Abstract
Tongkat ali commonly known as Malaysian Ginseng (Eurycoma longifolia) is a herbal root worldwide available in nutraceuticals, either as a crude powder or capsules blended with other herbal products. Herein, a multiplexed metabolomics approach based on nuclear magnetic resonance (NMR) and solid-phase microextraction combined with gas chromatography–mass spectrometry (SPME–GC–MS) was applied for authentic tongkat ali extract vs some commercial products quality control analysis. NMR metabolite fingerprinting identified 15 major metabolites mostly ascribed to sugars, organic and fatty acids in addition to quassinoids and cinnamates. Following that, multivariate analysis as the non-supervised principal component analysis (PCA) and supervised orthogonal partial least squares-discriminant analysis (OPLS-DA) were applied revealing that differences were related to fatty acids and 13,21-dihydroeurycomanone being more enriched in authentic root. SPME–GC–MS aroma profiling led to the identification of 59 volatiles belonging mainly to alcohols, aldehydes/furans and sesquiterpene hydrocarbons. Results revealed that aroma of commercial products showed relatively different profiles being rich in vanillin, maltol, and methyl octanoate. Whereas E-cinnamaldehyde, endo-borneol, terpinen-4-ol, and benzaldehyde were more associated to the authentic product. The present study shed the light for the potential of metabolomics in authentication and standardization of tongkat ali and identification of its true flavor composition.
Similar content being viewed by others
Introduction
Recent advances in biological and analytical sciences applied for traditional herbal medicines as well as innovations in omics can undoubtedly play a leading role in the quality control and validation of commercial herbal products. Particularly, tongkat ali (Malaysian Ginseng) is the roots of Eurycoma longifolia tree (family Simaroubaceae) that is native to Southeast Asian countries, but it is widely consumed by millions of people all over the world, competing with Panax Ginseng (Chinese Ginseng)1,2. It is mostly grown in Malaysia for its therapeutic benefits to preserve wild plants3. Despite of its bitter taste, it has been consumed as coffee and tea additives based on its well reported effect in natural wellness and unique bioactive contents4. Moreover, folk medicine has documented the use of its roots extract for sexual dysfunction, and many other health disorders1,2,5. However, more accessible formulas are marketed nowadays either as a raw crude powder or capsules blended with other herbs2.
The aroma profile of tongkat ali roots as a main predictor of their hot beverage flavor, as well as impact of heating/roasting on their chemical compositions have been characterized in preliminary research based on soxhlet and conventional steam extraction methods which revealed few number of volatiles6. Nevertheless, solid phase microextraction (SPME) is an ultimate novel, simple, solvent-free, and sensitive approach for detecting volatiles at low temperatures that can be used to evaluate the true aroma of in E. longifolia roots7,8. Compared to steam distillation for aroma analysis, there are numerous advantages for non-exhaustive SPME technique compared to exhaustive extraction resulting in more representative information of volatiles composition9. SPME presents an optimum sample pretreatment process that eliminates any compounds that are incompatible with the instrument such as matrix compounds10. Thus, SPME presents a potential tool for VOCs analysis in the roots of tongkat ali especially considering its medium aroma. Coupled to SPME; gas chromatography with mass spectrometry (GC–MS) is the most suited for identification of aroma components11,12.
Asides from tongkat ali aroma components contributing to its flavor, root encompasses a wide range of non-volatile bioactive chemicals, including phenolics, triterpenes, alkaloids, and squalene derivatives13,14. One of the best platforms that can characterize such chemicals is NMR with numerous advantages of being high-throughput, highly reproducible, non-destructive, and universal with minimal sample preparation steps15. In addition, the NMR signal intensities are directly proportional to the corresponding real molar levels of the detected metabolites, which makes NMR an absolute quantitative method without any need for calibration curves of individual analytes. Besides, the comparison of the overall metabolic composition can be performed for different specimens using a data matrix produced from 1H NMR spectra aided by multivariate models15,16,17.
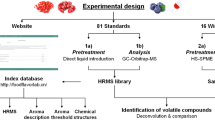
Hence, metabolomics research that employs NMR and MS as complimentary approaches may provide significant insights into tongkat ali root metabolome. The current study applies a complementary approach of NMR and GC–MS coupled with chemometric analyses for untargeted metabolite fingerprinting of E. longifolia roots extracts in comparison with some of its commercial products. The aim of this study was further to use NMR finger printing to standardize E. longifolia extract by quantifying its major components for future incorporation in quality control of nutraceuticals.
Materials and methods
Plant material and commercial preparations
Root specimen of cultivated E. longifolia was obtained from the Ethno Resources Sdn, Sungai Buloh. Malaysia. The plant material was authenticated by Dr. Idder Boubaker, Botanist of Plant Taxonomy at the Laboratory of microbiology and plant biology, University Abdulhamid Ibn Badis, Mostaganem, Algeria. In the laboratory's herbarium, voucher specimens (LMPB0017-2021) were kept. All procedures were conducted in conformity with the pertinent rules and regulations. Three commercial E. longifolia products, viz. Nu-prep LELAKI® (each capsule contains 100 mg E. longifolia extract), Naturelle® (each capsule contains 100 mg E. longifolia extract) and Long jack plus® (each capsule contains 425 mg E. longifolia, 50 mg Nigella sativa and 25 mg Cassia angustifolia extracts) were obtained from retail stores in Kuala Lumpur Malaysia.
Chemicals and reagents
Hexamethyldisiloxane (HMDS) and methanol-d4 (CD3OD) were purchased for NMR measurement as an internal standard from Deutero GmbH (Kastellaun, Germany). In addition, divinylbenzene-carboxen-polydimethylsiloxane (DVB-CAR-PDMS) and polydimethylsiloxane (PDMS) fibers provided in 1 cm long and 50/30 µm, from Supelco (Bellefonte, PA, USA), were used for SPME aroma sampling.
