Abstract
In this work, CoxZn1−xFe2O4 (x = 0.0–0.4) nanoparticles (NPs) were successfully synthesized by a hydrothermal method at 200 °C for 12 h. X-ray diffraction revealed a pure cubic spinel phase of all samples with space group Fd-3m. Fourier transform infrared spectrometry disclosed the vibrational modes of metal oxides in the spinel structure. Scanning electron microscopy and transmission electron microscopy disclosed a uniform distribution of cuboidal shape NPs with a decreased average NPs size from 22.72 ± 0.62 to 20.85 ± 0.47 nm as the Co content increased. X-ray absorption near edge spectroscopy results confirmed the presence of Zn2+, Co2+ and Fe2+/Fe3+ in Co-doped samples. The pore volume, pore size and specific surface area were determined using N2 gas adsorption/desorption isotherms by the Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) techniques. Electrochemical properties of supercapacitors, having active CoxZn1−xFe2O4 (x = 0.0–0.4) NPs as working electrodes, indicated pseudo-capacitor performance related to the Faradaic redox reaction. Interestingly, the highest specific capacitance (Csc), 855.33 F/g at 1 A/g, with a capacity retention of 90.41% after 1000 GCD cycle testing was achieved in the Co0.3Zn0.7Fe2O4 electrode.
Similar content being viewed by others

Introduction
Currently, multi-functional nanomaterials with intriguing properties are very important and necessary for many emerging novel technologies. They are essential parts of many electronic devices, specifically as electrodes in the energy collectors of fuel cells, batteries and supercapacitors (SCs). Moreover, increasing need for them is seen due to the immense increase in the demand for clean and sustainable energy. Particularly, SCs are a unique type of electrochemical device that stores energy via a collection of charges/ions on the electrodes surface, which can offer many benefits over conventional batteries and capacitors1,2,3. Generally, a SC cell consists of two separate electrodes immersed in an electrolyte. The chemical reactions between charges/ions in the electrolyte and electrodes materials are key functions that directly impact the performance of a SC cell. Practically, two important types of SCs are pseudo-capacitors (PCs) and electric double layer capacitors (EDLCs)4,5. Principally, each type of SC has similar cell structure, but a different charge storage mechanism and electrode material. For EDLCs, charges are stored in a very thin double layer on electrodes surface. Whereas in PCs, the reversible redox reactions are the major response for charge storage. In both types of SCs, the cell efficiency strongly depends on many parameters such as surface area, pore structure, and conductivity of active materials used for electrode fabrication4. Thus, a search for novel electrode materials with these good properties is an active area for researchers6. Usually, for PCs, various transition metal oxides (TMOs) and conducting polymers are generally employed for electrode fabrication because they can provide higher specific capacitance (Csc) and energy density (Ed). This due to their fast Faradaic redox reaction during charging/discharging compared with carbon materials7. Moreover, numerous nanosized (5–50 nm) TMOs have been extensively studied. Many of them were found to be suitable for SCs electrodes with high electrochemical performance. This is due to their increased electroactive sites which offer appropriate conductive pathways and extensive nanopaths to promote efficient transportation of charges/ionic species2,8,9,10. During the past decade, spinel TMOs of the AB2O4 structure (A and B represent metal elements at tetrahedral and octahedral sites, respectively ) of CuFe2O42, MnFe2O44, NiFe2O43,6, CoFe2O41,6,7,9 and ZnFe2O410,11,12,13,14,15 were extensively studied as electrode materials for PCs. This is due to their advantage of a polymorphic structure of normal, inverse and mixed spinel forms in which metal ions with a + 2 oxidation state (M2+) and (Fe2+/Fe3+) can be distributed at A or/and B sites. In normal spinel, M2+ and (Fe2+/Fe3+) ions are at A and B sites, respectively. For inverse spinel, M2+ ions replace some of (Fe2+/Fe3+) ions at the B site, while (Fe2+/Fe3+) ions can occupy both the A and B sites. In the case of mixed spinel, M2+ and (Fe2+/Fe3+) ions can be distributed at both A and B sites. As reported by earlier researchers, the Csc values of various AB2O4 ferrites of different morphologies vary over a wide range from a hundred to more than a thousand. For example, Ni0.4Co0.6Fe2O4 nanoparticles (NPs) (237 F/g at 1 A/g)5, CoFe2O4/NiFe2O4 nanospheres (269 F/g at 1 A/g)6, electrospun carbon/CuFe2O4 nanofibers (191 F/g at 10 mV/s)2, NiFe2O4@CoFe2O4 core–shell nanofibers (480 F/g at 1 A/g)3, ZnFe2O4 NPs (712 F/g at 2 mV/s)15, CoFe2O4 NPs (1,210 F/g at 1 F/g)1 and Ni1−xMnxFe2O4 NPs (1,221 F/g at 0.5 A/g)4. Among numerous AB2O4 materials, spinel zinc ferrite (ZnFe2O4) is of great interest. It has been extensively investigated due to its fascinating properties, natural richness, low cost, and environmental friendliness10,11,15. However, the development of high-performance ZnFe2O4 electrode materials remains a greater challenge due to their poor stability in cycling tests, as reported by Vadiyar et al.12. Owing to this problem, different approaches such as using material composites13 and structure modifications by doping with various transition metals (TMs) in MxZn1−xFe2O4 (for instance M = Co, Cu, Ni, Mn) have been attempted. However, doping with TMs was suggested as the effective method because it can significantly affect the ion distribution at A and B sites of spinel crystals of these materials and on the surface of ferrites. This makes them more active and sensitive to Faradaic redox reactions during charging/discharging process16. From literatures, Co was considered to be one of the effective TM that had been intensively used as dopant with successful improvement many properties of materials17,18,19.
