Abstract
In recent years, the report of loggerhead sea turtle (Caretta caretta) Mediterranean nesting range has expanded together with new records of nests becoming northward on the Italian coastline of the Tyrrhenian and Adriatic seas. These areas are characterized by intensive human activities, such as tourism, fishery, and marine traffic, all possibly involved in the influence of the use of coastal habitat by marine species. These anthropic threats, in addition to the natural ones and the changing environmental characteristics of the beach, may influence the growth of microorganisms causing hatching failures. Among microorganisms, fungal infection by the genus Fusarium (Link, 1809) is considered one of the main causes of globally declining sea turtle populations. In summer 2021, the two northernmost worldwide loggerhead sea turtle nests were monitored along the Northern Adriatic coastline (Veneto, Italy). These first records may potentially candidate this area as suitable for a large part of the loggerhead turtle’s life cycle and it could represent a minor sea turtle nesting area that, according to Prato and colleagues, remained unnoticed due to the lack of specific monitoring. Sea Turtle Egg Fusariosis (STEF) was deemed to have deeply compromised the hatching success of the northmost one. Climate change and anthropogenic impacts have been scored as one of the highest hazards to sea turtle health and could have played a role in the STEF development. Environmental changes, human activities, and emerging pathogens deserve the highest attention in terms of health research, and conservation management.
Similar content being viewed by others
Introduction
The loggerhead turtle, Caretta caretta (Linnaeus, 1758), is the most abundant sea turtle species in the Mediterranean Sea, representing a distinct population with specific demographic and genetic features1,2. Due to its conservation interest, it is listed in different international conventions for its protection (Habitat Directive 92/43/CEE; Convention on International Trade of Endangered Species; Bonn Convention; Bern Convention), and it has been deemed as “Vulnerable” in the International Union for Conservation of Nature (IUCN) Red List assessment.
In the Mediterranean Sea, every year loggerhead turtle nesting activities are reported with an estimation of more than 8000 eggs clutches annually laid3, mainly located in the easternmost region (i.e. Greece, Turkey, Cyprus, and Libya). The presence of nesting activities is also reported along the Italian coastlines4, with more frequent reports along the southern coastlines (i.e. Calabria, Sicily, Campania). In recent years, the report of loggerhead turtle nesting activity has increased with a total of 244 nests in 2021 in Italy (tartapedia.it), together with an expansion of the nesting range in the Mediterranean Sea2, and with new records of nests northward the Tyrrhenian and Adriatic coastlines5,6. These areas are characterized by more intensive human activities, such as tourism, fishery, and marine traffic, all possibly involved in the influence of the use of coastal habitat by marine species7,8,9,10,11,12. These anthropic activities, in addition to the natural threats, could impair egg-laying, embryotic development, carapacial abnormalities, and hatchlings survival on the beach13. For instance, during the embryotic stages, the hatchling success could be affected by a combination of factors such as human disturbance (i.e. beach cleaning, tourist activities), pollution, depredation, beach erosion, sediment granulometry, plant root invasion, excessive rainfall, tidal inundation, gas flow, salinity, humidity, and pathogenic infections14,15,16,17. All these factors may influence the physical parameters crucial for healthy embryonic development18 and the growth of microorganisms15, such as fungi and bacteria, reducing the hatchling success and causing embryonal death. Among microorganisms, fungal infections by the genus Fusarium (Link 1809) is considered one of the main causes of globally declining turtle populations15,19,20 also known as Sea Turtle Egg Fusariosis (STEF). Fusarium spp. members, including those belonging to the F. solani species complex (FSSC) and F. oxysporum species complex, have been isolated from the eggshells and embryonic tissue of failed sea turtle eggs for decades, with several hypotheses concerning their ecological role ranging from decomposition to pathogenicity21. Approximately 75% of fusariosis is caused by members of the FSSC, two closely related fungal species, F. keratoplasticum and F. falciforme22. They have been isolated from undeveloped eggs and embryos and deemed to be the causative agents of STEF15,23,20 causing mass mortalities in natural and relocated nests of the loggerhead turtles worldwide15.