Extraction procedure and samples preparation for NMR analysis
Samples extraction were performed following18. Briefly, 15 mg of the plant extract and the commercial preparations (according to the labels) were homogenized with 6 mL of MeOH using a Turrax mixer (11,000 rpm) for five 20 s periods. Extracts were vortexed vigorously to remove plant debris for 1–2 min and centrifuged at 3000×g for 30 min. Four mL of the root extracts and commercial products were dried under a stream of nitrogen, then resuspended with 700 µL CD3OD containing HMDS (0.94 mM). Afterwards, centrifugation at 13,000×g for 1 min, and finally the supernatant was transferred to a 5 mm NMR tube and subjected to NMR analysis. To account for biological variability, three biological replicates for each group were extracted and analyzed under identical conditions.
NMR analysis
Agilent VNMRS 600 NMR spectrometer was used to record all NMR spectra operating at a 1H NMR frequency of 599.83 MHz and combined with implemented in Varian VNMRJ 2.2C spectrometer software. For 1H NMR experiments, the instrument’s parameters were adjusted to a relaxation delay = 17.95 s; pulse angle = 90°, pulse width (pw) = 6.25 μs and number of transients = 120. For 2D-NMR experiments, 45° pulse width was used within the standard CHEMPACK 4.1 pulse sequences (gDQCOSY, gHSQCAD, gHMBCAD). The heteronuclear single quantum coherence spectroscopy (HSQC) experiment was adjusted to 1JCH = 146 Hz with DEPT-like editing and 13C-decoupling during acquisition time. In addition, the heteronuclear multiple bond correlation (HMBC) experiment was optimized for a long-range coupling of 8 Hz; a two-step 1JCH filter was utilized (130–165 Hz).
NMR data processing and quantification
The 1H NMR spectra were automatically fourier transformed, phase and baseline corrected with MestReNova software (9.0.1, Mestrelab Research, Santiago, Spain). The spectra were referenced to internal HMDS at 0.062 ppm for 1H NMR and to internal CD3OD signals at 49 ppm for 13C-NMR, respectively. Spectral intensities were then reduced to integrated sections of identical width (0.04 ppm), referred to as buckets, within the region of 10.94 to 0.0 ppm. The regions belonging to residual water between 5.0 and 4.7 ppm and methanol signals 3.26–3.20 ppm were removed prior and the data were subjected to multivariate analyses.
For metabolites quantification using 1H NMR spectroscopy (qNMR), the equation previously described by19 was followed. Manual integration of the peak areas of selected proton signals assigned to compounds and internal standard HMDS was performed.
Headspace SPME volatiles analysis
Except for Long jack plus®, 20 mg of finely pulverized specimens were placed in an SPME screw cap vials and spiked with 2 µg (Z)-3-hexenyl acetate as an internal standard. The analysis of volatile compounds was carried out on a Schimadzu GC-17A gas chromatograph equipped with DB-5 column (30 m × 0.25 mm i.d. × 0.25 µm film thickness; Supelco) and coupled to Schimadzu QP5050A mass spectrometer. GC–MS analysis was performed following exact conditions previously reported in20. Two biological replicates for each group were analyzed under identical conditions for accounting the biological variability of the samples.
GC–MS data processing
Volatile components were identified based on various paramenter, including their Kovat retention indices (KI) relative to C6-C20 n-alkane series, mass matching with the NIST and WILEY libraries, and with reference standards (when available). Before mass spectral matching, peaks were first deconvoluted through AMDIS software (http://www.amdis.net) and their peaks abundance data were extracted using MS-Dial tool (http://prime.psc.riken.jp/compms/msdial/main.html) applying the following parameters: retention time (0–20 min), mass range (0–1000 Da), accurate mass tolerance (MS1) 0.01, MS2, 0.025 Da, minimum peak height (1000), sigma (0.7), maximum charge number (2), MS1 tolerance for alignment (0.015 Da), and peak height 1000.
Multivariate and univariate statistical analysis
The datasets generated from the NMR and GC–MS were subjected to multivariate data analysis. Prior to data analysis, normalization by pareto-scaling have been performed to allow adjustment of differences among the samples. Then, unsupervised exploratory analysis has been applied i.e. principal component analysis (PCA) and hierarchical clustering analysis (HCA) followed by supervised analysis i.e. orthogonal partial least squares-discriminant analysis (OPLS-DA) using SIMCA-P Version 13.0 (Umetrics, Umeå, Sweden). Differential variables were subsequently identified based on by analyzing the S-plot, which was declared with covariance (p) and correlation (pcor) in addition to the variable influence in the projection (VIP).
For the univariate analysis, the data are presented as mean ± SD. The quantified NMR metabolites were analyzed by one-way ANOVA followed by Dunnett's test for multiple group comparison of the commercial against the authentic samples (GraphPad Prism, version 8.0, GraphPad Software, La Jolla, CA). Differences were considered statistically significant when p ≤ 0.05. Volcano plots were employed for analysis of the the GC–MS metabolites under R version 4.1.0 where differential metabolites were subsequently identified based on compiling the results of unpaired two-tailed t-test with p values ≤ 0.05 and fold changes above 1.5 or below 0.66.
Results and discussion
In the present study, a multiplexed approach of NMR and GC–MS metabolomics fingerprinting of E. longifolia root extract was employed to facilitate the identification and quantification of its major metabolites. Hence, reliable multivariate chemometric models can be developed for the authentication, standardization, and quality control of commercial E. longifolia preparations.
NMR identification of E. longifolia metabolites
1H NMR analysis of E. longifolia root extract exhibited characteristic signals at two main regions; an up-field region (δH 0.5 to 6.0 ppm) of high intensity peaks ascribed mostly to sugars, organic and fatty acids in addition to quassinoids (Fig. 1A), and a down-field region (δH 6.0 to 9.5 ppm) with signals mainly belonging to aromatic compounds viz., cinnamaldehyde and scopoletin (Fig. 1B). A total of 15 metabolites were identified (Fig. S1) mostly based on their 2D-NMR spectra to overcome overlapped peaks in such crude extract including 1H–1H COSY, 1H–13C HSQC and 1H–13C HMBC as listed in (Table 1).
