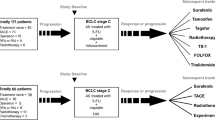
Abstract
Adenosine-to-inosine RNA editing is a process mediated by adenosine deaminases that act on the RNA (ADAR) gene family. It has been discovered recently as an epigenetic modification dysregulated in human cancers. However, the clinical significance of RNA editing in patients with liver metastasis from colorectal cancer (CRC) remains unclear. The current study aimed to systematically and comprehensively investigate the significance of adenosine deaminase acting on RNA 1 (ADAR1) expression status in 83 liver metastatic tissue samples collected from 36 patients with CRC. The ADAR1 expression level was significantly elevated in liver metastatic tissue samples obtained from patients with right-sided, synchronous, or RAS mutant-type CRC. ADAR1-high liver metastasis was significantly correlated with remnant liver recurrence after hepatic metastasectomy. A high ADAR1 expression was a predictive factor of remnant liver recurrence (area under the curve = 0.72). Results showed that the ADAR1 expression level could be a clinically relevant predictive indicator of remnant liver recurrence. Patients with liver metastases who have a high ADAR1 expression requires adjuvant chemotherapy after hepatic metastasectomy.
Similar content being viewed by others
Introduction
RNA editing is a mechanism in which the RNA sequence is altered but the DNA sequence is not, thereby resulting in phenotypic changes. The RNA editing enzymes include the ADAR and APOBEC families, which play important roles in embryonic development and immunity1,2. Interestingly, RNA editing can promote carcinogenesis3. The expression of adenosine deaminase acting on RNA 1 (ADAR1), an RNA editing enzyme, is upregulated in primary colorectal cancer (CRC), and this phenomenon promotes lymph node and distant metastasis. Thus, ADAR1 can be a prognostic marker4. In addition, in CRC, cancer-associated fibroblasts receive signals from cancer cells by humoral factors and upregulate RNA editing to promote invasive migration, thereby leading to cancer invasion5. Thus, RNA editing can contribute to malignant transformation and can be a potential novel therapeutic target in CRC.
The most important aspect in CRC treatment is distant metastasis control. Recent advancements in chemotherapy have prolonged the life expectancy of patients with CRC who developed distant metastases6. Molecular targeted therapy with anti-epidermal growth factor receptor and anti-vascular endothelial growth factor antibodies is associated with a life expectancy of 3 years in unresectable advanced-stage recurrent CRC7,8. However, to achieve a longer life expectancy, distant metastatic tumors should be resected without leaving any remnants. In particular, the resection of liver metastatic tumors and the prevention of recurrence are the key to a successful procedure. If liver metastatic tumors can be resected and liver recurrence can be controlled, the life expectancy of patients will be prolonged.
Therefore, the current study aimed to analyze the effects of RNA editing on the development of liver metastasis in patients with CRC and to investigate its therapeutic application. We evaluated the ADAR1 expression of patients with CRC who developed liver metastases via immunostaining. Further, a predictive model for remnant liver recurrence after hepatic metastasectomy was constructed.
Results
Remnant liver recurrence after hepatic metastasectomy is associated with a shorter survival
We included a total of 83 resected liver metastases in our study. These liver metastases were resected from 36 patients with CRC liver metastases. Table 1 shows data on the characteristics of patients. The median age of the participants was 68 years. There were 20 male and 16 female patients. In total, 25 patients presented with synchronous liver metastases and 11 with metachronous liver metastases. Further, 11 and 25 patients developed right- and left-sided CRC, respectively, and 16 and 15 patients had RAS mutant- and RAS wild-type CRC, respectively. However, five patients could not be evaluated due to poor DNA quality. The significance of ADAR1 expression in patients with CRC who developed liver metastases was evaluated.
Patients with right-sided CRC who developed liver metastasis had worse overall survival (p < 0.01; Fig. 1a). Right-sided CRC has a high-malignant potential9, and this finding is consistent with our result. In addition, patients with remnant liver recurrence after liver metastatic tumor resection had a significantly short survival (p < 0.01; Fig. 1b). In a multivariate analysis, liver metastasis from right-sided CRC (p = 0.03) and remnant liver recurrence (p = 0.01) were independent predictors of worse prognosis (Table 2).