The present study reports the case of the two northmost loggerhead turtles nesting sites reported to the best of our knowledge, which occurred along the northern Adriatic coastline (Veneto, Italy) in the summer of 2021. Furthermore, it describes how STEF has deeply compromised the hatching success of the northernmost one.
Results
Nests’ characteristics and reproductive output are described in Table 1. In Nest 1, rainfalls and tides were constantly monitored to promptly face flooding of the area by permanent barricades and temporary sheltering. Collected data compared to the 2021 pluviometric data from the Regional Environmental Protection Agency confirmed an increase in rainfalls (+ 32%) in Jesolo compared to the period 1993–2020.
During the inspection of Nest 1, 43 unhatched eggs, 1 embryo and 2 pipped eggs showed gross evidence consistent with fusariosis, including abnormal pink discoloration and incomplete development (later stage 23–31) (Fig. 1). No carapacial abnormalities were recorded on the hatchlings nor on the unhatched turtles. No additional gross findings were reported during the post-mortem examination of collected embryos.
The affected eggs were located at the periphery of the clutch, while the hatched eggs occupied the core. Eggs with yolk without any and the hatched eggs (25) occupied the deeper layer and the core of the incubation chamber respectively.
The microscopic examination of embryos revealed mild to moderate cellular degeneration in all the layers of the epidermis, mildly affecting also the level of the basement membrane and the superficial dermis, which appeared edematous and with loosely arranged collagen fibers. A severe diffuse thickening of the keratin layer with preserved keratinocyte maturation (orthokeratotic hyperkeratosis) was also evident (Fig. 2a). No other histological and microbiological findings consistent with any viral and/or bacterial infection were noted during the examination of the embryos. PAS and Grocott’s methenamine silver stains revealed an extensive fungal growth with myriads of yeasts and hyphae attached to the eggshell and the skin of the developing carapace, intermingled within the epidermal layers and, in less number, also in the dermis (Fig. 2b). The numerous hyaline hyphal elements were characterized by septation, acute-angle dichotomous or random branching, constrictions at branch points, and parallel thin walls, consistent with Fusarium spp. No evident inflammatory infiltrate was associated to these findings.
Nest 1 microscopical findings. Histological appearance of a section of an embryo’s skin (a) affected by multifocal degeneration of epidermal cells and orthokeratotic hyperkeratosis. (b) PAS stain underlined the presence of myriads of hyaline branched septate hyphae, 2 to 7 μm in diameter with thin, predominantly parallel wall attached to the skin, embedded within the epidermal layers (arrowhead) and in the superficial dermis (arrow). (a): hematoxylin and eosin staining, scale bar = 50 µm; (b): PAS staining, scale bar = 20 µm.
Fungal culture tested positive for filamentous fungi, growing from both eggshells, embryo, and yolk showing an intensive growth of Fusarium organisms. F. oxysporum was identified with similarity of 100% by blasting 28S rRNA sequencing in Genbank database. The mortality rate associated with Fusarium spp. was deemed to be 56.25%.
Discussion
Marine biodiversity is affected by the changes related to global warming in different ways. Among all the species, sea turtles at any life stage are particularly susceptible to environmental changes and human activities24.
In the context of climate change, migration patterns, habitat use, sex ratio and embryonic development could be influenced by the effects of increased temperature25. Furthermore, these two nesting episodes added some concerns regarding the challenges in the management and monitoring of sea turtle nests in terms of interaction with human activities and health problems.