1H-NMR spectra of E. longifolia root extract (A) at δ 0.5–6.5 ppm, and (B) at δ 6.5–11.0 ppm showing characteristic signals for primary and secondary metabolites. Peaks assigned in the spectrum are labeled as listed in Table 1.
Sugars were the dominant primary metabolites identified in E. longifolia root extract (Table 1) including α-glucose (N1), β-glucose (N2), sucrose (N3), α-fructofuranose (N4), β-fructofuranose (N5) and glycerol (N6). In detail, α- and β-glucose were assigned based on their anomeric protons’ signals displayed at δH 5.10 (d, J = 3.6 Hz) and 4.47 (d, J = 7.9 Hz), respectively. These signals showed cross peaks correlations with their corresponding carbons at δ13C of 94.1 and 97.8 ppm in the 1H-13C HSQC spectra. Sucrose was identified based on its characteristic anomeric proton at δH 5.38 (d, J = 3.81 Hz) and further confirmed by examining anomeric proton 2D-NMR correlations, i.e., COSY with cross peak at δH 3.40 and TOCSY with cross peak at δH 3.71 for H-2 and H-3 of the glucose moiety, respectively. In addition, α-fructofuranose and β-fructofuranose were identified from their H-5 and H-3 signals resonating as a doublet at δH 4.01 (J = 1.4 Hz) and at δH 4.10 (J = 8.34 Hz), respectively. Sugar alcohol exemplified by glycerol was identified based on its signal at δH 3.58 assigned to the diastereotropic methylene protons at C-1 position of glycerol (Fig. S1) and to contribute to roots sweet taste.
NMR analysis also revealed the presence of many organic acids including acetic acid (N8), succinic acid (N9), and syringic acid (N10) in agreement with reported literature21. Acetic acid and succinic acid were identified based on their corresponding singlet protons at δH 1.97 and 2.50 ppm with HSQC cross-peak correlation at δ13C 21.9 and 32 ppm, respectively. Another phenolic acid identified as syringic acid displayed characteristic signal for its methoxy protons at δH 3.91 showing correlation to δ 13C 56 in the HSQC spectra and long-range coupling to the aromatic methine carbons at δ 13C 149.3 in HMBC spectra. Other than organic acids, few peaks for fatty acids (N11) could be readily assigned in the 1H NMR spectrum such as two signals at δH 0.90 and δH 1.28–1.30 ppm for the terminal methyl and repeated methylene of fatty acids, respectively.
Compared to primary metabolites to impart tongkat ali typical flavor, quassinoids, the major secondary metabolites of E. longifolia roots were detected in this study including eurycomanone (N12) and 13,21-dihydroeurycomanone (N13) and to account more for roots hormonal effects in agreement with reported literature21. Eurycomanone was readily assigned from characteristic signal of its olefinic proton H-3 at δH 6.05 which exert cross peak correlation to its carbon at δ 13C 125.9 ppm in the HSQC spectra (Fig. S1). In addition, the deshielded signal of H-7 at δH 4.72, showed a long-range coupling with the carbonyl carbon (C-16) at δ 13C 175.1 ppm in HMBC and cross peak correlation to its direct carbon at δ 13C 77.6 ppm in HSQC spectra. Interestingly, H-21 signal of eurycomanone can be identified as the differential signal from that of 13,21-dihydroeurycomanone (Fig. S1). This signal was observed at δH 5.43 due to the presence of olefinic double bond (C13 = C21) for eurycomanone, while in 13,21-dihydroeurycomanone signal appeared at δH 1.241. It has been reported that both compounds displayed significant increase in spermatogenesis mostly via direct effect on the hypothalamic–pituitary–gonadal axis which aid in the fertility-enhancing property of the E. longifolia22.
Compared to quassinoids as major secondary metabolites in E. longifolia, aromatic region showed key signals in the downfield region 6.5–10.0 ppm for cinnamates ascribed to scopoltin (N14) and (E)-cinnamaldehyde (N15), respectively and to add to roots unique flavor as key components. Scopoltin, a hydroxycoumarin with 6-methoxy substitution previously identified in E. longifolia23, was identified from its characteristic olefinic protons H-2 and H-3 (Fig. S1) appearing as a pair of doublets at δH 6.18 and 7.85 ppm (J = 9.4), respectively. These peaks displayed distinct correlation signals in 1H-1H COSY experiment, and cross peak correlations with the carbonyl carbon (C-1) at δ 13C 163.8 ppm in the HMBC spectra confirming the coumarin nucleus. The methoxy group substitution was detected at δH 3.87 ppm correlated to δ 13C 57.1 ppm in HSQC spectra. Cinnamaldehyde, a major aroma compound in cinnamon, was detected for the first time in E. longifolia based on its three-spin systems i.e. a doublet aldehyde peak at δH 9.66 (J = 7.87 Hz), olefinic signals at δH 6.78 (dd, J = 16.0, 7.87 Hz) and 7.68 (d, J = 15.5) corresponding to olefinic H-2 and H-3, respectively (Fig. S1)24. The remaining aromatic protons were observed as multiplets at δH 7.45/7.68 ppm and further confirmed using HSQC and HMBC experiments (Table 1). The 16.0 Hz coupling of the olefinic protons indicated its (E)-geometry.
