Abstract
As a specific predictor of ovarian reserve, serum anti-Müllerian hormone (AMH) has become an area of intense research interest in the field of assisted reproductive technology. We assessed the relationship between AMH levels and pregnancy outcomes in Chinese patients and investigate the influencing factors of cumulative live birth in patients with high AMH levels. A total of 1379 patients starting their IVF/ICSI cycle were divided into normal (Group A, 1.1–4.0 ng/ml, n = 639) and high (Group B, > 4.0 ng/ml, n = 740) groups by serum AMH levels. Live birth rate (LBR), cumulative live birth rate (CLBR) and cumulative clinical pregnancy rate (CCPR) were also investigated. Compared with Group A, Group B had a significantly higher CLBR (65.80% vs. 43.95%) and CCPR (76.77% vs. 57.14%), respectively. Binomial logistic regression analysis showed that age over 40 years, LH/FSH > 2.5, total Gn dose and Gn duration, and greater than 4000 ng/ml serum E2 levels on HCG day were significantly associated with CLBR in Group B. The AUC value of CLBR averaged 0.664 (ranging from 0.621 to 0.706) (p < 0.001). The patients with high AMH levels had higher CPR, higher LBR, and lower MR with no statistically significant differences, although there were significant improvements in CLBR. Advanced age (> 40 years) still impacted CLBR, even in women with good ovarian reserves. Consequently, it is still recommended that patients over 40 years old with high AMH levels actively receive IVF treatment if they seek to become pregnant. PCOS diagnoses did not influence the CLBR. In summary, this study showed that serum AMH levels could positively predict patient ovarian responses and further affect pregnancy outcomes.
Similar content being viewed by others
Introduction
Ovarian reserves play an important role in predicting in vitro fertilization (IVF) /Intracytoplasmic sperm injection (ICSI) outcomes1. Previous studies have used the ovarian reserve markers antral follicle count (AFC), follicle stimulating hormone (FSH), and anti-Mullerian hormone (AMH)1 for this purpose. However, it remains unclear which is the most sensitive marker of ovarian reserve. Over time, as more relevant studies have been published, the classic joint markers of AFC and AMH have been replaced by AMH alone1,2. In recent years, AMH has become an area of intense research interest in the field of assisted reproductive technology (ART). AMH is a glycoprotein dimer that belongs to the transforming growth factor-β (TGF-β) superfamily; it is produced by granulosa cells (GCs) in the preantral, antral, and preovulatory follicles in the ovary3,4. AMH prevents follicular growth by inhibiting aromatase and downregulating luteinizing hormone receptor until these follicles are stimulated by other factors5. Compared to AFC and FSH, AMH is more reliable as an indicator of ovarian reserve because it shows minimal variation between menstrual cycle stages, menstrual cycles, and individuals4,6,7,8,9. Because of this consistency, AMH has become a widely-used tool for assessing ovarian reserves6,10. It has also been studied as a predictor of potential oocyte production before ovulation induced by ART. One study aiming to demonstrate a reliable correlation between AMH and oocyte production showed that patients with 11 or more oocytes in the ART cycle had 2.5-fold higher AMH levels than patients with six or fewer oocytes. This correlation between ART success and AMH extended to the live birth rate (LBR); a retrospective study observed the results of 1230 IVF-ICSI cycles and found that the probability of live birth in those with AMH ≥ 2.94 ng/ml increased in a logarithmic manner11. Many studies have confirmed the correlation between higher AMH levels and better ART outcomes12,13,14. However, other studies have shown that AMH levels > 3.5 ng/ml are not associated with significant increases in LBR15,16,17,18. The true relationship between AMH levels and LBR is thus controversial. With the widespread application of efficient embryo freezing technologies, the cumulative live birth rate (CLBR), including the LBR of fresh embryo transfer and subsequent frozen embryo thawing transfer (FET) after a single ovarian stimulation cycle, has gradually come to be considered an important indicator of ART success19. In practice, infertile couples may have to undergo several IVF/ICSI attempts to reach the desired outcome, and their expected prognosis is based on the chance of success within a number of years from the start of treatment20. However, to date, no study has comprehensively explored the factors that influence CLBR after IVF/ICSI in patients with high AMH levels. Therefore, this study was conducted to evaluate the relationship between serum AMH levels and pregnancy outcomes in Chinese patients receiving IVF/ICSI, and to investigate the factors influencing CLBR in patients with high AMH levels.