The two turtle nests hereby described, the episodes reported in Marche Region (Pesaro) in 2019, and the two in Liguria (Finale Ligure in 2021 and Levanto in 2022), as well as the confirmed increase in sea temperature in the Adriatic basin26,27 contribute to strengthen the hypothesis of the nesting activity expansion toward the northern coastline of the Western Mediterranean in the period 2010 – 20202,24. In this context, Jesolo Lido and Scano Boa can be considered the northernmost nesting sites in the Mediterranean Sea ever monitored and, most likely, worldwide28. As shown by the data reported in Table 1, the two nests showed different hatching success with the lower results (11%) in the most urbanized location (Jesolo Lido) compared to the average (66%) reported in the Western Mediterranean2. Human presence and activities, such as the urbanization, the touristic preparation of the beach, and pollution, could have also compromised embryonal survival24,29.
The histopathological investigations reveal the presence of a F. oxysporum infection in Nest 1 which has been considered the cause of the lower hatchling success in this area. The infection was detected in the shallowest layer of the incubation chamber, while the hatched eggs occupied the deepest part of the core suggesting that they might have been protected by those more superficial. Moreover, Nest 1 compared to Nest 2 was shallower, with a different substrate composition, a lower slope, and a more intense impact of the tide, hence, these factors may have also influenced the hatchling success. No particular differences were reported on the hatchlings size also due to the few numbers of hatchlings monitored in Nest 2.
In marine ecosystems, the prevalence of infectious diseases caused by fungi has dramatically increased during the past two decades, likely due to the transmission of emerging pathogens into new environments and the rapid rate of global climate change30,31. Among these, F. solani has been recognized as the most frequent fungus in sea turtle mycotic diseases, and it is normally isolated and referred to as a “species complex” including more than 60 phylogenetic species15. These fungi can be found in the turtles’ digestive system and they could colonize eggs during their deposition, but they have also been reported on floating particles of plant tissues, silt, and plastics in the ocean which are carried by wind and currents to the beaches where the turtles lay their eggs32. Environmental factors can help the mycotic dissemination on failed eggs: the disease incidence and mortality are strongly affected by tidal inundation and sand with a high percentage of silt and clay23. Nest 1 area is a heavily urbanized beach with a large tidal range as shown by the distance of the nesting area from the shore (from 2 to 50 m depending on the tide) and a gentle slope artificially created by beach nourishment using sand from inland areas of riverine origin, including silt, clay and organic material to cope with the coastal erosion. Beach nourishment activities, which are constantly performed from early spring in the area of Nest 1, may also have influenced on hatchling success33,34,29. Also, the cleaning constantly performed using mechanical systems could have influenced the hatching success by inducing a shallower deposition and reducing the nest dimensions due to soil compaction, as evident by comparing the two different beaches in Table 135. The aforementioned environmental factors (i.e. sand composition with organic matter, small and superficial nest chamber, tidal occurrence), besides being associated to a lower hatchling success, could have also influenced the development and diffusion of FSSC36 which has been globally recognized as a potential global threat to sea turtle eggs37. On the opposite side, Nest 2 occurred in a wilder area without human activities ongoing and showed a clearly higher hatching success rate (89%).
In conclusion, climate change and anthropogenic impacts have been scored as one of the highest hazards to sea turtle health and need the highest attention in terms of research and conservation management38. Additionally, identifying potential pathogens threatening endangered sea turtle species, also influenced by global warming and human activities, is crucial for developing conservation plans. The results of the present study contribute to the recent hypothesis of an expansion of the loggerhead sea turtle nesting area occurring in the Mediterranean basin, likely due to global warming2,24.