Herein, it is our aim to synthesize CoxZn1−xFe2O4 (x = 0.0–0.4) NPs using a hydrothermal method, because it is a facile process that can generally occur at low temperature, without toxicity due to the use of metallic salts that can be dissolved in water20,21. Moreover, a high yield NPs of narrow size distribution can be obtained as compared to other method22. The influence of Co doping on the physical properties of the materials such as their phase, crystal structure, morphology, functional groups, surface area and oxidation states of metal ions, were studied using various techniques. Furthermore, the electrochemical properties of the obtained products were studied for SCs application. To the best of our knowledge, the electrochemical properties of hydrothermally obtained Co-doped ZnFe2O4 have never been reported.
Experimental
Material synthesis
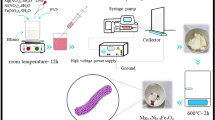
CoxZn1−xFe2O4 (x = 0.0–0.4) NPs were prepared by a hydrothermal method, as schematically shown in Fig. 1. Initially, the stoichiometric amounts of iron nitrate (Fe(NO3)3.9H2O, 5 mmol), zinc nitrate (Zn(NO3)2.6H2O, with 2.5-x mmol) and cobalt nitrate (Co(NO3)2.6H2O, with x = 0.0, 0.1, 0.2, 0.3 and 0.4 mmol) were dissolved in 25 mL deionized (DI) water, using a magnetic stirrer for 2 h at room temperature (RT). Subsequently, 10 ml ethylene glycol (EG) and 6 M KOH were added into the solution and magnetically stirred for 12 h. The obtained solution was hydrothermally treated in a tightly closed autoclave at 200 °C for 12 h in an oven (Memmert, UF55, USA). The obtained products were rinsed with DI water until they became neutral. Finally, the materials were dried in an oven at 80 °C for 6 h and ground to fine powders for further study.
Characterization
The phase and crystal structure of CoxZn1−xFe2O4 (x = 0.0–0.4) NPs were characterized using X-ray diffraction (XRD, a Philips PW3040, Cu Kα, \(\lambda\) = 0.15406 nm). Transmission electron microscopy (TEM, JEOL2010) and scanning electron microscopy (SEM, M-SEM, SNE-4500) were performed to reveal the morphology and structure of the materials which were used to calculate particle size. Fourier transform infrared spectrophotometry (Bruker, Senterra) was used to verify the vibrational modes of bonding between metals and oxygen molecules in the crystal structure. The oxidation states of Zn, Co and Fe cations were analyzed using X-ray absorption near edge spectroscopy (XANES) performed at the K-edge in a transmission mode using the synchrotron light of Beamline 1.1W (2 GeV). Pore volume, pore size and specific surface area were investigated using N2 gas adsorption/desorption isotherms by the Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) techniques (Autosorb-1, Quantachrome). A potentiostat/galvanostat station (CS350 electrochemical workstation; Corrtest, Hubei) was employed for electrochemical property studies via cyclic voltammetry (CV), galvanostatic charge–discharge (GCD) and electrochemical impedance spectroscopy (EIS) measurements in an aqueous 3 M KOH electrolyte using a three-electrode configuration.
Electrode fabrication
The main parts of a cell for electrochemical measurements consists of a working electrode, having CoxZn1−xFe2O4 (x = 0.0–0.4) NPs as active materials with platinum wire and Ag/AgCl as counter and reference electrodes, respectively. CV testing was done at various scan rates (5–100 mV/s) in a potential range from −0.2 to 0.6 V. GCD measurements were performed at 1, 1.5, 2, 2.5, 3, 4, 5, 7.5 and 10 A/g. The stability of electrodes was evaluated after 1000 GCD cycle testing at 5 A/g. Specific capacitance (Csc) was determined using Eq. (1),4
where Csc is obtained in F/g, I is the current density (A/g), Δt is the charging/discharging time (s), m and ΔV represent mass of active materials (g) and working potential window (V), respectively. The specific energy density (Ed, Wh/kg) of electrode was directly related to the Csc value and evaluated using Eq. (2),
In principle, the specific power density (Pd, W/kg) can be derived from Ed and discharge time t as shown in Eq. (3),
Results and discussion
Phase and structure analysis
In Fig. 2, the XRD results of CoxZn1−xFe2O4 (x = 0.0–0.4) NPs in a 2\(\theta\) range from 15° to 70° degree display well distinguishable crystalline peaks, consisting of the (111), (220), (311), (222), (400), (422), (511), and (440) planes. This matches the cubic spinel structure of crystalline ZnFe2O4 standard data (JCPDS Card No. 22–1012) within the Fd-3m space group, in agreement with the work of Perumal et al.10 and Mohamed et al.18. Moreover, all these XRD patterns correspond to a cubic spinel structure of crystalline Fe3O4 and CoFe2O4, as well, since these TMOs have the same crystalline structure shown in Fig. 3a–e.23 These structures illustrate a typical spinel model of Fe3O4, a normal spinel of ZnFe2O4, an inverse spinel of CoFe2O4 and a mixed spinel of CoxZn1−xFe2O4 ferrites, respectively. According to our previous study on the magnetic properties of hydrothermally synthesized CoxZn1−xFe2O4 ferrites24 and similar work of Gözüak et al.25, Mohamed et al.18, Mathew and Juang26 and Feng et al.27, it can be concluded that our hydrothermally synthesized CoxZn1−xFe2O4 (x = 0.1–0.4) NPs are ferrites of a mixed spinel structure. Moreover, the absence of impurities and other secondary phases confirms the purity of all samples and implies that Co ions can be successfully substituted for Zn ions in the ferrite structure. Furthermore, the lattice parameter (a) and average (Av.) crystallite size (DXRD) of all samples were determined by Rietveld refinement using the dominant (220), (311), (400), (422), (511), (440) reflection planes and Scherrer’s formula presented as Eq. (4):7
where the constant k is associated with crystalline shape and is generally taken as 0.9. β is the full width at half maximum of the involved diffraction peaks in radians and X-ray wavelength λ is in nanometers (nm). Calculated a and DXRD values of CoxZn1−xFe2O4 NPs are tabulated in Table 1 and their plots versus Co content are shown in Fig. 4. In this figure, both a and DXRD decrease with increasing Co content, in agreement with the work of Malik et al.28. Slight decreases in a and DXRD with Co loading are subjected to substitution of the smaller ionic radii Co2+ ions (0.580 Å at A site, 0.745 Å at B site) on Zn2+ ions (0.600 Å at A site, 0.740 Å at B site), including the displacement and redistribution of Fe2+ ions (0.63 Å at A site, 0.78 Å at B site) and Fe3+ ions (0. 49 Å at A site, 0.645 Å at B site), as reported by Mohamed et al. (2019) in a study of CoxZn1−xFe2O4 NPs18. The oxidation states of Co, Zn and Fe ions will be further discussed with the XANES analysis below.