Methods
Participant selection
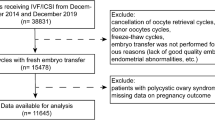
This was a retrospective, single-center cohort study. We collected clinical data for 1379 patients who received the gonadotropin-releasing hormone (GnRH) antagonist protocol at the Reproductive Medicine Center of Peking University People’s Hospital between January 2016 and December 2020. Exclusion criteria included: (1) serum AMH levels < 1.1 ng/ml; (2) a diagnosis of hypothalamic amenorrhea; (3) uterine disease, including uterus submucous myoma, uterine mediastinum, uterine adhesions, a thin endometrium, and immature uterus. Serum AMH concentrations were determined using a UniCel DxI 800 Access automatic chemiluminescence immunoassay instrument (Beckman Coulter, Brea, CA, USA) and an AMH microparticle enzymatic luminescence kit (Beckman Coulter). Patients were divided into two groups based on serum AMH levels: normal (Group A, 1.1–4.0 ng/ml; n = 639) and high (Group B, > 4.0 ng/ml; n = 740). Other patient data collected included age, body mass index (BMI), duration of infertility, polycystic ovary syndrome (PCOS) status, baseline follicle-stimulating hormone (bFSH) levels, baseline luteinizing hormone (bLH) levels, AFC levels, initial gonadotropins (Gn) dose, total Gn dose, duration of Gn treatment, serum concentrations of estradiol (E2), progesterone (P), and luteinizing hormone (LH) on human chorionic gonadotrophin (HCG) day, the number of retrieved oocytes, and the number of metaphase II (MII) oocytes. Endpoint measurements included the clinical pregnancy rate (CPR), early miscarriage rate (MR), LBR, cumulative clinical pregnancy rate (CCPR), and CLBR. PCOS was classified based on the presence of two of the three 2003 Rotterdam PCOS criteria: (1) oligo- or anovulation; (2) clinical and/or biochemical signs of hyperandrogenism; and (3) polycystic ovaries combined with the exclusion of other etiologies (e.g., congenital adrenal hyperplasia, androgen-secreting tumors, or Cushing’s syndrome)21. This study was approved by the Ethics Committee of Peking University People’s Hospital (reference number 2021PHB083-001) and performed in accordance with the Declaration of Helsinki. All participants provided informed consent.
IVF/ICSI procedure
The baseline values of FSH, LH, E2, and P were measured. All patients received a vaginal ultrasound on the second day of menstruation, except those with bilateral ovarian cysts or pregnancy. The initial dose of r-FSH (Gonal-F; Merck Serono Biopharma) was determined based on maternal age, BMI, and levels of AFC, bFSH, and AMH. Beginning on the second day of the menstrual cycle, all patients received r-FSH at an initial dose of 150–300 IU for 4 or 5 d. The daily dose was adjusted based on the ovarian response. When the diameter of one follicle reached ≥ 13–14 mm, a GnRH antagonist (ganirelix [Organon] or cetrorelix acetate [Merck Serono Biopharma]) was administered. When at least two leading follicles reached 18 mm in diameter, 250 μg recombinant human chorionic gonadotropin (rhCG) (Ovidrel, Merck Serono Biopharma) was administered to induce oocyte maturation. Oocytes were retrieved 36 h later. Embryos were then classified according to morphological criteria. Day 3 embryos had at least six to 10 cells and a fragmentation rate < 20%. Blastocysts showed a distinct trophectoderm and inner cell mass on Day 522. All embryos were transferred on the third or fifth day after egg retrieval. The number of embryos transferred (one or two) was determined based on maternal age and embryo quality. Patients received progesterone supplements for luteal phase support. Individuals with serum β-HCG levels > 5 IU/ml at 14 d after embryo transfer were considered pregnant. Clinical pregnancy was defined as having an intrauterine sac detected by ultrasonic examination at 4 weeks after embryo transfer. Early miscarriage was defined as a loss of clinical pregnancy before 12 weeks, a lack of fetal heart activity, or disappearance of fetal heart activity. LBR was defined as the delivery of one live baby after the 28th week of pregnancy22. For all cases of clinical pregnancy, luteal phase support continued until the 12th week of gestation. Patients who were not pregnant after a fresh embryo transfer could undergo frozen-thawed replacement cycles in either a natural or an artificial cycle.
Outcome measurements
The main outcome measured was CLBR, which was defined as the number of deliveries with at least one live birth resulting from one initiated ART cycle. For each individual, this comprised a one-year treatment period including all cycles in which fresh and/or frozen embryos were transferred, until either one delivery with a live birth occurred or all embryos were used. CCPR was defined as the total number of clinical pregnancies arising from fresh or frozen embryo transfer following one initiated ART cycle per patient during the one-year treatment period23,24,25.
Data analysis
The required sample size for statistical power at 99% with a two-sided alpha level of 0.05 and a dropout rate of 10% was calculated using PASS v15.0. The actual sample sizes (639 in group A and 740 in group B) achieved 99% power to detect differences between the groups at a proportion of − 0.2185. The proportion in group A was assumed to be 0.6580 under the null hypothesis and 0.4395 under the alternative hypothesis. The proportion in group B was 0.6580. The test statistic used was the two-sided Z-test with unpooled variance. The significance level of the test was 0.050.
Statistical Package for the Social Sciences (SPSS, v19.0) was used to perform statistical analyses. Continuous variables were analyzed by calculating the mean and standard deviation (SD). Normal and nonnormal distributions were analyzed using independent t-tests and Mann–Whitney U tests, respectively. In comparisons between two groups, significant differences were explored using the Bonferroni correction method to obtain an α value. Categorical variables were compared using a chi-square test or a Fisher’s exact test and presented as raw frequencies with corresponding percentages; a p-value of < 0.05 was considered statistically significant. The potential factors associated with pregnancy outcomes were analyzed with a binary logistic regression model. An odds ratio (OR) with 95% confidence intervals (CIs) indicated the strength of relevance. A receiver operating characteristic (ROC) curve analysis was performed to evaluate the predictive ability of AMH levels for CLBR and CCPR. The area under the ROC curve (AUC) was calculated to judge the predictive value of these influencing factors for CLBR and CCPR in patients with high AMH levels26.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Peking University People's Hospital (reference number 2021PHB083-001) and performed in accordance with the Declaration of Helsinki. All participants provided informed consent.