Association between remnant liver recurrence and a shorter survival. (a) Patients with liver metastasis from right-sided colorectal cancer had worse overall survival (p < 0.01). (b) Patients with remnant liver recurrence after liver metastatic tumor resection had a significantly shorter survival (p < 0.01). (c) The level of ADAR1 staining was evaluated using staining scores ranging from 1 to 5.
Remnant liver recurrence after hepatic metastasectomy is an indicator of worse prognosis. Further, it is important in identifying patients who are at high risk of remnant liver recurrence and in strengthen treatment application. Therefore, we aimed to identify whether the ADAR1 expression can be a predictive biomarker of remnant liver recurrence after hepatic metastasectomy (Fig. 1c). Predicting the risk of remnant liver recurrence may help in its prevention and the identification of adjuvant chemotherapy indications after liver metastatic tumor resection.
Analysis of the ADAR1 expression in each metastatic site
Although most patients had multiple liver metastases, the intensity of ADAR1 immunostaining differed in each tumor. Therefore, we initially characterized 83 liver metastases as independent tumor tissues (Fig. 2a).
ADAR1 expression in each metastatic site. (a) In total, 83 liver metastases were characterized as independent tumor tissues. (b–e) The ADAR1 expression was upregulated in patients with liver metastases from right-sided colorectal cancer, concurrent liver metastases, RAS mutant-type cancer, and remnant liver recurrence. (f) A high ADAR1 expression was a predictive factor of remnant liver recurrence (area under the curve = 0.72). *p < 0.05, **p < 0.01, ***p < 0.001.
The ADAR1 expression in patients with liver metastases was analyzed. Results showed that it was upregulated in patients with liver metastases from right-sided CRC (cytoplasm: p < 0.05; Fig. 2b), concurrent liver metastases (nucleus: p < 0.01, cytoplasm: p < 0.001; Fig. 2c), RAS mutant-type CRC (nucleus: p < 0.01; Fig. 2d), and remnant liver recurrence after hepatic metastasectomy (cytoplasm: p < 0.001; Fig. 2e). Right-sided colon cancer and RAS mutant-type carcinomas are associated with poor prognosis9. Interestingly, the current study showed that patients with CRC who had poor prognosis had a high ADAR1 expression. This finding is consistent with the fact that ADAR1 is correlated with increased malignant potential in CRC based on a previous study4. Clinically, it is important to predict remnant liver recurrence after hepatic metastasectomy. A high ADAR1 expression was a predictive factor of remnant liver recurrence (area under the curve [AUC] = 0.72; Fig. 2f).
ADAR1 expression in one representative metastatic site in each patient
If a patient has several liver metastatic tumors, these lesions might have different ADAR1 staining intensities. Averaging the ADAR1 staining intensities could diminish the characteristics of liver metastatic tumors. Therefore, in each patient, we selected one liver metastatic site with the highest ADAR1 staining intensity (Fig. 3a). Then, the association between ADAR1 staining intensity and clinicopathological features was examined.
ADAR1 expression in each representative metastatic site in each patient. (a) One liver metastasis with the highest ADAR1 staining intensity was selected among liver metastases. (b–e) The ADAR1 expression was upregulated in patients with liver metastases from concurrent liver metastases, RAS mutant-type cancer, and remnant liver recurrence. (f) A high ADAR1 expression was a predictive factor of remnant liver recurrence (area under the curve = 0.68). *p < 0.05, ***p < 0.001.
The results were similar to those obtained by analyzing each independent liver metastatic tumor. The ADAR1 expression was upregulated in patients with liver metastases related to concurrent liver metastases (cytoplasm: p < 0.001), RAS mutant-type CRC (nucleus: p < 0.05), and remnant liver recurrence after hepatic metastasectomy (cytoplasm: p < 0.05; Fig. 3b–e). A high ADAR1 expression was a predictive factor of remnant liver recurrence (AUC = 0.68; Fig. 3f). Using the log-rank test, patients with liver metastases who have high ADAR1 levels had earlier remnant liver recurrence after hepatic metastasectomy (p = 0.04; Fig. 4). The multivariate analysis was also performed using the Cox hazard model. Results showed that high ADAR1 levels remained an independent high risk factor for remnant liver recurrence in patients with liver metastases (p = 0.05, Table 3).