Morphological study
SEM micrographs of CoxZn1−xFe2O4 (x = 0.0–0.4) NPs in Fig. 5a–e show a uniform distribution of particles of a cuboidal shape. The particle sizes were estimated, and the obtained average (Av.) values are listed in Table 1. Moreover, plots of Av. particle size vs. Co content is also shown in Fig. 5f. As seen in Table 1 and the plot in Fig. 5f, the Av. nanometer particle sizes decrease from 41.74 ± 0.22 to 40.33 ± 0.13 nm with increasing Co content from x = 0.1–0.4. The decreased particle sizes of Co1−xZnxFe2O4 presented the lowest value for x = 0.2 and then increase with Co content (for x = 0.3 and 0.4). This might be dependent on different growth rates. Co cations may be substituted at A and B sites in different amounts during the preparation process. The homogeneous distribution of NPs was suggested to provide an enhanced active surface area with beneficial pathways for the electrolyte penetration and fast ion/electron transfer. This would result in enhanced electrochemical performance, as reported by Feng et al.6.
TEM images of CoxZn1−xFe2O4 (x = 0.0–0.4) NPs with insets displaying selected area electron diffraction (SAED) patterns and the histograms of estimated Av. particle size are shown in Fig. 6a–e. In these figures, NPs of a cuboidal shape with a uniform dispersion of particle sizes over a narrow range are observed, which is also seen in the SEM images. In the TEM images, the Av. particle sizes of all samples could be more precisely measured using the Image J program. A plot of the obtained values as a function of Co content is shown in Fig. 6f. In this figure, the average particle size decreases with Co loading, corresponding to the SEM results. However, the decreased particle sizes of Co1−xZnxFe2O4 to its lowest value for x = 0.2 and then its increase with Co content (for x = 0.3 and 0.4), as shown in Figs. 5f and 6f, might be dependent on the different growth rates. Additionally, Co cations might be substituted at A and B sites in different amounts during the preparation process. Additionally, all the observed SAED patterns display halo rings with bright spots on their circumferences. These rings can be indexed to the (220), (311), (400), (422), (511) and (440) planes of a ZnFe2O4 phase, indicating the polycrystalline nature of these materials. Moreover, these indexed diffraction rings have been verified to correspond with a Fd-3m space group, agreeing well with the XRD results and the study of Mohamed et al.18. From the XRD, SEM and TEM results, Co doping can significantly reduce the crystallite and particle sizes of CoxZn1−xFe2O4 (x = 0.0ؘ–0.4) NPs.
FTIR study
FTIR measurements were performed over a wide range of wavenumbers from 400 to 4000 cm−1. The obtained spectra are displayed in Fig. 7. As illustrated in this figure, all spectra are similar. The observed spectra at around 3442 and 1626 cm−1 correspond to O–H stretching and bending modes, respectively. This may be due to the absorption of water molecules by KBr pellets during spectroscopic studies. In the case of Co0.3Zn0.7Fe2O4 and Co0.4Zn0.6Fe2O4 NPs, the peak at 1626 cm−1 shifts to a slightly lower frequency upon substitution of Co, implying that both samples are sensitive to moisture and water molecules29. Additionally, the peak at 1435 cm−1 corresponds to the bending modes of anionic carboxylate C-H by ethylene glycol (EG) coated on the surfaces of nanoparticles30,31. EG can function as a stabilizer that uses surfactant molecules to form coordination bonding or strong interaction. This can prevent aggregation of mixed spinel Co0.3Zn0.7Fe2O4 and Co0.4Zn0.6Fe2O4 NPs. Moreover, it can kinetically control the growth rates of crystals and the morphology of NPs, as reported by Gözüak et al.25. Additionally, it is established that small particle sizes and good dispersion are helpful for electrolyte ions to penetrate the porous surfaces of the electrodes and to provide for high specific capacitance. The spectra at 448 to 562 cm−1 were attributed to the characteristic vibrational modes of metals ion and oxygen atoms in the spinel structure32,33,34. Specifically, the wavenumber at 448 cm−1 was ascribed to the stretching mode of metal and oxygen atoms at the B site (octahedral site). However, the same vibration mode at 562 cm−1 was attributed to the stretching mode of metal and oxygen atoms at the A site (tetrahedral site). Moreover, as the Co content increases, the peak position of vibrational mode at A site slightly shifts to a higher frequency region due to displacement of a heavy atom (Zn) by a lighter atom (Co)18,35.