Results
Significant differences in age, duration of infertility, PCOS, bFSH, bLH, AFC, total Gn dose , Gn duration, serum E2, P, and LH levels on HCG day, the numbers of retrieved oocytes and MII oocytes were noted in Group B compared with Group A. Compared with Group A, Group B exhibited the following features: younger maternal age, shorter duration of infertility, more PCOS patients, lower bFSH, higher bLH, more AFC, longer Gn duration, less total Gn dose, higher E2 levels on HCG day, greater number of retrieved oocytes and MII oocytes (Tables 1 and 2).
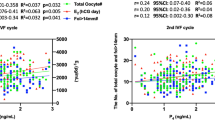
The age range of all the patients is 21–45 years. And the age range of normal AMH and high AMH patients are 21–45 years and 24–44 years. No significant differences were noted between Group A and Group B at LBR (37.07% vs. 41.25%, respectively) and CPR (44.79% vs. 48.13%, respectively). Compared with Group A, Group B had a significantly higher CLBR (65.80% vs. 43.95%, respectively) and CCPR (76.77% vs. 57.14%, respectively) (Table 2, Fig. 1). Binomial logistic regression analysis showed that age greater than 40 years, LH/FSH > 2.5, total Gn dose, Gn duration, and E2 levels greater than 4000 ng/ml on HCG day were significantly associated with CLBR in Group B (Table 3, Fig. 2). The AUC values of CLBR and CCPR averaged 0.664 (ranging from 0.621 and 0.706) and 0.661 (ranging from 0.613 and 0.708) (p < 0.001), respectively, demonstrating moderate predictive value of AMH levels for CLBR and CCPR. (Figs. 3 and 4).
Discussion
The present study is the first to comprehensively explore the relationship between serum AMH levels and pregnancy outcomes in the population of Chinese individuals receiving IVF/ICSI; it is also the first to consider the factors influencing CLBR in patients with high AMH levels. The results of this study suggested that AMH levels were good predictors of CCPR and CLBR in Chinese patients undergoing IVF/ICSI. Maternal age, duration of infertility, PCOS status, bFSH levels, bLH levels, AFC levels, total Gn dose, Gn duration, serum E2, P, and LH levels on HCG day, the number of retrieved oocytes, and the number of MII oocytes were significantly different in patients with normal serum AMH levels compared to those with high serum AMH levels. There was a significant correlation between serum AMH levels and controlled ovarian stimulation outcomes.
High AMH levels and CLBR
Compared with the normal-AMH group, the pregnancy outcomes of fresh embryo transfer in the high-AMH group were higher in the LBR and CPR groups and lower in the MR group; however, the values were not significantly different. In contrast, CLBR was significantly increased in the high-AMH group. Consistent with these results, Tal et al. showed in 2014 that increased AMH levels were correlated with higher fresh CPR27. A recent study suggested that higher serum AMH levels were associated with a poorer LBR in a fresh cycle28. However, with a larger sample size, the present study found that the LBR of the fresh cycle in the high-AMH group was improved (although the difference was not significant), and that the CLBR was significantly increased. Therefore, CLBR tended to increase with increasing AMH levels. Previous studies have disagreed regarding whether AMH levels predict oocyte quality. There are two main hypotheses on this point. The first is that AMH only affects the ovarian response29,30; the second is that AMH also affects oocyte quality and reproductive outcomes31,32,33,34,35. We here found that the number of retrieved oocytes and the number of MII oocytes were increased in those with higher AMH levels. In addition, as AMH levels increased, the E2 levels on HCG day increased to > 4000 ng/ml, and CLBR increased significantly. These results could thus partially explain the increased CLBR associated with increasing AMH levels. In addition, although some studies have indicated that high AMH levels may cause disturbed folliculogenesis36,37,38,39,40, others show that ovarian hyperstimulation and the associated high estrogen-induced endometrial receptivity damage do not affect the FET cycle outcome19. The results of the present study suggested that higher AMH levels may have a greater effect on oocyte production and quality than normal AMH levels. The possible impact of AMH on folliculogenesis and oocyte quality should be further investigated in clinical studies and in molecular biological experiments. Additional studies designed to evaluate blastocysts and Preimplantation Genetic Testing-Aneuploidy could provide further information regarding oocyte quality. The current study included a large sample size, and the main outcomes were objective indexes unaffected by subjective patient factors. Thus, the results showing significant differences can be considered highly reliable.