Based on these results, we hypothesized the following clinical applications: If patients with liver metastases from CRC undergo surgery, ADAR1 immunostaining should be performed, and the expression intensity must be assessed. Patients whose highest ADAR1 immunostaining intensity exceeds the cutoff value were at high risk of remnant liver recurrence after hepatic metastasectomy.
Immunostaining results of ADAR1 in the primary lesion cannot be a predictive factor of remnant liver recurrence
We evaluated the expression of ADAR1 in the primary tumor (Supplementary Fig. 1a). However, no correlation with clinicopathological features was observed (Supplementary Fig. 1b–e). Moreover, the predictive ability of ADAR1 expression in the primary tumor was poor (AUC = 0.59; Supplementary Fig. 1f). This finding could be attributed to primary tumor heterogeneity. Thus, primary tumors contain a mixture of cells with high and low ADAR1 expressions. Due to heterogeneity, this was not evident in the analysis of the primary tumor. We hypothesized that highly malignant cancer cells with a high ADAR1 expression are more likely to cause liver metastases, and even a small number of these cells can easily metastasize, thereby resulting in remnant liver recurrence after hepatic metastasectomy (Fig. 5).
Highly malignant colorectal cancer cells with a high ADAR1 expression cause liver metastases. High-malignant cancer cells with a high ADAR1 expression are more likely to cause liver metastases, and even a small number of cells can easily metastasize, thereby resulting in remnant liver recurrence over time.
Discussion
Patients with CRC presented with a series of genetic and epigenetic alterations in colon tissues10. RNA editing has emerged as an important epigenetic modification involved in the evolution of different types of cancers and disease progression. Adenosine-to-inosine RNA editing, which is associated with oncogenes and tumor suppressor genes, can alter tumor characteristics to promote a more aggressive phenotype11. AZIN1, which is aberrant in different types of cancers, is a major target of ADAR1. Further, it is significantly edited in different types of cancers, including hepatocellular carcinoma, esophageal cancer, and CRC4,11,12. Emerging evidence has shown that edited AZIN1 is highly oncogenic, and it inhibits ornithine decarboxylase degradation and induces polyamine accumulation and invasion, migration, and stemness12.
On the other hand, Liver metastasis in CRC requires special attention. Patients with CRC who developed liver metastases have poor prognosis. The median survival of patients with hepatic metastasis from CRC who did not receive treatment is 5–20 months13. The prognosis is extremely poor if liver metastatic tumors become unresectable, particularly in right-sided CRC, which has a 5-year OS rate of 4.3%14. Therefore, CRC treatment aims to control liver metastases.
High-grade malignant liver metastases are more likely to be correlated with remnant liver recurrence and extrahepatic lymph node metastases. Such extremely malignant liver metastases are challenging to control by surgery alone, and they require preoperative or postoperative chemotherapy15. However, grading of liver metastases from CRC is still technically challenging.
The current study had an extremely important finding. ADAR1 was highly expressed in patients with liver metastases from CRC, which resulted in remnant liver recurrence after hepatic metastasectomy. Patients with CRC who developed ADAR1-expressing liver metastases had an earlier and higher rate of remnant liver recurrence. Thus, ADAR1 immunostaining at the time of liver metastasis resection may identify patients at high risk for remnant liver recurrence after hepatic metastasectomy. The results of the current study will be useful in the evaluation of adjuvant chemotherapy indications after liver metastatic tumor resection.