Multivariate data analysis of E. longifolia commercial preparations based on 1H NMR dataset
To further determine how commercial preparation NMR fingerprint compare to authenticated tongkat ali root, three of its commercial preparations i.e., Naturelle®, Long jack plus®, and Nu-prep LELAKI® were modelled alongside roots based on their acquired NMR dataset. Details on products´ composition are presented in the experimental section. PCA was first employed to determine the pattern among examined samples in an unsupervised manner. The score plot revealed for complete segregation of authenticated root from commercial products suggesting for metabolic differences with PC1 and PC2 accounting for 89.23% of the variance (Fig. 2A). NMR signals that had major contribution to such pattern can be identified from the corresponding loading plot with peaks of fatty acids (δH 0.88, 1.28 ppm) and 13,21-dihydroeurycomanone (δH 1.24 ppm) found more enriched in authentic root as evident from their positive PC1 values (Fig. 2B). The signals related to commercial products (δH 3.45–4.69 ppm) appeared to belong to sugars in the upper-mid 1H NMR spectra. Similar results were also observed from HCA dendrogram (Fig. 2C) with two main cluster: cluster (a) for authentic root and cluster (b) for commercial product samples.
Principal component analysis (PCA) and hierarchical cluster analysis (HCA) of E. longifolia commercial preparations NMR dataset. (A) Score plot of PC1 vs. PC2, complete segregation of the authentic samples from the commercial products. (B) Loading plot for PC1 and PC2 showing signals of fatty acids (δ 0.88, 1.28 ppm) and 13,21-dihydroeurycomanone (δ 1.24 ppm) as the major signals contributing to samples discrimination. (C) HCA of NMR dataset shows two main cluster: cluster (a) for the authentic samples and cluster (b) for the commercial product specimens.
Another multivariate analysis model i.e. OPLS-DA was employed for confirming PCA results and identifying markers. OPLS-DA exhibit its advantage from finding the maximum separation among the studied classes and identifying differential biomarkers in a supervised approach. In agreement with PCA results, complete separation was attained along the predictive components between the authentic samples and the commercial products with a fitness of data R2(X) of 94.8%, R2(Y) of 99.2% and predictive ability Q2 of 98.5% (Fig. 3A). Metabolites that influence such pattern were verified form loading S-plot (Fig. 3B) and VIP plot (Fig. 3C) where peaks related to fatty acids (δH 0.88, 1.28 ppm) and 13,21-dihydroeurycomanone (δH 1.24 ppm) were found abundant in authenticated root compared to the commercial products in agreement with the PCA results. Regarding validity of the developed model, a valid model was obtained using permutation test with negative Q2 intercept value (Fig. 3D) and CV-ANOVA with p < 0.05.
(A) OPLS-DA score plot and (B) loading S-plots derived from E. longifolia authentic root modelled against other commercial samples analysed by 1H-NMR, n = 3. The p(corr)[1] axis represents the correlation of the bin towards the predictive variation. The p[1] axis represents the magnitude of the spectral bins. (C) Top 10 variables that have the greatest influence on the projection as revealed from the developed OPLS-DA. (D) Permutation test (n = 200) as a validation criterion for the OPLS-DA model, the test verifies the validity of the model with negative intercept for the Q2 values.
1H NMR-based quantification of identified metabolites
The absolute concentrations of identified metabolites were determined both in the authentic root and commercial products to aid in standardization of E. longifolia nutraceuticals. Out of the 15 identified metabolites, 10 metabolites (7 primary and 3 secondary metabolites) were successfully quantified considering they showed no signal overlap, where quantification and precise metabolites measurement were calculated as μg/mg extract based on the integration of their corresponding peaks in the 1H NMR spectra as shown in (Table S1). Representative 1H NMR spectra of the commercial preparations i.e., Naturelle®, Long jack plus®, and Nu-prep LELAKI® is depicted in (Fig. S2).
The authentic E. longifolia extract showed α- and β-glucose at 2.8 µg/mg and 4.3 µg/mg, respectively. However, commercial products, especially Nu-prep LELAKI® showed the highest contents at 38.0 and 49.0 µg/mg, while the Long jack plus® contained the lowest levels at 1.1 and 3.8 µg/mg, respectively. β-Glucose is the building monomer of cellulose which is the major polysaccharide in plant cell walls. Interestingly, the β-glucose showed non-significant difference with the authentic root at p < 0.05. Also, a similar pattern was observed with other primary metabolites such as sucrose, α-fructofuranose, β-fructofuranose, glycerol, and acetic acid. Moreover, the highest and significant content of β-fructofuranose was detected in Naturelle® and Nu-prep LELAKI® products at 28.3 and 15.1 µg/mg, respectively, at more than fivefold levels compared to authentic roots suggestive that industrial processing to lead to more sweet taste in products. In contrast, the sweet taste of authentic roots may originate from the highest sucrose content (14.0 µg/mg).
On the other hand, the secondary metabolites scopoletin and (E)-cinnamaldehyde demonstrated the highest levels in authenticated root at 4.7 and 1.9 µg/mg, respectively, with a significant difference (p < 0.05) compared to other commercial products. However, the other identified quassinoid eurycomanone was detected at higher levels in commercial products, specifically in Naturelle® and Nu-prep LELAKI® at 37.0 and 18.9 µg/mg, respectively. The richness of commercial products in eurycomanone is recognized as a superior objective and encourages better marketing of the tongkat ali herbal products as sexual function and fertility-promoting products helping to improve serum testosterone levels25. Furthermore, the lowest abundance of quassinoids in Long jack plus® preparations proposed its nutritional and medicinal effects resulting from the combined herbal mixture present in its constituents.
Since the root is the organ of storage of metabolites that provide energy source. The investigated samples showed richness in sugars and their products of metabolism. These findings might be inferred from the low abundance either in primary sugars or secondary metabolites in Long jack plus® product which contains additional plant extracts other than tongkat ali root, including Nigella sativa seeds. Nevertheless, other differences of authentic roots compared with Naturelle® and Nu-prep LELAKI® are likely attributed to the processing protocols and excipients added during manufacturing. Yet, other products from different companies worldwide and authentic samples from different geographical origins ought to be analyzed to provide insights into the further processing procedures followed by companies.