High AMH levels, maternal age, and CLBR
Chinese women under age 25 tend to have significantly higher AMH levels than white women do. We here noted a positive correlation between maternal age and AMH levels in patients younger than 25 years old and a decline in AMH levels after 34 years of age until menopause. Previous studies have revealed no consistent linear relationship between age and AMH level, but that AMH decreases from age 30 until menopause41. The age-related decline in AMH levels is greater in Chinese women, resulting in 28% and 80% reductions in AMH levels by 30 and 45 years of age, respectively42. These prior results were consistent with the findings of this study. The mean age was significantly lower in the high-AMH group than in the normal-AMH group (31.67 ± 4.08 vs. 34.24 ± 4.21 years; p < 0.001). Previous studies have suggested that maternal age has a substantial impact on ovarian reserves. Thus, advanced age decreases oocyte production during ART cycles43. However, for patients with high AMH levels, ovarian reserves are generally good. It is currently unclear whether advanced age has an impact on CLBR of patients with high AMH levels25.The results of the present study suggested that CLBR was significantly lower in high-AMH patients over 40 years old compared with other age subgroups. In contrast to the results of this study, a study by Kai Lun Hu44 showed that the CLBR decreased in women younger than 35 years with serum AMH levels > 5 ng/ml (when the number of oocytes retrieved was > 20) compared with women who had lower AMH levels. Another study by Dang Kien Nguyen20 examined the relationship between AMH levels and the CLBR after fresh and subsequent FET during the 36-month follow-up period, and found that serum AMH levels were significantly associated with the CLBR following IVF/ICSI, independent of maternal age20. However, these studies failed to completely consider a variety of confounding factors that may influence CLBR, e.g., whether patients had PCOS; bFSH and bLH levels; Gn dose and duration in the ART cycle; and E2 levels on HCG day. In the present study, the AUC values of CLBR and CCPR averaged 0.664 (ranging from 0.621 and 0.706) and 0.661 (ranging from 0.613 and 0.708) (p < 0.001), respectively, demonstrating moderate predictive value of AMH levels for CLBR and CCPR. This study included as many potentially influencing factors as possible to reduce the bias caused by confounding factors, and showed that the CLBR was significantly higher in the high-AMH group than in the normal-AMH group. Furthermore, binomial logistic regression analysis indicated that maternal age > 40 years was significantly associated with CLBR in the high-AMH group, excluding other confounding factors. Age stratification revealed that maternal age over 40 years had a significant negative impact on CLBR compared with other age subgroups (OR = 0.137 [0.028–0.680], p = 0.015). These results were consistent with previous studies suggesting that advanced maternal age substantially affects CPR and LBR45,46,47,48,49. Therefore, even if the ovarian reserve was good in patients with high AMH levels, age (especially > 40) still markedly influenced CLBR. Age-related changes in oocyte quantity and quality, embryo quality, and even endometrial receptivity have a negative impact on pregnancy outcomes, thereby reducing CLBR 50. Consequently, patients over 40 years old seeking to become pregnant are still recommended to receive IVF treatment even if they have high AMH levels.
High AMH levels and PCOS
An earlier study proposed a correlation between high AMH levels and adverse pregnancy outcomes in patients with PCOS. They found that increased AMH levels led to abnormal folliculogenesis, endometrial receptivity changes, and placental abnormalities28. In 2021, another study showed that higher baseline AMH levels in women with PCOS resulted in lower LBR and CPR, but did not influence CLBR19. However, the sample sizes of these studies were relatively small. Interestingly, the present study showed that significantly more patients in the high-AMH group than in the normal-AMH group had been diagnosed with PCOS (3.76% vs. 23.65%; p < 0.001), although a binomial logistic regression analysis indicated that whether patients with high AMH levels also had PCOS had no effect on CLBR. Using CLBR as a pregnancy outcome index may be more effective than single fresh embryo transfer in PCOS patients with high AMH levels. Because more embryos could be transferred overall when pregnancy outcomes were counted within a one-year treatment period, the impacts of the single fresh embryo transfer on endometrial receptivity due to PCOS and of high AMH levels could ultimately be minimized.
High AMH levels and ovarian responses
A study by Tal et al.27 showed that increased AMH levels were correlated with greater ovarian stimulation. Consistent with previous studies51,52,53,54,55, we here found that the number of retrieved oocytes and the number of MII oocytes were significantly increased in the high-AMH group compared to the normal-AMH group. Moreover, the total Gn dose was lower in the high-AMH group than the normal-AMH group (2654.14 ± 908.48 vs. 1896.41 ± 689.52 IU; p < 0.001). Total gonadotrophin consumption was inversely correlated with AMH levels. These results indicated that AMH levels could positively predict ovarian responsiveness to gonadotrophin28,56,57,58.
High AMH and E2 levels on HCG day
In the present study, E2 levels on HCG day were significantly positively correlated with serum AMH levels. However, previous studies on this topic have yielded conflicting results. In 2010, an randomized controlled trial(RCT) study of 60 patients with PCOS showed that E2 levels on HCG day were significantly negatively correlated with AMH levels among different groups (< 2.54 ng/ml, 2.54–3.85 ng/ml, and > 3.85 ng/ml AMH)56. In 2020, a retrospective cohort study of 184 women with PCOS undergoing their first fresh IVF/ICSI cycle found no correlation between AMH and E2 levels on HCG day28. However, a different study of 164 patients with PCOS suggested a positive relationship between AMH and E2 levels57; this is consistent with the findings of the present study, which had a larger sample size. Here, the population with high AMH levels served as the research subject, and PCOS was only one of many potential influencing factors. The range of AMH levels studied here was also broader, ranging from 1.1 and 66.6 ng/ml. Furthermore, the mechanism of interaction between AMH and E2 needed to be explored. GCs can synthesize E2 through the action of aromatase. Many studies have shown that AMH can inhibit aromatase expression, which is induced by FSH and LH in GCs, thereby significantly reducing E2 production36,59,60. These previous findings seem to contradict our results. However, other studies have also shown that E2 and AMH are positively correlated57,58,61,62. The AMH level is assumed to be lower in mature follicles than in preantral and small antral follicles, explaining this finding; it also contributes to the relief of aromatase expression inhibition and subsequent E2 synthesis57. AMH is only expressed by GCs with mitotic activity60,61. Therefore, these findings indicate that E2 may reflect GC proliferation during FSH-stimulated follicle growth, which could neutralize AMH inhibition of FSH-induced aromatase activity58.