Oxidation state analysis
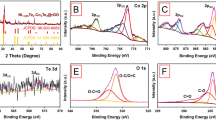
Figure 8a displays the normalized Zn K-edge XANES spectra of CoxZn1−xFe2O4 (x = 0.0–0.4) NPs, as well as Zn foil and ZnO standards. As seen in Fig. 8a, the Zn foil and ZnO (having oxidation states of 0 and + 2, respectively) show the maximum gradient of their spectra at different energy positions, 9659.0 and 9661.83 eV, respectively36,37,38. For our samples, all the spectra display their maximal gradients in the range of 9660.20–9661.67 eV, implying that the oxidation state of the Zn cation is + 2, in agreement with the work of Wiriya et al.37. In Fig. 8b, the Co foil, Co(SO4), and Co3O4 standards reveal, respectively, the maximum gradient Co K-edge XANES spectra at energy positions of 7709, 7720.35, and 7726.68 eV. These energy positions correspond to the oxidation states of 0, + 2, and (+ 2, + 3), respectively. Similarly, our samples (x = 0.1–0.4) reveal their maximum gradient Co K-edge XANES spectra at energy positions of 7721.75, 7722.56, 7722.84 and 7722.88 eV, respectively, which are close to that of Co(SO4). Therefore, it is suggested that the oxidation state of the Co cation is + 2 with redistribution of the ions at A- and B-sites in the spinel structure39,40. In Fig. 8c, the maximum gradient Fe K-edge XANES spectra of Fe foil, Fe(SO4), Fe3O4, and Fe2(SO4)3 appear, respectively, at 7112, 7122.74, 7125.07 and 7127.54 eV, corresponding to the oxidation states of 0, + 2, (+ 2, + 3) and + 3, respectively. In the case of our samples with x = 0.0, 0.1, 0.2, 0.3 and 0.4, the maximum gradient Fe K-edge spectra appear at energy position of 7125.52, 7125.23, 7125.18, 7125.20 and 7125.16 eV, respectively, suggesting oxidation states + 2 and + 3 of Fe cations for these samples. Regarding our results and others related to the studies of Nilmoung et al.2, Wiriya et al.37,40 and Liang et al.41, Co2+ ions can replace Zn2+ ions at both the A and B sites, while Fe2+ and Fe3+ ions can be distributed at both sites of a mixed spinel CoxZn1−xFe2O4 (x = 0.1–0.4) NPs, as shown in Fig. 3d. Additionally, the probable distribution of these ions at A and B sites is proposed as the following:
-
[A] site [B] site
-
Normal spinel ZnFe2O4 [Zn2+] [Fe2+/Fe3+]O42-
-
Inverse spinel CoFe2O4 [Fe2+/Fe3+] [Co2+, Fe2+/Fe3+]O42-
-
Mixed spinel CoxZn1−xFe2O4 [(Cox2+, Zn1−x2+)y, Fe2+/Fe3+] [(Cox2+, Zn1−x2+)1−y, Fe2+/Fe3+]O42−
Surface area study
Figure 9a displays N2 adsorption–desorption isotherms of CoxZn1−xFe2O4 (x = 0.0–0.4) NPs. These results were found to be typical type IV isotherms with small hysteresis loops, representing a mesoporous nature1. Clearly, Co0.3Zn0.7Fe2O4 (x = 0.3) NPs exhibit the maximum BET surface area, 84.30 m2 g−1. Other electrodes, with x = 0.0, 0.1, 0.2 and 0.4, show lower surface areas, 37.05, 58.84, 64.38 and 75.31 m2 g−1, respectively. Based on the BJH model and the plot of pore volume vs. pore width shown in Fig. 9b, the pore volumes of CoxZn1−xFe2O4 (x = 0.0–0.4) NPs were calculated and found to be 0.01833, 0.02491, 0.2306, 0.03127 and 0.0322 cm3/g, respectively. Moreover, the pore size of CoxZn1−xFe2O4 (x = 0.0–0.4) NPs were determined to be 16.93, 16.90, 14.33, 14.84 and 17.11 nm, respectively. It is clear that Co doping can significantly impact the surface area, pore volume and pore size of CoxZn1−xFe2O4 (x = 0.1–0.4) NPs, and gives evidence of increased active sites and shortened transport paths at electrodes surfaces7.
Electrochemical properties study
The CV results of CoxZn1−xFe2O4 (x = 0.0–0.4) NPs electrodes recorded at a scan rate 5 mV/s over a potential window −0.2 to 0.6 V are shown in Fig. 10a at different scan rates of 5–100 mV/s in Fig. 10b–f. In Fig. 10a, a pair of reversible redox or faradaic redox reaction peaks can be observed in every curve, indicating a pseudocapacitive behavior of these electrodes1,5,15,19,42. For the ZnFe2O4 (x = 0) electrode, anodic and cathodic peaks appear at around 0.341 V and 0.184 V, respectively, which can be ascribed to stepwise oxidation and reduction of Zn2+ and Fe3+/Fe2+ in a KOH electrolyte, similar to earlier literature reports10,15,42. The charging process in ZnFe2O4 normal spinel is due to coupling between Zn2+ at its A site and Fe3+/Fe2+ at the B site. During discharge, Fe3+/Fe2+ and Zn2+ can become distributed among both sites, introducing a reversible redox reaction. However, in CoxZn1−xFe2O4 (x = 0.1–0.4) NPs electrodes, the anodic peaks were shifted from 0.372 to 0.422 V, while the cathodic peaks ranged from 0.186 to 0.139 V with increasing Co content. These anodic and cathodic peaks are related to the Co2+ and Zn2+ ion occupancy at the B site and Fe3+/Fe2+ at both A and B sites. This is a result of redox couples with the assistance of OH− in the KOH electrolyte42,43. Furthermore, it is observed that Co0.3Zn0.7Fe2O4 (x = 0.3) electrode shows the largest CV area, corresponding to the highest Csc value among these products. Figure 10b–f show CV curves the CoxZn1−xFe2O4 NPs electrodes performed at various scan rates from 5–100 mV/s. With increasing Co content and scan rate, the anodic and cathodic peaks reveal increased current values and areas of redox peaks. Optimal values were observed in the x = 0.3 electrode, indicating that the charge storage process is dominated by a diffusion-controlled redox reaction5,43. Moreover, the anodic and cathodic peaks at all potential values of every electrode were slightly shifted towards higher and lower potentials, respectively. This might be caused by a polarization effect due to an increased Co content. As seen in Fig. 10e, the closed CV curve of the Co0.3Zn0.7Fe2O4 (x = 0.3) electrode is larger than those of other electrodes at different sweep rates, implying the best electrochemical performance.