GC–MS based aroma profiling of E. longifolia and its commercial preparations
Considering that cinnamaldehyde appeared as major component in E. longifolia using NMR, aroma profiling was attempted using more sensitive technique targeting volatile organic compounds (VOCs) using SPME coupled with GC–MS in roots as a more sensitive technique alongside two of its available commercial products, i.e., Nu-prep LELAKI® and Naturelle®. However, Long jack plus® specimen was excluded from aroma profiling by SPME coupled with GC–MS since such product contained other aromatic herbal extracts, including N. sativa and Cassia angustifolia, which might interfere with tongkat ali original aroma constituents giving unreliable results.
This study assessed tongkat ali aroma directly from the authentic roots powder in contrast to the previous studies and likewise the effect of industrial processing and pharmaceutical formulation, including excipients, which have not been investigated yet. Previous aroma analysis revealed a number of VOCs extracted by either water or methanol and detected by electronic nose response and SPME coupled with GC–MS. However, non-specific volatiles as 2-hexadecanol, nonanal, acetaldehyde, acetic acid, 2-methylhexanol and 2(5H)-furanone were identified6,26,27.
The results of the current study showed that the GC total ion chromatograms (TIC) of the three investigated samples, i.e., E. longifolia and Nu-prep LELAKI®, and Naturelle® products showed relatively different patterns (Fig. 4). A total of 59 VOCs were identified listed in Table 2 belonging to acids (3 compounds), alcohols (14), aldehyde/furans (10), aliphatic hydrocarbons (3), aromatic hydrocarbons (1), phenol/ethers (4), fatty acids/esters (9), ketones (4), lactones (2), nitrogenous compounds (2), and sesquiterpene hydrocarbons (7). A total of 133 volatiles were previously identified in E. longifolia extracts via SPME (carboxen/polydimethylsiloxane fiber) using a smell sensor equipped with a gas chromatographic stationary phases and lipid materials similar to the olfactory system27. Difference in volatiles recovery may be attributed to such pre-treatment for volatiles collection and the higher number and diversity of analyzed samples, i.e., 10 samples of freeze-dried and spray-dried. Fatty acids/esters and aldehydes/furans amounted for the most abundant classes in investigated samples and in agreement with NMR results revealing for predominance of aldehydes i.e., E-cinnamaldehyde. Authentic powder and Naturelle® were rich in aldehydes/furans at 77.1% and 54.8% of total aroma compounds, respectively. In contrast, Nu-prep LELAKI® was found to be enriched in fatty acids/esters (74.6%), Table 2. These findings indicated that industrial processing and/or pharmaceutical formulation affect VOCs profile to various extent in investigated products of tongkat ali. The following subsections shall highlight the major classes of volatiles and differences observed among samples.
Aldehydes/furans
Aldehyde/furan compounds were represented by ten metabolites ranging from 14.3% in Nu-prep LELAKI product to 77.1% in authentic powder. However, in the Naturelle product they amounted to 54.8% (Table 2). The high abundance of aldehyde/furan in the authentic powder was attributed to E-cinnamaldehyde (P26) which accounted for 73.0% of the total volatiles’ abundance, Fig. 4A, and in agreement with NMR results, Fig. 1B and Table 1. In contrast, (E)-cinnamaldehyde was detected at much lower levels in commercial preparations 8.1% and 39.1% in Nu-prep LELAKI and Naturelle commercial products, Fig. 4B,C. Decrease was also observed in other aldehydes including benzaldehyde (P18) detected at 1.22% in authentic root powder versus 0.45% and 0.25% in Naturelle® and Nu-prep LELAKI, respectively. In contrast, alletone/furaneol (P23) which is a furan derivative detected in commercial products and was completely absent in authentic powder, Table 2. The presence of this compound may indicate its production during the processing step from heating of sugars.
Aldehydes showing opposite accumulation pattern included vanillin detected at 10.8% and 3.0% in Naturelle® and Nu-prep LELAKI® products, respectively versus only 0.35% in authenticated root powder. The last finding may be linked with the addition of vanillin-containing flavor for taste improvement. The results were in agreement with previous reports for aroma extraction using soxhlet extraction of tongkat ali root, where vanillin (2.22%) and benzaldehyde (0.87%) were among the identified aldehydes6.
Alcohols
Alcohols were represented by 14 compounds, which were the most diverse chemical class ranging from 5.4% in Nu-prep LELAKI to 12.6% in authentic E. longifolia powder represented by endo-borneol (P10), 2-ethyl hexanol (P5), and terpinen-4-ol (P11) at 3.3%, 2.8%, and 1.8%, respectively (Table 2). endo-Borneol or isoborneol and terpinen-4-ol are monoterpene alcohols to possess a fragrant odor and28,29. The identification of both compounds in tongkat ali is reported for the first time and likely to contribute to the overall aroma in E. longifolia root.
However, in other commercial preparations, i.e., Nu-prep LELAKI® and Naturelle®, endo-borneol (P10) was not a major alcohol component, while 2-ethyl hexanol (P5) was the most abundant alcohol detected at 1.4% and 2.6%, respectively (Table 2).
Previous reports showed only 1-propanol and 1-hexadecanol as volatile alcohols in tongkat ali extracted by conventional steam extraction6, in addition to 2-hexadecanol and 2-methylhexanol extracted by microwave-assisted extraction26. These findings infer that SPME coupled with GC–MS is more efficient for volatiles extraction and providing the true aroma of medicinal herbs.
Fatty acids/esters
Fatty acids/esters were also detected as major constituents especially in commercial products amounting for 74.6 and 13.6% for Nu-prep LELAKI® and Naturelle®, respectively and less abundant in root powder (Table 2). 9 Compounds were identified including methyl octanoate (P36), Fig. 4B as major form detected at 74.0% in Nu-prep LELAKI especially compared to other specimens, Table 2. Methyl octanoate is a major food flavoring agents possessing a winey, fruity, orange odour30 and is likely to be added in the commercial product of Nu-prep LELAKI® intensifying the product's odour though not indicated on its label. Other esters, including 2-propyl tetradecyl ester and 1-methylundecyl ester previously reported26 were found absent in this study. These findings could be attributed to different extraction methods. Moreover, myristic acid methyl ester (P40) was found to be rich in Naturelle® preparation at 8.2% and absent in authentic and Nu-prep LELAKI® products.