Conclusions
In this cohort study of 1379 women undergoing IVF/ICSI, we found that patients with high AMH levels had higher CPR, higher LBR, and lower MR with no statistically significant differences, although there were significant improvements in CLBR. This may have been due to the improvements in oocyte quantity and quality and changes in endometrial receptivity and homeostasis associated with high AMH levels. Advanced age (> 40 years) still impacted CLBR, even in women with good ovarian reserves. Consequently, it is still recommended that patients over 40 years old with high AMH levels actively receive IVF treatment if they seek to become pregnant. PCOS diagnoses did not influence the CLBR. In summary, this study showed that serum AMH levels could positively predict patient ovarian responses and further affect pregnancy outcomes. Larger, multicenter prospective studies and molecular experiments are required to further clarify this relationship.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AMH:
-
Anti-Müllerian hormone
- LBR:
-
Live birth rate
- CLBR:
-
Cumulative live birth rate
- CCPR:
-
Cumulative clinical pregnancy rate
- CPR:
-
Clinical pregnancy rate
- IVF/ICSI:
-
In vitro fertilization/Intracytoplasmic sperm injection
- AFC:
-
Antral follicle count
- FSH:
-
Follicle stimulating hormone
- ART:
-
Assisted reproductive technology
- FET:
-
Frozen embryo thawing transfer
- E2:
-
Estradiol
- P:
-
Progesterone
- MII:
-
Metaphase II
- MR:
-
Early miscarriage rate
- PCOS:
-
Polycystic ovarian syndrome
- rHCG:
-
Recombinant human chorionic gonadotropin
- GnRH-a:
-
Gonadotropin-releasing hormone agonist
- GCs:
-
Granulosa cells
References
Granger, E. & Tal, R. Anti-Müllerian hormone and its predictive utility in assisted reproductive technologies outcomes. Clin. Obstet. Gynecol. 62(2), 238–256. https://doi.org/10.1097/GRF.0000000000000436 (2019).
La Marca, A. & Sunkara, S. K. Individualization of controlled ovarian stimulation in IVF using ovarian reserve markers: From theory to practice. Hum. Reprod. Update 20(1), 124–140. https://doi.org/10.1093/humupd/dmt037 (2014).
Josso, N., di Clemente, N. & Gouédard, L. Anti-Müllerian hormone and its receptors. Mol. Cell Endocrinol. 179, 25–32. https://doi.org/10.1016/s0303-7207(01)00467-1 (2001).
Seifer, D. B. et al. Gonadotropin-releasing hormone agonist-induced differences in granulosa cell cycle kinetics are associated with alterations in follicular fluid Müllerian inhibiting substance and androgen content. J. Clin. Endocrinol. Metab. 76(3), 711–714. https://doi.org/10.1210/jcem.76.3.8445031 (1993).
Caglar, G. S., Kahyaoglu, I., Pabuccu, R., Demirtas, S. & Seker, R. Anti-Müllerian hormone and insulin resistance in classic phenotype lean PCOS. Arch. Gynecol. Obstet. 288(4), 905–910. https://doi.org/10.1007/s00404-013-2833-9 (2013).
Tal, R. & Seifer, D. B. Ovarian reserve testing: A user’s guide. Am. J. Obstet. Gynecol. 217(2), 129–140. https://doi.org/10.1016/j.ajog.2017.02.027 (2017).
Fleming, R., Seifer, D. B., Frattarelli, J. L. & Ruman, J. Assessing ovarian response: Antral follicle count versus anti-Müllerian hormone. Reprod. BioMed. Online 31(4), 486–496. https://doi.org/10.1016/j.rbmo.2015.06.015 (2015).
van Disseldorp, J. et al. Comparison of inter- and intra-cycle variability of anti-Müllerian hormone and antral follicle counts. Hum. Reprod. 25(1), 221–227. https://doi.org/10.1093/humrep/dep366 (2010).
de Koning, C. H. et al. The endocrine and follicular growth dynamics throughout the menstrual cycle in women with consistently or variably elevated early follicular phase FSH compared with controls. Hum. Reprod. 23(6), 1416–1423. https://doi.org/10.1093/humrep/den092 (2008).
Seifer, D. B. & Tal, R. (eds) Anti-Mullerian hormone: Biology, role in ovarian function and clinical significance (Nova, 2016).