These first recordings of nesting activity and the historical data on loggerhead turtles39 may potentially candidate the Northern Adriatic Sea as a suitable area for large part of the loggerhead turtle’s life cycle, and it could represent a minor sea turtle nesting area as defined by Prato and colleagues (2022), which could have remained unnoticed due to the lack of specific monitoring4,40. The expansion in highly urbanized areas, with several anthropic activities having a negative impact on hatchling success, requires wider monitoring coverage as a priority action for sea turtle conservation. To cope with this possible menace, a dialogue between economical and conservation stakeholders should be focused on a management plan ensuring the coexistence of sustainable economic activities and the conservation of endangered species. These plans should include: (1) development of nest suitability models, real-time monitoring, nest protection, and inspection to cope with the negative effects of anthropic activities; (2) effective management strategies for the control of emerging diseases including their epidemiology23; and (3) the possible application of relocation practice, even if it remains unclear whether such an approach increases pathogens contamination or carry, hence the risk of FSSC infection and mortality in sea turtle eggs41.
Materials and methods
Nesting sites and monitoring
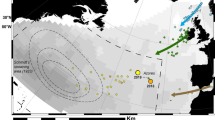
The present study was performed along the Veneto coastline, in the northwestern Adriatic Sea, Italy. The Northern Adriatic Sea is considered an important foraging and overwintering habitat, especially for subadult and adult female loggerhead turtles39. Sandy beaches and shallow waters characterized the coastal area, where no sea turtle nesting activity has ever been recorded. In July 2021, sea turtle nesting activity was reported by locals in Jesolo Lido (Venezia), one of the major touristic beaches in Italy with 1.491.296 and 1.738.396 tourists respectively in July and August 2021 (Statistical Data of Tourism from Veneto Region). After examination and confirmation of the nest presence (Nest 1), the area was protected and systematically monitored. Environmental parameters (temperature, humidity, weather, and tidal trends) were continuously monitored to prevent or face any flooding and record possible environmental influences on the hatchling success. Weather conditions were compared with historical data from the Regional Environmental Protection Agency (ARPAV)42.In September 2021, the presence of hatchlings was reported also in Scano Boa (Rovigo), a wild beach included in the Parco Regionale Veneto del Delta del Po (WDPA 178,945; EUAP 1062). This nest (Nest 2) was examined and confirmed too. After 13 days of the conclusion of the hatching period, no hatching, and a total of 81 days from the nesting of Nest 1, both nests were inspected, according to the Italian ministerial guidelines43. The main characteristics and reproductive outputs were recorded.
Nest sample collection and characteristics
During the inspection, samples of sand were collected for granulometry analysis and to assess further characteristics of both nests44. All the eggs of the nests were collected to assess any possible changes in their color as a possible indication of the presence of microorganisms15. Furthermore, the eggs were examined to determine the development stage and the external morphological characteristics; those still unhatched were finally opened to assess the presence of any dead embryo according to the description by Miller and colleagues45.
Post-mortem investigations
Gross inspection has been performed on embryos in order to assess any pathological change according to standardized post-mortem procedures46,47.
Eggshell, embryo and yolk samples from all unhatched eggs both with macroscopic changes and from 3 specimens (1 embryo and 2 pipped eggs) without apparent macroscopic lesions were collected for additional analysis. The samples from eggshell, embryo and yolk for microscopic examination were fixed in 10% formalin, embedded in paraffin, sectioned at 5 µm and mounted onto TOMO Adhesion Microscope Slides (Matsunami Glass), stained with hematoxylin and eosin (HE) using a semi-automatic histo-stainer (Leica Autostainer XL, Leica Biosystems Nussloch GmbH). Additionally, periodic acid–Schiff (PAS) and Grocott’s methenamine silver stain were performed in case of suspected fungal infections. Additionally, standard bacteriological examinations were routinely performed on the above-mentioned fresh eggshell, embryo, and yolk samples.
Fungal culture and Fusarium species identification
Since gross pathological findings of the Nest 1 were consistent with Fusariosis, samples from eggshell, embryo and yolk were cultured on Sabouraud dextrose agar and incubated at 25 °C for at least 10 days. All fungal colonies identified morphologically belonging to the Fusarium genus were further molecularly processed for identification at species level. DNA extracted from Fusarium colonies was amplified by using SYBR Green Real-Time PCR (rtPCR) with a set of primers targeting a portion of the D1-D2 domain of the 28S rRNA gene using primers NL1/NL4 as previously described48. All amplicons were sequenced for fungal identification by using Blast in the GenBank database.