The GCD results measured over a potential window 0–0.45 V at 1 A/g and various current densities of 1–10 A/g were used to determine the capacitive performance of CoxZn1−xFe2O4 (x = 0.0–0.4) NPs electrodes, as shown in Fig. 11a–f. In these figures, all GCD curves show a relatively asymmetric shape, implying a favorable pseudocapacitive behavior8,19,44 for all electrodes. The GCD results of the Co0.3Zn0.7Fe2O4 (x = 0.3) electrode show the highest specific capacitance among these products, agreeing well with the CV results. Moreover, the GCD curves of all CoxZn1−xFe2O4 NPs electrodes display a very low internal resistance, suggesting good conductivity of electrode materials4,15,21.
Furthermore, the specific capacitances (Csc) of CoxZn1−xFe2O4 (x = 0.0–0.4) NPs electrodes were calculated with Eq. (1), using the integral discharge curves at various current densities from 1–10 A/g. The obtained data are plotted in Fig. 12a and summarized in Table 2. In this figure, the Csc values decease with increasing current density due to the decay of electrodes during redox reactions at high current density3,15. Clearly, the Co0.3Zn0.7Fe2O4 (x = 0.3) electrode exhibits the maximum Csc value, 855.33 F/g, at a current density of 1 A/g, while other electrodes with x = 0.0, 0.1, 0.2 and 0.4 show lower Csc values of 391.51, 530.86, 662.75 and 733.53 F/g, respectively. Figure 12b shows a Ragone plot of CoxZn1−xFe2O4 (x = 0.0–0.4) NPs electrodes over a potential window of 0.0–0.45 V and at various current densities of 1–10 A/g in a KOH electrolyte. It is clearly seen in Fig. 12b that the Co0.3Zn0.7Fe2O4 (x = 0.3) electrode delivers the highest energy density. This enhanced electrochemical performance is suggested to arise from improved electroactive sites with shortened conduction pathways, as well as numerous nanopaths that promote transport of various ionic species due to the presence of Co2+ ions in the structure21. Furthermore, Fig. 12c shows the capacity retention of CoxZn1−xFe2O4 (x = 0.0–0.4) NPs electrodes after 1000 GCD cycle testing at 5 A g−1. The capacity retention was found to be 54.83, 73.80, 81.63, 90.41 and 82.70% for CoxZn1−xFe2O4 electrodes with x = 0.0, 0.1, 0.2, 0.3 and 0.4, respectively. As can be seen, the Co0.3Zn0.7Fe2O4 (x = 0.3) electrode exhibits better cycling stability among the electrodes. It is suggested that this might be due to more effective contact of the Co0.3Zn0.7Fe2O4 electrode and KOH electrolyte. This promotes simultaneous faster ion/charge transport in the bulk of electrode and at the electrode/electrolyte interface to these achieve excellent properties6,8,12,38. Moreover, this electrode has a higher active area via a uniform distribution of nanosized particles that prevents particle agglomeration, as shown in the SEM, TEM, and BET results. This enhances the electrical conductivity of the electrode10. The highest Csc value obtained in the Co0.3Zn0.7Fe2O4 (x = 0.3) electrode is superior to values reported in the literature on spinel TMOs (AB2O4), as illustrated in Table 3.
The highest Csc value obtained in Co0.3Zn0.7Fe2O4 (x = 0.3) electrode is superior to earlier related work reported in literature on spinel TMOs (AB2O4), as illustrated in Table 3. Clearly in Table 3, the Co0.3Zn0.7Fe2O4 (x = 0.3) electrode prepared by a hydrothermal method, shows a higher Csc value than those obtained in many of TMOs (AB2O4) ferrites. The high specific capacitance of the Co0.3Zn0.7Fe2O4 (x = 0.3) electrode might be due to a homogeneous distribution of nanosized particles and the mesoporous nature of the material, which could promote electrolyte access. Moreover, with the high cycling stability retention of Co0.3Zn0.7Fe2O4 (x = 0.3) electrode, this material is a good candidate for supercapacitor applications. It is remarkable that Co ions in Co-doped ZnFe2O4 NPs can play a significant role in improving the electrochemical properties of these materials. Co ions can influence the interstitial sites of a ZnFe2O4 lattice, which can enhance the electrochemical activities of the electrode material. Moreover, the morphology of nanosized cuboidal shape-particles with good dispersion are supportive of electrolyte ion penetration of the porous electrode surface. This could result in a high specific capacitance. Additionally, Co doping could possibly increase cation transfer (Co2+, Zn2+ and Fe3+/Fe2+) between A and B sites, resulting in more redox couples with the assistance of OH− in KOH electrolyte.