Brodin, T., Hadziosmanovic, N., Berglund, L., Olovsson, M. & Holte, J. Anti-Müllerian hormone levels are strongly associated with live-birth rates after assisted reproduction. J. Clin. Endocrinol. Metab. 98(3), 1107–1114. https://doi.org/10.1210/jc.2012-3676 (2013).
Reichman, D. E., Goldschlag, D. & Rosenwaks, Z. Value of anti-Müllerian hormone as a prognostic indicator of in vitro fertilization outcome. Fertil. Steril. 101(10), 1012-1018.e1. https://doi.org/10.1016/j.fertnstert.2013.12.039 (2014).
Arce, J. C., La Marca, A., Mirner Klein, B., Nyboe Andersen, A. & Fleming, R. Anti-Müllerian hormone in gonadotropin releasing-hormone antagonist cycles: Prediction of ovarian response and cumulative treatment outcome in good-prognosis patients. Fertil. Steril. 99(6), 1644–1653. https://doi.org/10.1016/j.fertnstert.2012.12.048 (2013).
Nelson, S. M., Klein, B. M. & Arce, J. C. Comparison of anti-Müllerian hormone levels and antral follicle count as predictor of ovarian response to controlled ovarian stimulation in good-prognosis patients at individual fertility clinics in two multicenter trials. Fertil. Steril. 103(4), 923–930. https://doi.org/10.1016/j.fertnstert.2014.12.114 (2015).
Wang, S. et al. Discordant anti-Müllerian hormone (AMH) and follicle stimulating hormone (FSH) among women undergoing in vitro fertilization (IVF): Which one is the better predictor for live birth?. J. Ovarian. Res. 11(1), 60. https://doi.org/10.1186/s13048-018-0430-z (2018).
Lukaszuk, K. et al. Anti-Müllerian hormone (AMH) is a strong predictor of live birth in women undergoing assisted reproductive technology. Reprod. Biol. 14(3), 176–181. https://doi.org/10.1016/j.repbio.2014.03.004 (2014).
Leijdekkers, J. A. et al. Predicting the cumulative chance of live birth over multiple complete cycles of in vitro fertilization: An external validation study. Hum. Reprod. 33(9), 1684–1695. https://doi.org/10.1093/humrep/dey263 (2018).
Lyttle Schumacher, B. M., Jukic, A. M. Z. & Steiner, A. Z. Anti- Müllerian hormone as a risk factor for miscarriage in naturally conceived pregnancies. Fertil. Steril. 109(6), 1065-1071e1. https://doi.org/10.1016/j.fertnstert.2018.01.039 (2018).
Guo, Y., Liu, S., Hu, S., Li, F. & Jin, L. High serum anti-Müllerian hormone concentrations are associated with poor pregnancy outcome in fresh IVF/ICSI cycle but not cumulative live birth rate in PCOS patients. Front. Endocrinol. 12, 673284. https://doi.org/10.3389/fendo.2021.673284 (2021).
Nguyen, D. K. et al. Anti-Müllerian hormone is a predictor of medium-term cumulative live birth following in vitro fertilization/intracytoplasmic sperm injection: A retrospective study. Eur. J. Obstet. Gynecol. 272, 220–225. https://doi.org/10.1016/j.ejogrb.2022.03.043 (2022).
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 81(1), 19–25. https://doi.org/10.1016/j.fertnstert.2003.10.004 (2004).
Hou, Y. et al. The effect of serum luteinizing hormone on trigger day with a GnRH antagonist protocol in IVF/ICSI treatment. Gynecol. Obstet. Clin. Med. 1, 205–210. https://doi.org/10.1016/j.gocm.2021.11.004 (2021).
Zegers-Hochschild, F. et al. The international glossary on Infertility and fertility care. Fertil Steril 108, 393–406. https://doi.org/10.1016/j.fertnstert.2017.06.005 (2017).
Hamdine, O. et al. Antimüllerian hormone: prediction of cumulative live birth in gonadotropin-releasing hormone antagonist treatment for in vitro fertilization. Fertil. Steril. 104(4), 891-898.e2. https://doi.org/10.1016/j.fertnstert.2015.06.030 (2015).
Zhang, B. et al. IVF outcomes of women with discrepancies between age and serum anti-Müllerian hormone levels. Reprod. Biol. Endocrinol. 17, 58. https://doi.org/10.1186/s12958-019-0498-3 (2019).
Liu, P. et al. A new method to predict anatomical outcome after idiopathic macular hole surgery. Graefes. Arch. Clin. Exp. Ophthalmol. 254, 683–688. https://doi.org/10.1007/s00417-015-3116-x (2016).
Tal, R. et al. Characterization of women with elevated anti-müllerian hormone levels (AMH): Correlation of AMH with polycystic ovarian syndrome phenotypes and assisted reproductive technology outcomes. Am. J. Obstet. Gynecol. 211(1), 59.e1–8. https://doi.org/10.1016/j.ajog.2014.02.026 (2014).
Tal, R., Seifer, C. M., Khanimov, M., Seifer, D. B. & Tal, O. High serum antimullerian hormone levels are associated with lower live birth rates in women with polycystic ovarian syndrome undergoing assisted reproductive technology. Reprod. Biol. Endocrinol. 18(1), 20. https://doi.org/10.1186/s12958-020-00581-4 (2020).