Animal ethics
All methods were carried out in accordance with relevant Italian ministerial guidelines and regulations (Linee guida per il recupero, soccorso, affidamento e gestione delle tartarughe marine ai fini della riabilitazione e per la manipolazione a scopi scientifici. ISPRA-MATTM, Manuali e Linee Guida 89, 2013).
Data availability
All data generated or analyzed during this study are included in this published article.
References
Wallace, B. P. et al. Regional management units for marine turtles: A novel framework for prioritizing conservation and research across multiple scales. PLoS One 5(12), e15465 (2010).
Hochscheid, S. et al. Nesting range expansion of loggerhead turtles in the Mediterranean: Phenology, spatial distribution and conservation implications. Glob. Ecol. Conserv. 38, e02194 (2022).
Casale, P. et al. Mediterranean sea turtles: Current knowledge and priorities for conservation and research. Endanger. Species Res. 36, 229–267 (2018).
Prato, O. O. et al. Minor sea turtle nesting areas may remain unnoticed without specific monitoring: The case of the largest Mediterranean Island (Sicily, Italy). Animals 12, 1221 (2022).
De Silva, M. et al. Mediterranean Sea: Caretta caretta nestings at high latitudes, minimally invasive approach to promote their survival. 7th Mediterranean Conference on Marine Turtles, Morocco, October 18–21 (2022).
Masotti, C. et al. Sea turtle in Liguria: state of the art and future perspectives. III National congress of C.Re.Ta.M. (National Reference Center on Welfare, Monitoring and Diagnostics of Sea Turtle Diseases), Italy, December 1–2 (2022).
Depellegrin, D. et al. Multi-objective spatial tools to inform maritime spatial planning in the Adriatic Sea. Sci. Total Environ. 609, 1627–1639 (2017).
Niavis, S. et al. Revealing the potential of maritime transport for ‘Blue Economy’ in the Adriatic-Ionian Region. Case Stud. Transp. Policy 5(2), 380–388 (2017).
Dimitriadis, C. et al. Reduction of sea turtle population recruitment caused by nightlight: Evidence from the Mediterranean region. Ocean Coast. Manag. 153, 108–115 (2018).
Vlachogianni, et al. Marine litter on the beaches of the Adriatic and Ionian Seas: An assessment of their abundance, composition and sources. Mar. Pollut. Bull. 131, 745–756 (2018).
Fortuna, C. et al. The coherence of the European Union Marine Natura 2000 network for wide-ranging charismatic species: A Mediterranean case study. Front. Mar. Sci. 5, 356 (2018).
European Commission 2021 “The Potential of Maritime Spatial Planning in the Mediterranean Sea” Case Study Report: The Adriatic Sea.
Casale, P. & Margaritoulis, D. Sea Turtles in the Mediterranean: Distribution Threats and Conservation Priorities (IUCN, 2010).
Santoro, M., Hernández, G., Caballero, M. & Garcı́a, F. Aerobic bacterial flora of nesting green turtles (Chelonia mydas) from Tortuguero National Park, Costa Rica. J. Zoo Wildl. Med. 37, 549–552 (2006).
Sarmiento-Ramírez, J. et al. Global distribution of two fungal pathogens threatening endangered sea turtles. PLoS One 9, e85853 (2014).
Durmuş, S. H., Ilgaz, Ç., Gü çlü, Ö. & Özdemir, A. The effect of the predicted air temperature change on incubation temperature, incubation duration, sex ratio and hatching success of loggerhead turtles. Anim. Biol. 61, 369–383 (2011).
Caracappa, S. et al. Incidental catch of loggerhead sea turtles (Caretta caretta) along the sicilian coasts by longline fishery. PeerJ 6, e5392 (2018).