To date, the need for adjuvant chemotherapy after liver metastatic tumor resection in CRC remains controversial6. Most recently, the JCOG 0603 trial was conducted, and the results were as follows: from March 2007 to January 2019, 300 patients were randomly assigned to undergo either liver resection-alone or liver resection, followed by adjuvant chemotherapy16. In the combined phase II and phase III study, 149 patients were included in the surgery alone group and 151 in the chemotherapy group. The 5-year disease-free survival rates were 38.7% in the liver resection-alone group and 49.8% in the adjuvant chemotherapy group. That is, the adjuvant chemotherapy group had a better disease-free survival than the liver resection-alone group. By contrast, the adjuvant chemotherapy group had a lower 5-year overall survival rate than the liver resection-alone group (71.2% vs. 83.1%). This controversial result may be attributed to the fact that there are no adequate eligibility criteria for adjuvant therapy. The ADAR1 expression can accurately predict remnant liver recurrence after hepatic metastasectomy in patients with liver metastases. Thus, more intensive adjuvant chemotherapy may be effective in patients with liver metastases with a high ADAR1 expression. The ADAR1 expression may affect the choice of adjuvant therapy protocol.
This retrospective study had several limitations. That is, it was performed at a single center. We are currently planning to perform a clinical trial to reduce remnant liver recurrence with adjuvant chemotherapy in patients with liver metastases who have a high ADAR1 expression, and we hope to report the results in the near future.
In conclusion, a high ADAR1 expression is associated with a greater risk of remnant liver recurrence after hepatic metastasectomy in patients with liver metastases from CRC. Therefore, it may be a good indicator of multimodality treatment, including chemotherapy, in patients with liver metastases from CRC who have high ADAR1 expression levels.
Methods
Patients and sample collection
This study examined 83 cases of liver metastases resected from 36 patients at Okayama University. Patients who did or did not receive neoadjuvant chemotherapy were both included. CRC diagnosis was confirmed in all patients based on clinicopathological findings. The Tumor Node Metastasis staging system of the American Joint Committee on Cancer was used for pathological staging. The current research was approved by the Ethics Committee of Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences and Okayama University Hospital (1903-037). A written informed consent was obtained from each patient. All methods were performed in accordance with the relevant guidelines and regulations.
Immunohistochemical analysis
Paraffin-embedded sections were deparaffinized using xylene and ethanol, and endogenous peroxidase activity was eliminated with H2O2, as previously described5. After antigen retrieval by autoclaving the tissues at 121 °C for 15 min, the slides were incubated overnight with an anti-ADAR1 antibody at a 1:100 dilution (Abcam, Cambridge, MA, the USA). Color development was achieved using the EnVision + Dual Link Kit (DAKO, Carpinteria, CA, the USA), and the slides were counterstained with hematoxylin. Negative controls were run in parallel. The level of ADAR1 staining was evaluated using the staining score ranging from 1 to 54 and measured three times by three independent investigators who were blinded to the nature of the specimens and antibodies used.
Statistical analysis
Data were expressed as mean ± standard deviation. The JMP software (version 10.0, SAS Institute Inc., Cary, NC, the USA) was used to perform statistical analyses. Between-group differences were assessed using the Wilcoxon’s rank-sum test, χ2 test, and Steel test, as appropriate. The correlations between two groups were evaluated via Spearman’s rank correlation analysis. For time-to-event analyses, survival estimates were calculated using the Kaplan–Meier method, and groups were compared with the log-rank test. Two-sided p-values of < 0.05 were considered statistically significant.
Ethics approval and consent to participate
A written informed consent was obtained from each patient, and the current study was approved by the Ethics Committee of Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences and Okayama University Hospital (1903-037).
Data availability
All data generated or analyzed during this study are included in the published article.
References
Wang, Q., Khillan, J., Gadue, P. & Nishikura, K. Requirement of the RNA editing deaminase ADAR1 gene for embryonic erythropoiesis. Science 290, 1765–1768. https://doi.org/10.1126/science.290.5497.1765 (2000).
Mangeat, B. et al. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature 424, 99–103. https://doi.org/10.1038/nature01709 (2003).
Dominissini, D., Moshitch-Moshkovitz, S., Amariglio, N. & Rechavi, G. Adenosine-to-inosine RNA editing meets cancer. Carcinogenesis 32, 1569–1577. https://doi.org/10.1093/carcin/bgr124 (2011).
Shigeyasu, K. et al. AZIN1 RNA editing confers cancer stemness and enhances oncogenic potential in colorectal cancer. JCI Insight 3, 12. https://doi.org/10.1172/jci.insight.99976 (2018).