Peñarrubia, J. et al. Basal and stimulation day 5 anti- Müllerian hormone serum concentrations as predictors of ovarian response and pregnancy in assisted reproductive technology cycles stimulated with gonadotropin-releasing hormone agonist—gonadotropin treatment. Hum. Reprod. 20(4), 915–922. https://doi.org/10.1093/humrep/deh718 (2005).
van Rooij, I. A. et al. Women older than 40 years of age and those with elevated follicle-stimulating hormone levels differ in poor response rate and embryo quality in in vitro fertilization. Fertil. Steril. 79(3), 482–488. https://doi.org/10.1016/s0015-0282(02)04839-2 (2003).
Ebner, T. et al. Basal level of anti-Mullerian hormone is associated with oocyte quality in stimulated cycles. Hum. Reprod. 21(8), 2022–2026. https://doi.org/10.1093/humrep/del127 (2006).
Irez, T. et al. Different serum anti- Müllerian hormone concentrations are associated with oocyte quality, embryo development parameters and IVF-ICSI outcomes. Arch. Gynecol. Obstet. 284, 1295–1301. https://doi.org/10.1007/s00404-011-1979-6 (2011).
Baird, D. D. & Steiner, A. Z. Anti- Müllerian hormone: A potential new tool in epidemiologic studies of female fecundability. Am. J. Epidemiol. 175, 245–249. https://doi.org/10.1093/aje/kwr439 (2012).
Steiner, A. Z. et al. Antimullerian hormone as a predictor of natural fecundability in women aged 30–42 years. Obstet. Gynecol. 1117, 798–804. https://doi.org/10.1097/AOG.0b013e3182116bc8 (2011).
Fréour, T. et al. Anti-Müllerian hormone: Clinical relevance in assisted reproductive therapy. Ann. Endocrinol. 67, 567–574. https://doi.org/10.1016/s0003-4266(06)73008-6 (2006).
Catteau-Jonard, S. et al. Changes in serum anti-Mullerian hormone level during low-dose recombinant follicular-stimulating hormone therapy for anovulation in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 92(11), 4138–4143. https://doi.org/10.1210/jc.2007-0868 (2007).
Dewailly, D., Barbotin, A. L., Dumont, A., Catteau-Jonard, S. & Robin, G. Role of Anti-Müllerian hormone in the pathogenesis of polycystic ovary syndrome. Front. Endocrinol. (Lausanne) 11, 641. https://doi.org/10.3389/fendo.2020.00641 (2020).
Das, M., Gillott, D. J., Saridogan, E. & Djahanbakhch, O. Anti-Müllerian hormone is increased in follicular fluid from unstimulated ovaries in women with polycystic ovary syndrome. Hum. Reprod. 23(9), 2122–2126. https://doi.org/10.1093/humrep/den185 (2008).
Seifer, D. B. & Merhi, Z. Is AMH a regulator of follicular atresia?. J. Assist. Reprod. Genet. 31(11), 1403–1407. https://doi.org/10.1007/s10815-014-0328-7 (2014).
Webber, L. J. et al. Prolonged survival in culture of preantral follicles from polycystic ovaries. J. Clin. Endocrinol. Metab. 92(5), 1975–1978. https://doi.org/10.1210/jc.2006-1422 (2007).
Kotlyar, A. M. & Seifer, D. B. Ethnicity/race and age-specific variations of serum AMH in women—A review. Front. Endocrinol. 11, 593216. https://doi.org/10.3389/fendo.2020.593216 (2021).
Nelson, S. M. et al. Ethnic discordance in serum anti-Müllerian hormone in healthy women: A population study from China and Europe. Reprod. Biomed. Online 40(3), 461–467. https://doi.org/10.1016/j.rbmo.2019.11.013 (2020).
Yavas, Y. Curvilinear relationship between age and assisted reproduction technique success: Retrospective analyses of US National ART surveillance system data from 2010–2014. Reprod. BioMed. Online 35(6), 657–668. https://doi.org/10.1016/j.rbmo.2017.07.018 (2017).
Kai-Lun, Hu., Liu, F.-T., Huiyu, Xu., Li, R. & Qiao, J. Association of serum anti-Müllerian hormone and other factors with cumulative live birth rate following IVF. Reprod. Biomed. Online 40(5), 675–683. https://doi.org/10.1016/j.rbmo.2020.01.024 (2020).
Serour, G. et al. Analysis of 2386 consecutive cycles of in vitro fertilization or intracytoplasmic sperm injection using autologous oocytes in women aged 40 years and above. Fertil. Steril. 94(5), 1707–1712. https://doi.org/10.1016/j.fertnstert.2009.09.044 (2010).
Raz, N. et al. Cumulative pregnancy and live birth rates through assisted reproduction in women 44–45 years of age: Is there any hope?. J. Assist. Reprod. Genet. 35(3), 441–447. https://doi.org/10.1007/s10815-017-1094-0 (2018).
Bellver, J., Albert, C., Soares, S. R., Alvarez, C. & Pellicer, A. The singleton, term gestation, and live birth rate per cycle initiated: A 1-year experience in in vitro fertilization cycles with native and donated oocytes. Fertil. Steril. 83(5), 1404–1409. https://doi.org/10.1016/j.fertnstert.2004.11.047 (2005).