Figure 12d shows the EIS spectra of CoxZn1−xFe2O4 (x = 0.0–0.4) NPs electrodes, where the plots of the real part (Z') vs. an imaginary part (Z'') were obtained of a frequency range of 0.01 Hz to 10 MHz using an applied AC voltage of 10 mV. The EIS analysis data for each electrode is given in Table 2. Additionally, the Randle’s equivalent circuit employed for EIS analysis, primarily consisting of the solution resistance (Rs), charge transfer resistance (Rct), constant phase element (CPE) and Warburg resistance (Rw), is displayed in the inset of Fig. 12d. The plots shown in Fig. 12d display three regions, depending on the applied frequency1,7. Initially, the Z' axis intercept in the high frequency range represents the solution resistance (Rs) of the 3 M KOH electrolyte and working electrode interface6,15,38,43. The Rs value for each CoxZn1−xFe2O4 (x = 0.0, 0.1, 0.2, 0.3 and 0.4) NPs electrode was found to be 0.55, 0.67, 0.71, 0.68 and 0.82 Ω, respectively. However, the slight increase of Rs causes a reduction in the conductivity of the KOH electrolyte6,38. In the next frequency range, where semicircular loops are observed, the diameter of each semicircular loop is related to electron charge transfer resistance (Rct) at the electrode and electrolyte interface, resulting from the Faradaic redox process6,38,43,44. Accordingly, the Rct values were respectively found to be 4.56, 4.46, 3.83, 3.63 and 3.72 Ω for CoxZn1−xFe2O4 (x = 0.0, 0.1, 0.2, 0.3 and 0.4) NPs electrodes. It is clear that the Rct value of the Co0.3Zn0.7Fe2O4 (x = 0.3) electrode is lower than that of other electrodes, indicating higher electrolytic ion diffusion. This might be due to the homogeneous surface of electrode that could provide area for effective active sites for the OH− ions of the KOH electrolyte, promoting their easy access. This is in good agreement with the lowest Rct value15,42,43. Last, in the low frequency range, the observed slope of the straight-line portion of the curve reflects the Warburg resistance (Rw). This represents the ion diffusion process of redox material in the KOH electrolyte6,38. The increased slope in this frequency range indicates that the materials have become more purely capacitive2,32. The slope values of CoxZn1−xFe2O4 (x = 0.0, 0.1, 0.2, 0.3 and 0.4) NPs electrodes were determined to be 4.08, 5.31, 6.75, 7.32 and 5.60, respectively. As seen, the slope value of the Co0.3Zn0.7Fe2O4 (x = 0.3) electrode is highest among the electrodes, suggesting its faster ion transfer from the electrolyte and providing a greater Csc value7,44.
Conclusion
CoxZn1−xFe2O4 (x = 0.0–0.4) NPs samples were successfully synthesized using a hydrothermal method. All CoxZn1−xFe2O4 NPs have a cubic spinel structure as revealed by XRD. The a, DXRD and Av. particles sizes of CoxZn1−xFe2O4 NPs slightly decreased with increasing Co content (8.402 to 8.353 Å for a, 19.81 ± 4.8 to 14.23 ± 2.9 nm for DXRD and 22.72 ± 0.62 to 20.85 ± 0.47 nm for the Av. particles sizes). This was due to the substitution of Co2+ ions of smaller ionic radius at the A and B sites in a suggested mixed spinel structure of CoxZn1−xFe2O4 NPs. SEM and TEM images displayed the homogeneous distribution of NPs of a cuboidal shape, which could significantly increase the active surface area of electrodes (supported by the BET results) and beneficially provide optimal penetration paths for fast ion/electron transfer. The results in electrochemical enhancement of electrodes. The presence of Zn2+, Co2+ and Fe2+ and Fe3+ ions in all samples was confirmed by the XANES results. According to the suggested mixed spinel ferrites of CoxZn1−xFe2O4 NPs, Co2+ could replace Zn2+ ions at A and B sites, while Fe2+ and Fe3+ ions could be redistributed at both sites as well. The distribution of these ions could well provide for the Faradaic redox reactions of a pseudo-capacitive characteristic of the materials, as illustrated by the CV, GCD and EIS results. From these results, it is emphasized that Co ions could significantly influence the structure, morphology and electrochemical properties of CoxZn1−xFe2O4 NPs. The highest Csc value, 855.33 F/g at 1 A/g with a 90.41% capacity retention after 1000 GCD cycle testing, was achieved in a working Co0.3Zn0.7Fe2O4 (x = 0.3) NPs electrode. Based on these results, the Co0.3Zn0.7Fe2O4 NPs are suggested as a suitable candidate material for supercapacitor electrodes.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Vijayalakshmi, S., Elaiyappillai, E., Johnson, P. M. & Lydia, I. S. Multifunctional magnetic CoFe2O4 nanoparticles for the photocatalytic discoloration of aqueous methyl violet dye and energy storage applications. J. Mater. Sci. Mater. Electron. 31, 10738–10749. https://doi.org/10.1007/s10854-020-03624-z (2020).
Nilmoung, S. et al. Electrospun carbon/CuFe2O4 composite nanofibers with improved electrochemical energy storage performance. J. Alloy. Compd. 688, 1131–1140. https://doi.org/10.1016/j.jallcom.2016.06.251 (2016).
Wang, Q., Gao, H., Qin, X., Dai, J. & Li, W. Fabrication of NiFe2O4@CoFe2O4 core-shell nanofibers for high-performance supercapacitors. Mater. Res. Express 7, 015020. https://doi.org/10.1088/2053-1591/ab61ba (2020).
Sharifi, S., Yazdani, A. & Rahimi, K. Incremental substitution of Ni with Mn in NiFe2O4 to largely enhance its supercapacitance properties. Sci. Rep. 10, 10916. https://doi.org/10.1038/s41598-020-67802-z (2020).
Malima, N. M. et al. Solventless synthesis of nanospinel Ni1−xCoxFe2O4 (0 ≤ x ≤ 1) solid solutions for efficient electrochemical water splitting and supercapacitance. RSC Adv. 11, 31002–31014. https://doi.org/10.1039/D1RA04833C (2021).
Feng, X. et al. Hierarchical CoFe2O4/NiFe2O4 nanocomposites with enhanced electrochemical capacitive properties. J. Mater. Sci. 53, 2648–2657. https://doi.org/10.1007/s10853-017-1735-9 (2018).
Kotutha, I. et al. Electrochemical properties of rGO/CoFe2O4 nanocomposites for energy storage application. Ionics 25, 5401–5409. https://doi.org/10.1007/s11581-019-03114-1 (2019).
Aktara, M. N., Nayim, S., Sahoo, N. K. & Hossain, M. The synthesis of thiol-stabilized silver nanoparticles and their application towards the nanomolar-level colorimetric recognition of glutathione. New J. Chem. 43, 13480–13490. https://doi.org/10.1039/C9NJ01360A (2019).