These differences pointed out that industrial processing and formulation affected volatile profile, especially fatty acid/ester class in tongkat ali preparation. Furthermore, radar plot (Fig. S3) showed the unique aroma profile of Nu-prep LELAKI® and its richness in fatty acid/ester and different from authentic root powder and Naturelle® which were abundant in aldehyde/furan, i.e., E-cinnamaldehyde. SPME results fall in accordance with NMR results, Table 2.
Sesquiterpene hydrocarbons
Sesquiterpene hydrocarbons (C15) represented another major class, Table 2, detected at comparable levels ca. 5% in authentic root and Naturelle®. β-Caryophyllene (P55) was the most abundant within that class amounting for 3.0%, 2.5%, and 0.3% in the authentic root, Naturelle®, and Nu-prep LELAKI®, respectively.
Miscellaneous
Other classes were also detected and may contribute to root aroma and flavor. Particularly, the ketone compound maltol (P47) at 4.6% and nitrogenous compound 2-acetylpyrrole (P51) at 6.2% were more abundant in Naturelle® preparation in contrast to other investigated samples (Table 2). Maltol was also found in the other commercial product Nu-prep LELAKI® though at lower levels 1.7%. Maltol exhibits a fragrant caramel-butterscotch odor, while 2-acetylpyrrole is a Maillard reaction product, which is associated with foods drying/roasting and contributes to herbal aroma31. The presence of these compounds can be used as an index for thermal processing and drying step level in tongkat ali. Finally, tonkalide (P49) was detected in all samples at relatively low levels ranging from 0.4 to 1.5%. To the best of our knowledge, these volatiles are identified in tongkat ali for the first time either in authentic or commercial products.
In conclusion, the aroma of the authentic tongkat ali root is mainly attributed to cinnamaldehyde, endo-borneol, and β-caryophyllene, while Naturelle® preparation is a mixture of volatile constituents acquired during industrial formulation from cinnamaldehyde, vanillin, maltol, and 2-acetylpyrrole. Whereas Nu-prep LELAKI® preparation showed the least similar aroma profile to that of tongkat ali being dominated by methyl octanoate. These findings are in accordance with that of NMR quantification, which showed the less quality of Nu-prep LELAKI® compared with Naturelle®, Table S2. Also, analysis of commercial preparations from other origins shall aid identifying how formulation and/or processing steps can impact root aroma attributes.
Multivariate data analysis of tongkat ali commercial preparations based on GC–MS dataset
Multivariate data analysis of the GC–MS dataset was also employed to assess compositional differences between tongkat ali authentic roots and its two commercial preparations i.e., Naturelle® and Nu-prep LELAKI® based on their VOC profiles. A similar segregation pattern to NMR model (Fig. 2) was observed confirming the validity of the findings. PCA score plot showed that authentic samples were completely segregated away from commercial products along the first principal component with negative PC1 values (Fig. 5A). In contrast, commercial products with positive PC1 values were separated along the second components (PC2) from each other with both PC1 and PC2 accounting for 80.3% of the total variance (Fig. 5A). In terms of VOCs that signify such pattern, loading plot revealed that signals related to benzaldehyde, endo-borneol and terpinen-4-ol in addition to cinnamates were enriched in the authentic root (Fig. 5B) in agreement with results presented in (Table 2). Asides, peaks related to methyl octanoate and nonanoic were more enriched in the Nu-prep LELAKI® preparation while peaks related to maltol and myristic acid were more abundant in Naturelle® samples and posing these volatiles as potential differential biomarkers for tongkat ali QC analysis in the future. HCA analysis comes in agreement with PCA results with two main clusters: cluster (I) for the authentic root and cluster (II) for the Nu-prep LELAKI® and Naturelle® samples signifying their heterogeneity and compositional differences in terms of their VOCs with the authentic group (Fig. 5C).
Principal component analysis (PCA) and Hierarchical cluster analysis (HCA) of E. longifolia commercial preparations GC–MS dataset. (A) Score Plot of PC1 vs. PC2. (B) Loading plot for PC1 and PC2 showing features the have the major contributing to samples discrimination. (C) HCA of GC–MS dataset shows two main cluster: cluster (I) for the authentic samples and cluster II) for the Nu-prep LELAKI® and Naturelle® samples signifying their heterogeneity and compositional differences.
OPLS-DA was likewise employed as supervised model to identify showing likewise complete segregation of the authentic samples away from the commercial preparation with a fitness of data R2(X) of 70.6%, R2(Y) of 99.7% and predictive ability Q2 of 97.1% (Fig. 6A). The volatiles responsible for such clustering can be unraveled for the corresponding S-plot (Fig. 6B) and VIP plot (Fig. 6C) where peaks related to benzaldehyde, endo-borneol, terpinen-4-ol, cinnamaldehyde and cinnamyl alcohol were found to be more enriched in the authentic samples, while peaks of nonanoic acid, vanillin, alletone/furaneol and maltol were related to commercial products in agreement with (Table 2). Regarding the validity of OPLS model, permutation test with negative Q2 intercept value (Fig. 6D) and CV-ANOVA with p < 0.05 confirmed for its significance and no overfit. Interestingly, univariate analysis also showed comparable results with OLPS-DA model as revealed from the volcano plots (Fig. S4) where peaks related to endo-borneol, terpinen-4-ol and cinnamaldehyde were found enriched in the authentic samples. On the other hand, peaks related to benzyl alcohol was found significant and more enriched in the Naturelle® preparation while peaks of vanillin, alletone/furaneol, methyl octanoate and maltol were related to Nu-prep LELAKI® preparation posing these volatiles as differential metabolites for tongkat ali aroma profile.