Klipstein, S. et al. One last chance for pregnancy: A review of 2705 in vitro fertilization cycles initiated in women age 40 years and above. Fertil. Steril. 84(2), 435–445. https://doi.org/10.1016/j.fertnstert.2005.02.020 (2005).
Gunnala, V., Irani, M., Melnick, A., Rosenwaks, Z. & Spandorfer, S. One thousand seventy-eight autologous IVF cycles in women 45 years and older: The largest single-center cohort to date. J. Assist. Reprod. Genet. 35(3), 435–440. https://doi.org/10.1007/s10815-017-1088-y (2018).
Fang, G. et al. Can repeat IVF/ICSI cycles compensate for the natural decline in fertility with age? An estimate of cumulative live birth rates over multiple IVF/ICSI cycles in Chinese advanced-aged population. Aging 13(10), 14385–14398. https://doi.org/10.18632/aging.203055 (2021).
La Marca, A. et al. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART). Hum. Reprod. Update 16(2), 113–130. https://doi.org/10.1093/humupd/dmp036 (2010).
Hazout, A. et al. Serum Antimüllerian hormone/Müllerian-inhibiting substance appears to be a more discriminatory marker of assisted reproductive technology outcome than follicle-stimulating hormone, inhibin B, or estradiol. Fertil. Steril. 82(5), 1323–1329. https://doi.org/10.1016/j.fertnstert.2004.03.061 (2004).
Seifer, D. B., MacLaughlin, D. T., Christian, B. P., Feng, B. & Shelden, R. M. Early follicular serum Müllerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil. Steril. 77(3), 468–471. https://doi.org/10.1016/S0015-0282(01)03201-0 (2002).
Li, H. W. R. & Nelson, S. M. Clinical application of AMH measurement in assisted reproduction. Front. Endocrinol. (Lausanne) 11, 606744. https://doi.org/10.3389/fendo.2020.606744 (2020).
Broer, S. L. et al. Added value of ovarian reserve testing on patient characteristics in the prediction of ovarian response and ongoing pregnancy: An individual patient data approach. Hum. Reprod. Update 19(1), 26–36. https://doi.org/10.1093/humupd/dms041 (2013).
Kaya, C., Pabuccu, R. & Satiroglu, H. Serum Anti-Müllerian hormone concentrations on day 3 of the in vitro fertilization stimulation cycle are predictive of the fertilization, implantation, and pregnancy in polycystic ovary syndrome patients undergoing assisted reproduction. Fertil. Steril. 94(6), 2202–2207. https://doi.org/10.1016/j.fertnstert.2009.12.002 (2010).
Xi, W., Gong, F. & Lu, G. Correlation of serum anti-Mullerian hormone concentrations on day 3 of the in vitro fertilization stimulation cycle with assisted reproduction outcome in polycystic ovary syndrome patients. J. Assist. Reprod. Genet. 29(5), 397–402. https://doi.org/10.1007/s10815-012-9726-x (2012).
Dumesic, D. A. et al. Intrafollicular anti- Müllerian hormone levels predict follicle responsiveness to follicle-stimulating hormone (FSH) in normoandrogenic ovulatory women undergoing gonadotropin releasing-hormone analog/recombinant human FSH therapy for in vitro fertilization and embryo transfer. Fertil. Steril. 92(1), 217–221. https://doi.org/10.1016/j.fertnstert.2008.04.047 (2009).
Dewailly, D. et al. Interactions between androgens, FSH, anti-Mullerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum. Reprod. Update 22(6), 709–724. https://doi.org/10.1093/humupd/dmw027 (2016).
Pellatt, L. et al. Anti-Müllerian hormone reduces follicle sensitivity to follicle-stimulating hormone in human granulosa cells. Fertil. Steril. 96(5), 1246–1251. https://doi.org/10.1016/j.fertnstert.2011.08.015 (2011).
La Marca, A. et al. Anti-Müllerian hormone plasma levels in spontaneous menstrual cycle and during treatment with FSH to induce ovulation. Hum. Reprod. 19(12), 2738–2741. https://doi.org/10.1093/humrep/deh508 (2004).
Lee, J. R. et al. Anti-Müllerian hormone dynamics during controlled ovarian hyperstimulation and optimal timing of measurement for outcome prediction. Hum. Reprod. 25(10), 2597–2604. https://doi.org/10.1093/humrep/deq204 (2010).
Acknowledgements
We thank all the staff of the Reproductive Medicine Center for their contributions.
Funding
This work was supported by Society fund (2021-Z-45) and Peking University People’s Hospital Scientific Research Development Funds (RDE2021-03 and RDL2022-27).
Author information
Authors and Affiliations
Contributions
Y.H. and L.W.: Project development, Data Collection, Data analysis, Manuscript writing. L.T.: Project development, Data analysis, Manuscript editing. Y.L. and J.A.: Data Collection. Acknowledgements.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hou, Y., Wang, L., Li, Y. et al. Serum levels of anti-Müllerian hormone influence pregnancy outcomes associated with gonadotropin-releasing hormone antagonist treatment: a retrospective cohort study. Sci Rep 13, 2127 (2023). https://doi.org/10.1038/s41598-023-28724-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-28724-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.