Ackerman, R. A. The nest environment and the embryonic development of sea turtles. In The Biology of Sea Turtles Vol. 1 (eds Lutz, P. L. & Musick, J. A.) 83–106 (CRC Press, 1997).
Sáenz, V. et al. A one health perspective to recognize fusarium as important in clinical practice. J. Fungi 6, 235. https://doi.org/10.3390/jof6040235 (2020).
Cafarchia, C. et al. Fusarium spp. in loggerhead sea turtles (Caretta caretta): From colonization to infection. Vet. Pathol. 57(1), 139–146 (2020).
Phillott, A. D. & Parmenter, C. J. Influence of diminished respiratory surface area on survival of sea turtle embryos. J. Exp. Zool. 289, 317–321 (2001).
Zhang, N. et al. Members of the Fusarium solani species complex that cause infections in both humans and plants are common in the environment. J. Clin. Microbiol. 44, 2186–2190 (2006).
Smyth, C. W. et al. Unraveling the ecology and epidemiology of an emerging fungal disease, sea turtle egg fusariosis (STEF). PLoS Pathog. 15(5), e1007682 (2019).
Mancino, C., Canestrelli, D. & Maiorano, L. Going west: Range expansion for loggerhead sea turtles in the Mediterranean Sea under climate change. Glob. Ecol. Conserv. 38, e02264 (2022).
Laloë, J. O., Cozens, J., Renom, B., Taxonera, A. & Hays, G. C. Climate change and temperature-linked hatchling mortality at a globally important sea turtle nesting site. Glob. Change Biol. 23, 4922–4931 (2017).
García-Monteiro, S., Sobrino, J. A., Julien, Y., Sòria, G. & Skokovic, D. Surface temperature trends in the Mediterranean Sea from MODIS data during years 2003–2019. Reg. Stud. Mar. Sci. 49, 102086 (2022).
Zampollo, A. et al. Seasonal niche and spatial distribution modelling of the loggerhead (Caretta caretta) in the Adriatic and Ionian seas. Aquat. Conserv. Mar. Freshw. Ecosyst. 32(7), 1141–1155 (2022).
Sénégas, J. B., Hochscheid, S., Groul, J. M., Lagarrigue, B. & Bentivegna, F. Discovery of the northernmost loggerhead sea turtle (Caretta caretta) nest. Mar. Biodivers. Rec. 2, 1–4 (2009).
Patino-Martinez, J. et al. Light sandy beaches favour hatching success and best hatchling phenotype of loggerhead turtles. Front. Ecol. Evol. https://doi.org/10.3389/fevo.2022.823118 (2022).
Groner, M. L. et al. Managing marine disease emergencies in an era of rapid change. Philos. Trans. R. Soc. B 371(1689), 20150364 (2016).
Harvell, D., Altizer, S., Cattadori, I. M., Harrington, L. & Weil, E. Climate change and wildlife diseases: When does the host matter the most?. Ecology 90, 912–920 (2009).
Duncan, E. M. et al. Microplastic ingestion ubiquitous in marine turtles. Glob. Change Biol. 25(2), 744–752 (2019).
Grain, D. A., Bolten, A. B. & Bjorndal, K. A. Effects of beach nourishment on sea turtles: Review and research initiatives. Restor. Ecol. 3, 95–104 (1995).
Rumbold, D. G., Davis, P. W. & Perretta, C. Estimating the effect of beach nourishment on Caretta caretta (loggerhead sea turtle) nesting. Restor. Ecol. 9(3), 304–310 (2001).
Marco, A., Abella, E., Martins, S., López, O. & Patino-Martinez, J. Female nesting behaviour affects hatchling survival and sex ratio in the loggerhead sea turtle: Implications for conservation programmes. Ethol. Ecol. Evol. 30(2), 141–155 (2018).