(A) OPLS-DA score plot and (B) loading S-plots derived from E. longifolia authentic samples modelled against other commercial samples analysed by GC–MS. (C) top 10 variables that have the greatest influence on the projection as revealed from the developed OPLS-DA model. (D) Permutation test (n = 200) as a validation criterion for the OPLS-DA model, the test verifies the validity of the model with negative intercept for the Q2 values.
Conclusion
A comparative multiplexed metabolomics approach of NMR and GC–MS based profiling has been developed for the authentication, profiling, and standardization of tongkat ali compared to its commercial products. A total of 15 metabolites have been identified using NMR dataset mostly ascribed to sugars, organic and fatty acids, in addition to quassinoids as cinnamates as secondary metabolites. The multivariate analysis of the NMR fingerprint revealed for differences between the authentic and the commercial products with peaks related to fatty acids and 13,21-dihydroeurycomanone found to be more enriched in the authentic samples compared to commercial products. Asides, volatile profile of tongkat ali and two of its commercial products were characterized via SPME GC–MS analysis. The profile of the authentic root was relatively more similar to one commercial product Naturelle® than Nu-prep LELAKI® and indicating a potential contribution from the industrial processing and pharmaceutical formulation. The three products differed regarding the most abundant class, while the authentic product was rich in cinnamaldehyde and β-caryophyllene, Nu-prep LELAKI® was abundant in methyl octanoate (fatty acid/ester). Markers for processing and thermal treatment was suggested based on the detection of maltol and 2-acetylpyrroleThe current research might help in authentication of tongkat ali as major flavor component in food, i.e., coffee in addition to nutraceuticals based on such multi-analytical platforms. Application of other metabolomics tools such as liquid chromatography mass spectrometry should provide better insight on secondary metabolome composition and impact of formulation in that major drug.
Data availability
The data presented in this study are available on request from the corresponding author.
References
Bhat, R. & Karim, A. A. Tongkat Ali (Eurycoma longifolia Jack): A review on its ethnobotany and pharmacological importance. Fitoterapia 81, 669–679. https://doi.org/10.1016/j.fitote.2010.04.006 (2010).
Rehman, S. U., Choe, K. & Yoo, H. H. Review on a traditional herbal medicine, Eurycoma longifolia Jack (Tongkat Ali): Its traditional uses, chemistry, evidence-based pharmacology and toxicology. Molecules 21, 331 (2016).
Ang, H. H., Ikeda, S. & Gan, E. K. Evaluation of the potency activity of aphrodisiac in Eurycoma longifolia Jack. Phytother. Res. 15, 435–436. https://doi.org/10.1002/ptr.968 (2001).
Ahmad, N. et al. Eurycoma longifolia-infused coffee—An oral toxicity study. Nutrients 12, 3125. https://doi.org/10.3390/nu12103125 (2020).
Kotirum, S., Ismail, S. & Chaiyakunapruk, N. Efficacy of Tongkat Ali (Eurycoma longifolia) on erectile function improvement: Systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 23, 693–698. https://doi.org/10.1016/j.ctim.2015.07.009 (2015).
GhaziFaisal, N., Kamalanathan, N., Deeveeya, A. P. K. & GhasakGhazi, F. Comparison of Tongkat Ali root chemical composition extracted by soxhlet, conventional steam and microwave assisted extraction techniques. Pharmacogn. J. 10, 916–920 (2018).
Abd El-Kader, E. M., Serag, A., Aref, M. S., Ewais, E. E. A. & Farag, M. A. Metabolomics reveals ionones upregulation in MeJA elicited Cinnamomum camphora (camphor tree) cell culture. Plant Cell Tissue Organ Cult. (PCTOC) 137, 309–318. https://doi.org/10.1007/s11240-019-01572-z (2019).
Farag, M. A., Dokalahy, E. U., Eissa, T. F., Kamal, I. M. & Zayed, A. Chemometrics-based aroma discrimination of 14 Egyptian mango fruits of different cultivars and origins, and their response to probiotics analyzed via SPME coupled to GC–MS. ACS Omega 7, 2377–2390. https://doi.org/10.1021/acsomega.1c06341 (2022).
Xu, J. & Ouyang, G. Solid Phase Microextraction: Recent Developments and Applications (eds. Ouyang, G. & Jiang, R.). 17–61 (Springer, 2017).
Farag, M. A., Afifi, S. M., Rasheed, D. M. & Khattab, A. R. Revealing compositional attributes of Glossostemon bruguieri Desf. root geographic origin and roasting impact via chemometric modeling of SPME–GC–MS and NMR metabolite profiles. J. Food Compos. Anal. 102, 104073. https://doi.org/10.1016/j.jfca.2021.104073 (2021).
Baky, M. H., Shamma, S. N., Xiao, J. & Farag, M. A. Comparative aroma and nutrients profiling in six edible versus nonedible cruciferous vegetables using MS based metabolomics. Food Chem. 383, 132374. https://doi.org/10.1016/j.foodchem.2022.132374 (2022).
Farag, M. A., Khaled, S. E., El Gingeehy, Z., Shamma, S. N. & Zayed, A. Comparative metabolite profiling and fingerprinting of medicinal cinnamon bark and its commercial preparations via a multiplex approach of GC–MS, UV, and NMR techniques. Metabolites 12, 614 (2022).
Kuo, P.-C., Damu, A. G., Lee, K.-H. & Wu, T.-S. Cytotoxic and antimalarial constituents from the roots of Eurycoma longifolia. Bioorg. Med. Chem. 12, 537–544. https://doi.org/10.1016/j.bmc.2003.11.017 (2004).
Farag, M. A., Ajayi, A. O., Taleb, M., Wang, K. & Ayoub, I. M. A Multifaceted review of Eurycoma longifolia nutraceutical bioactives: Production, extraction, and analysis in drugs and biofluids. ACS Omega https://doi.org/10.1021/acsomega.2c06340 (2022).
Salem, M. A. et al. Metabolomics in the context of plant natural products research: From sample preparation to metabolite analysis. Metabolites 10, 37 (2020).