Takeda, S. et al. Activation of AZIN1 RNA editing is a novel mechanism that promotes invasive potential of cancer-associated fibroblasts in colorectal cancer. Cancer Lett. 444, 127–135. https://doi.org/10.1016/j.canlet.2018.12.009 (2019).
Martin, J. et al. Colorectal liver metastases: Current management and future perspectives. World J. Clin. Oncol. 11, 761–808. https://doi.org/10.5306/wjco.v11.i10.761 (2020).
Hurwitz, H. et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 350, 2335–2342. https://doi.org/10.1056/NEJMoa032691 (2004).
Feng, Q. Y. et al. Anti-EGFR and anti-VEGF agents: Important targeted therapies of colorectal liver metastases. World J. Gastroenterol. 20, 4263–4275. https://doi.org/10.3748/wjg.v20.i15.4263 (2014).
Baran, B. et al. Difference between left-sided and right-sided colorectal cancer: A focused review of literature. Gastroenterol. Res. 11, 264–273. https://doi.org/10.14740/gr1062w (2018).
Grady, W. M. & Markowitz, S. D. Genetic and epigenetic alterations in colon cancer. Annu. Rev. Genomics Hum. Genet. 3, 101–128. https://doi.org/10.1146/annurev.genom.3.022502.103043 (2002).
Qin, Y. R. et al. Adenosine-to-inosine RNA editing mediated by ADARs in esophageal squamous cell carcinoma. Can. Res. 74, 840–851. https://doi.org/10.1158/0008-5472.CAN-13-2545 (2014).
Chen, L. et al. Recoding RNA editing of AZIN1 predisposes to hepatocellular carcinoma. Nat. Med. 19, 209–216. https://doi.org/10.1038/nm.3043 (2013).
Valderrama-Trevino, A. I., Barrera-Mera, B., Ceballos-Villalva, J. C. & Montalvo-Jave, E. E. Hepatic metastasis from colorectal cancer. Eur. J. Hepato-Gastroenterol. 7, 166–175. https://doi.org/10.5005/jp-journals-10018-1241 (2017).
Engstrand, J., Nilsson, H., Stromberg, C., Jonas, E. & Freedman, J. Colorectal cancer liver metastases: A population-based study on incidence, management and survival. BMC Cancer 18, 78. https://doi.org/10.1186/s12885-017-3925-x (2018).
Adam, R. et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: A model to predict long-term survival. Ann. Surg. 240, 644–657. https://doi.org/10.1097/01.sla.0000141198.92114.f6 (2004) (Discussion 657–648).
Kanemitsu, Y. et al. Hepatectomy followed by mFOLFOX6 versus hepatectomy alone for liver-only metastatic colorectal cancer (JCOG0603): A phase II or III randomized controlled trial. J. Clin. Oncol. 39, 3789–3799. https://doi.org/10.1200/JCO.21.01032 (2021).
Acknowledgements
We want to thank Tae Yamanishi and Tomoko Sueishi for assisting us in performing the experiments. The authors would like to thank Enago (www.enago.jp) for the English language review.
Funding
This study was supported by grants from Takeda Science Foundation, Mochida Memorial Foundation for Medical and Pharmaceutical Research, and JSPS KAKENHI (20K17653) to KS, from JSPS KAKENHI (20K22848, 22K16533) to ST, and from JSPS KAKENHI (21K16422) to Y. Kondo.
Author information
Authors and Affiliations
Contributions
K.S., N.H., S.Y., S.T., Y.U., F.T., H.M., H.T., and T.F. conceived the study and designed the experiments. K.S., N.H., H.U., and T.T. performed the experiments. K.S., N.H., K. Yoshida, H.K., and H.Y. analyzed the data. K.S., N.H., Y.U., T.F., R.Y., K. Yasui, Y.M., Y.K., and K.N. contributed reagents, materials, and other analytical tools. KS, NH wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hata, N., Shigeyasu, K., Umeda, Y. et al. ADAR1 is a promising risk stratification biomarker of remnant liver recurrence after hepatic metastasectomy for colorectal cancer. Sci Rep 13, 2078 (2023). https://doi.org/10.1038/s41598-023-29397-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-29397-z
This article is cited by
-
Molekularpathologie kolorektaler Karzinome
Die Pathologie (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.