Gleason, F. H., Allerstorfer, M. & Lilje, O. Newly emerging diseases of marine turtles, especially sea turtle egg fusariosis (SEFT), caused by species in the Fusarium solani complex (FSSC). Mycology 11(3), 184–194 (2020).
Bailey, J. B., Lamb, M., Walker, M., Weed, C. & Craven, K. S. Detection of potential fungal pathogens Fusarium falciforme and F. keratoplasticum in unhatched loggerhead turtle eggs using a molecular approach. Endanger. Species Res. 36, 111–119 (2018).
Mashkour, N. et al. Disease risk analysis in sea turtles: A baseline study to inform conservation efforts. PLoS One 15(10), e0230760 (2020).
Casale, P., Laurent, L. & De Metrio, G. Incidental capture of marine turtles by the Italian trawl fishery in the north Adriatic Sea. Biol. Conserv. 119(3), 287–295 (2004).
Margaritoulis, D. An estimation of the overall nesting activity of the loggerhead turtle in Greece. In Proc. of the 18th International Sea Turtle Symposium. NOAA Technical Memorandum NMFS-SEFSC-436, 48–50 (2000).
Hoh, D. Z., Lin, Y. F., Liu, W. A., Sidique, S. N. M. & Tsai, I. J. Nest microbiota and pathogen abundance in sea turtle hatcheries. Fungal Ecol. 47, 100964 (2020).
Mo, G., Montalto, F., Serangeli, M.T. & Duprè, E. Linee guida per il recupero, soccorso, affidamento e gestione delle tartarughe marine ai fini della riabilitazione e per la manipolazione a scopi scientifici. ISPRA-MATTM, Manuali e Linee Guida 89 (2013).
Dick, R. P., Thomas, D. R. & Halvorson, J. J. Standardized methods, sampling, and sample pretreatment. In Methods for Assessing Soil Quality Vol. 49 (eds Doran, J. W. & Jones, A. J.) 107–121 (Madison, 1996).
Miller, J. D., Mortimer, J. A. & Limpus, C. J. A field key to the developmental stages of marine turtles (Cheloniidae) with notes on the development of Dermochelys. Chelonian Conserv. Biol. 16(2), 111–122 (2017).
Orós, J., Torrent, A. Manual de necropsia de tortugas marinas. Ediciones del Cabildo de Gran Canaria, LasPalmas de Gran Canaria (2001).
Flint, M. et al. Post mortem diagnostic investigation of disease in free-ranging marine turtle populations: A review of common pathological findings and protocols. J. Vet. Diagn. Investig. 21, 733–759 (2009).
Galosi, L. et al. Atypical mycosis in psittacine birds: A retrospective study. Front. Vet. Sci. 12(9), 883276 (2022).
Acknowledgements
This research would not have been possible without the exceptional support of Parco Regionale Veneto del Delta del Po, the Jesolo Municipality, the Coast Guard, and the Regional Environmental Protection Agency (ARPAV). We would also like to thank Legambiente Veneto, WWF Veneto, Plastic Free Veneto, Sea Shepherd Conservation Society Veneto, Marevivo Veneto, Museo Civico di Storia Naturale di Jesolo, and all the volunteers for their commitment in all the monitoring phases.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
G.P.: conceptualization; formal analysis, writing-original draft, writing-review and editing. C.C.: conceptualization, formal analysis, writing-original draft, writing-review and editing. G.S.: investigation, formal analysis, methodology, writing-review and editing. L.C.: investigation, formal analysis. P.D.: investigation, formal analysis, writing-review and editing. D.P.: investigation, formal analysis. S.M.: methodology, conceptualization, writing-original draft, writing-review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pietroluongo, G., Centelleghe, C., Sciancalepore, G. et al. Environmental and pathological factors affecting the hatching success of the two northernmost loggerhead sea turtle (Caretta caretta) nests. Sci Rep 13, 2938 (2023). https://doi.org/10.1038/s41598-023-30211-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-30211-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.