Otify, A. M., Serag, A., Porzel, A., Wessjohann, L. A. & Farag, M. A. NMR metabolome-based classification of Cymbopogon species: A prospect for phyto-equivalency of its different accessions using chemometric tools. Food Anal. Methods 15, 2095–2106. https://doi.org/10.1007/s12161-022-02257-8 (2022).
Md Nor, S., Ding, P., Abas, F. & Mediani, A. 1H NMR reveals dynamic changes of primary metabolites in purple passion fruit (Passiflora edulis Sims) juice during maturation and ripening. Agriculture 12, 156 (2022).
Farag, M. A., Porzel, A., Mahrous, E. A., El-Massry, M. M. & Wessjohann, L. A. Integrated comparative metabolite profiling via MS and NMR techniques for Senna drug quality control analysis. Anal. Bioanal. Chem. 407, 1937–1949. https://doi.org/10.1007/s00216-014-8432-1 (2015).
Zayed, A. et al. Dissecting coffee seeds metabolome in context of genotype, roasting degree, and blending in the Middle East using NMR and GC/MS techniques. Food Chem. 373, 131452. https://doi.org/10.1016/j.foodchem.2021.131452 (2022).
Farag, M. A., Rasheed, D. M. & Kamal, I. M. Volatiles and primary metabolites profiling in two Hibiscus sabdariffa (roselle) cultivars via headspace SPME–GC–MS and chemometrics. Food Res. Int. 78, 327–335. https://doi.org/10.1016/j.foodres.2015.09.024 (2015).
Ebrahimi, F., Ibrahim, B., Teh, C. H., Murugaiyah, V. & Lam, C. K. 1HNMR-based discriminatory analysis of Eurycoma longifolia from different locations and establishing a profile for primary metabolites identification and quassinoids quantification. Planta Med. 83, 172–182 (2017).
Low, B.-S., Das, P. K. & Chan, K.-L. Standardized quassinoid-rich Eurycoma longifolia extract improved spermatogenesis and fertility in male rats via the hypothalamic–pituitary–gonadal axis. J. Ethnopharmacol. 145, 706–714. https://doi.org/10.1016/j.jep.2012.11.013 (2013).
Ruan, J. et al. Bioactive constituents from the roots of Eurycoma longifolia. Molecules 24, 3157 (2019).
Farag, M. A., Labib, R. M., Noleto, C., Porzel, A. & Wessjohann, L. A. NMR approach for the authentication of 10 cinnamon spice accessions analyzed via chemometric tools. LWT 90, 491–498. https://doi.org/10.1016/j.lwt.2017.12.069 (2018).
Low, B. S., Choi, S. B., Abdul Wahab, H., Das, P. K. & Chan, K. L. Eurycomanone, the major quassinoid in Eurycoma longifolia root extract increases spermatogenesis by inhibiting the activity of phosphodiesterase and aromatase in steroidogenesis. J. Ethnopharmacol. 149, 201–207. https://doi.org/10.1016/j.jep.2013.06.023 (2013).
Najmuldeen, G. F., Fatmanathan, H. A., Ghazi, G. & Hassan, Z. Characterization of Eurycoma longifolia (Tongkat Ali) essential oils extracted by microwave assisted extraction. J. Appl. Pharmaceut. Sci. 7, 062–068. https://doi.org/10.7324/JAPS.2017.70609 (2017).
Shafiqul Islam, A. K. M. et al. Correlation studies between electronic nose response and headspace volatiles of Eurycoma longifolia extracts. Sens. Actuators B Chem. 120, 245–251. https://doi.org/10.1016/j.snb.2006.02.020 (2006).
Almeida, J. R. G. D. S. et al. Borneol, a bicyclic monoterpene alcohol, reduces nociceptive behavior and inflammatory response in mice. Sci. World J. 2013, 808460. https://doi.org/10.1155/2013/808460 (2013).
Shapira, S., Pleban, S., Kazanov, D., Tirosh, P. & Arber, N. Terpinen-4-ol: A novel and promising therapeutic agent for human gastrointestinal cancers. PLoS ONE 11, e0156540. https://doi.org/10.1371/journal.pone.0156540 (2016).
Nations, T. F. a. A. O. o. t. U. Food Safety and Quality: Online Edition “Specifications for Flavourings”. http://www.fao.org/food/food-safety-quality/scientific-advice/jecfa/jecfa-flav/details/en/c/220/ (2021).
Lasekan, O. & Teoh, L. S. Contribution of aroma compounds to the antioxidant properties of roasted white yam (Dioscorea rotundata). BMC Chem. 13, 133. https://doi.org/10.1186/s13065-019-0650-3 (2019).
Acknowledgements
Dr. Mohamed A. Farag acknowledges the financial support received from the Alexander von Humboldt Foundation, Germany.
Author information
Authors and Affiliations
Contributions
A.S. Data curation, Formal Analysis, Visualization, Writing—Original Draft; A.Z. Data curation, Visualization, Writing—Original Draft; A.M. Resources, Writing—Original Draft; M.A.F. Methodology, Resources, Writing—Review & Editing, Project Administration. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Serag, A., Zayed, A., Mediani, A. et al. Integrated comparative metabolite profiling via NMR and GC–MS analyses for tongkat ali (Eurycoma longifolia) fingerprinting and quality control analysis. Sci Rep 13, 2533 (2023). https://doi.org/10.1038/s41598-023-28551-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-28551-x
This article is cited by
-
A multiplex metabolomic approach for quality control of Spirulina supplement and its allied microalgae (Amphora & Chlorella) assisted by chemometrics and molecular networking
Scientific Reports (2024)
-
Tensor methods in data analysis of chromatography/mass spectroscopy-based plant metabolomics
Plant Methods (2023)
-
Aroma-based discrimination of Egyptian versus Indian guava fruits and in response to probiotics as analyzed via SPME/GC–MS and chemometric tools
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.