Silva, E. C. et al. Influence of temperature on the preparation of CoFe2O4 by the sol-gel method and its application in electrochemical energy storage. J. Solid State Electrochem. 24, 1961–1968. https://doi.org/10.1007/s10008-020-04616-z (2020).
Perumal, P., Kiruthika, T., Sivaraj, P., Lakshmi, D. & Selvin, P. C. Tamarind seed polysaccharide biopolymer-assisted synthesis of spinel zinc iron oxide as a promising alternate anode material for lithium-ion batteries. J. Mater. Sci. Mater. Electron. 31, 10593–10604. https://doi.org/10.1007/s10854-020-03608-z (2020).
Saraf, M. et al. Electrochemical energy storage properties of solvothermally driven ZnFe2O4 microspheres. Mater. Res. Express 6, 095534. https://doi.org/10.1088/2053-1591/ab3339 (2019).
Vadiyar, M. M. et al. Binder-free chemical synthesis of ZnFe2O4 thin films for asymmetric supercapacitor with improved performance. Ionics 23, 741–749. https://doi.org/10.1007/s11581-016-1833-8 (2017).
Li, L. et al. Uniformly dispersed ZnFe2O4 nanoparticles on nitrogen-modified graphene for high-performance supercapacitor as electrode. Sci. Rep. 7, 43116. https://doi.org/10.1038/srep43116 (2017).
Fan, C. et al. ZnFe2O4 nanoparticles for electrochemical determination of trace Hg(II), Pb(II), Cu(II), and glucose. ACS Appl. Nano Mater. 4, 4026–4036. https://doi.org/10.1021/acsanm.1c00379 (2021).
Roshani, R. & Tadjarodi, A. Synthesis of ZnFe2O4 nanoparticles with high specific surface area for high-performance supercapacitor. J. Mater. Sci. Mater. Electron. 31, 23025–23036. https://doi.org/10.1007/s10854-020-04830-5 (2020).
Heydari, N., Kheirmand, M. & Heli, H. A nanocomposite of CoFe2O4-carbon microspheres for electrochemical energy storage applications. Int. J. Green Energy 16, 476–482. https://doi.org/10.1080/15435075.2019.1580198 (2019).
Shanmugavani, A., Kalpana, D. & Selvan, R. K. Electrochemical properties of CoFe2O4 nanoparticles as negative and Co(OH)2 and Co2Fe(CN)6 as positive electrodes for supercapacitors. Mater. Res. Bull. 71, 133–141. https://doi.org/10.1016/j.materresbull.2015.04.018 (2015).
Mohamed, W. S. et al. Impact of Co2+ substitution on microstructure and magnetic properties of CoxZn1−xFe2O4 nanoparticles. Nanomaterials 9, 1602. https://doi.org/10.3390/nano9111602 (2019).
Acharya, J. et al. Facile one pot sonochemical synthesis of CoFe2O4/MWCNTs hybrids with well-dispersed MWCNTs for asymmetric hybrid supercapacitor applications. Int. J. Hydrogen Energy 45, 3073–3085. https://doi.org/10.1016/j.ijhydene.2019.11.169 (2020).
Nabi, G. et al. Role of cerium-doping in CoFe2O4 electrodes for high performance supercapacitors. J. Energy Storage 29, 101452. https://doi.org/10.1016/j.est.2020.101452 (2020).
Yue, L. et al. One-pot synthesis CoFe2O4/CNTs composite for asymmetric supercapacitor electrode. Solid State Ionics 329, 15–24. https://doi.org/10.1016/j.ssi.2018.11.006 (2019).
Bourrioux, S. et al. Evaluation of electrochemical performances of ZnFe2O4/γ-Fe2O3 nanoparticles prepared by laser pyrolysis. New J. Chem. 41, 9236–9243. https://doi.org/10.1039/C7NJ00735C (2017).
Zeng, X. et al. Direct observation of cation distributions of ideal inverse spinel CoFe2O4 nanofibres and correlated magnetic properties. Nanoscale 9, 7493–7500. https://doi.org/10.1039/C7NR02013A (2017).
Wongpratat, U., Maensiri, S. & Swatsitang, E. EXAFS study of cations distribution dependence of magnetic properties in Co1−xZnxFe2O4 nanoparticles prepared by hydrothermal method. Microelectron. Eng. 146, 68–75. https://doi.org/10.1016/j.mee.2015.03.059 (2015).
Gözüak, F., Köseoğlu, Y., Baykal, A. & Kavas, H. Synthesis and characterization of CoxZn1−xFe2O4 magnetic nanoparticles via a PEG-assisted route. J. Magn. Magn. Mater. 321, 2170–2177. https://doi.org/10.1016/j.jmmm.2009.01.008 (2009).
Mathew, D. S. & Juang, R.-S. An overview of the structure and magnetism of spinel ferrite nanoparticles and their synthesis in microemulsions. Chem. Eng. J. 129, 51–65. https://doi.org/10.1016/j.cej.2006.11.001 (2007).
Feng, J., Xiong, R., Liu, Y., Su, F. & Zhang, X. Preparation of cobalt substituted zinc ferrite nanopowders via auto-combustion route: An investigation to their structural and magnetic properties. J. Mater. Sci. Mater. Electron. 29, 18358–18371. https://doi.org/10.1007/s10854-018-9950-y (2018).
Malik, H. et al. Influence of cobalt substitution on the magnetic properties of zinc nanocrystals synthesized via micro-emulsion route. Ceram. Int. 40, 9439–9444. https://doi.org/10.1016/j.ceramint.2014.02.015 (2014).
Monisha, P., Priyadharshini, P., Gomathi, S. S., Mahendran, M. & Pushpanathan, K. Role of Zn dopant on superparamagnetic property of CoFe2O4 nanoparticles. Appl. Phys. A 125, 736. https://doi.org/10.1007/s00339-019-3014-x (2019).
