervising the overall research and the manuscript preparation. L.M. and Zh.A. performed the experiments. V.H.M., and M.R.G. supported the experiments and preparation of the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pourbasheer, E., Malekpour, L., Azari, Z. et al. Magnetic solid phase extraction of Sunitinib malate in urine samples assisted with mixed hemimicelle and spectrophotometric detection. Sci Rep 13, 3361 (2023). https://doi.org/10.1038/s41598-023-30404-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-30404-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.