Yuliantika, D. et al. Exploring structural properties of cobalt ferrite nanoparticles from natural sand. IOP Conf. Ser. Mater. Sci. Eng. 515, 012047. https://doi.org/10.1088/1757-899X/515/1/012047 (2019).
Chieng, B. W., Ibrahim, N. A., Yunus, W. M. Z. W. & Hussein, M. Z. Poly(lactic acid)/Poly(ethylene glycol) Polymer Nanocomposites: Effects of Graphene Nanoplatelets. Polymers 6, 93–104. https://doi.org/10.3390/polym6010093 (2014).
Huang, Z., Zhu, Y., Wang, S. & Yin, G. Controlled growth of aligned arrays of Cu−Ferrite nanorods. Crystal Growth Des. 6, 1931–1935. https://doi.org/10.1021/cg0505517 (2006).
Laokul, P., Amornkitbamrung, V., Seraphin, S. & Maensiri, S. Characterization and magnetic properties of nanocrystalline CuFe2O4, NiFe2O4, ZnFe2O4 powders prepared by the Aloe vera extract solution. Curr. Appl. Phys. 11, 101–108. https://doi.org/10.1016/j.cap.2010.06.027 (2011).
Liu, W. et al. Understanding the formation of ultrafine spinel CoFe2O4 nanoplatelets and their magnetic properties. J. Appl. Phys. 112, 104306. https://doi.org/10.1063/1.4765033 (2012).
Köseoğlu, Y., Alan, F., Tan, M., Yilgin, R. & Öztürk, M. Low temperature hydrothermal synthesis and characterization of Mn doped cobalt ferrite nanoparticles. Ceram. Int. 38, 3625–3634. https://doi.org/10.1016/j.ceramint.2012.01.001 (2012).
Sakurai, S., Sasaki, S., Okube, M., Ohara, H. & Toyoda, T. Cation distribution and valence state in Mn–Zn ferrite examined by synchrotron X-rays. Physica B 403, 3589–3595. https://doi.org/10.1016/j.physb.2008.05.035 (2008).
Wiriya, N., Bootchanont, A., Maensiri, S. & Swatsitang, E. Magnetic properties of Zn1−xMnxFe2O4 nanoparticles prepared by hydrothermal method. Microelectron. Eng. 126, 1–8. https://doi.org/10.1016/j.mee.2014.03.044 (2014).
Karaphun, A. et al. Influence of calcination temperature on structural, morphological, and electrochemical properties of Zn2P2O7 nanostructure. Surfaces Interfaces 23, 100961. https://doi.org/10.1016/j.surfin.2021.100961 (2021).
Sato Turtelli, R. et al. Interplay between the cation distribution and production methods in cobalt ferrite. Mater. Chem. Phys. 132, 832–838. https://doi.org/10.1016/j.matchemphys.2011.12.020 (2021).
Wiriya, N., Bootchanont, A., Maensiri, S. & Swatsitang, E. X-ray absorption fine structure analysis of Mn1-xCoxFe2O4 nanoparticles prepared by hydrothermal method. Jpn. J. Appl. Phys. 53, 69. https://doi.org/10.7567/jjap.53.06jf09 (2014).
Liang, X. et al. The valence and site occupancy of substituting metals in magnetite spinel structure Fe3−xMxO4 (M = Cr, Mn, Co and Ni) and their influence on thermal stability: An XANES and TG-DSC investigation. Solid State Sci. 15, 115–122. https://doi.org/10.1016/j.solidstatesciences.2012.10.005 (2013).
Wang, J. et al. Self-supported ultrathin mesoporous CoFe2O4/CoO nanosheet arrays assembled from nanowires with enhanced lithium storage performance. J. Mater. Sci. 51, 6590–6599. https://doi.org/10.1007/s10853-016-9902-y (2016).
Yao, J. et al. Preparation of ZnFe2O4/α-Fe2O3 nanocomposites from sulfuric acid leaching liquor of jarosite residue and their application in lithium-ion batteries. Front. Chem. 6, 442. https://doi.org/10.3389/fchem.2018.00442 (2018).
Guo, X. et al. Design and synthesis of CoFe2O4 quantum dots for high-performance supercapacitors. J. Alloy. Compd. 764, 128–135. https://doi.org/10.1016/j.jallcom.2018.06.054 (2018).
Rani, B. & Sahu, N. K. Electrochemical properties of CoFe2O4 nanoparticles and its rGO composite for supercapacitor. Diamond Rel. Mater. 108, 107978. https://doi.org/10.1016/j.diamond.2020.107978 (2020).
Acknowledgements
This research was funded by the Research and Graduate Studies of Khon Kaen University. The Rajamangala University of Technology Rattanakosin, Wang Klai Kangwon Campus, Hua Hin, Prachuap khiri khan, Thailand is also thanked for their co-financial support.
Author information
Authors and Affiliations
Contributions
E.S., A.K. and T.P. designed this research. A.K., S.P. and P.M. carried out the experiments. E.S., A.K., I.K., T.P. and P.M. analyzed data. E.S., A.K. and T.P. prepared the manuscript. All authors contributed to the scientific discussion and manuscript revisions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Putjuso, T., Putjuso, S., Karaphun, A. et al. Influence of Co doping on phase, structure and electrochemical properties of hydrothermally obtained CoxZn1−xFe2O4 (x = 0.0–0.4) nanoparticles. Sci Rep 13, 2531 (2023). https://doi.org/10.1038/s41598-023-29830-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-29830-3
This article is cited by
-
Comparative analysis of adsorption of Pb(II) ions by different hexagonal nanoferrites synthesized using the flash-combustion method
Applied Physics A (2023)
-
ZnFe-MOF derived ZnO/ZnFe2O4 nanocomposite as an electrode material for supercapacitor application
Journal of Materials Science: Materials in Electronics (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.